Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry
Abstract
:1. Introduction
- (1)
- search for new PI or PS being able (i) to absorb the visible lights that are very often lost when employing conventional mercury lamps and PISs and/or (ii) to move the system towards a UV-free exposure (doped Hg lamps, Xe-Hg lamps, Xe lamps).
- (2)
- use of newly developed high intensity LED or laser diodes operating at well-defined near UV/visible wavelengths avoiding the use of Hg-based lamps and the presence of more energetic UV wavelengths (254, 313 nm). Today, in industrial applications, LED technology allows highly packed arrays operating at 365 or 395 nm, together with a low heat generation, low energy consumption, low cost and low maintenance; the development of laser diode arrangements ensures high intensity monochromatic irradiations from the blue to the red part of the spectrum.
- (3)
- development of PISs for soft irradiation conditions and use of low visible light intensity sources, e.g., household devices: halogen lamp, fluorescent bulbs and LED bulbs.
- (4)
- use of sunlight, which is a cheap and inexhaustible energy source (but strongly affected by the weather and location) that might be of interest for (i) particular outdoor applications (e.g., for paint drying) and (ii) the possibility of curing large dimensioned pieces or surfaces without requiring any irradiation device.
- (5)
- search of natural products or renewable monomers (the plant oil derivatives present attractive features, such as versatility, biodegradability and low cost).
2. Drawbacks/Breakthroughs on the Way to Greener Photopolymerization Reactions
2.1. The Photopolymerization Reactions

2.2. Reactions under High Intensity Sources Emitting Visible Lights

2.3. The Oxygen Inhibition
2.4. The Soft Irradiation Conditions
2.5. The Development of New Photosensitive Systems
2.6. The Photoredox Catalysis
2.7. Renewable Monomers and Oligomers

3. Greener Photopolymerization Reactions: Attained Performance in Recent Laboratory Scale Experiments
3.1. 3a/ Soft or Eco-Friendly Photopolymerization of Synthetic Monomers
3.1.1. Design of New PIS Allowing a UV-Free Exposure and Ensuring the Use of Visible Light
3.1.2. Use of Newly Developed LEDs and Laser Diodes Avoiding Hg-Based Lamps
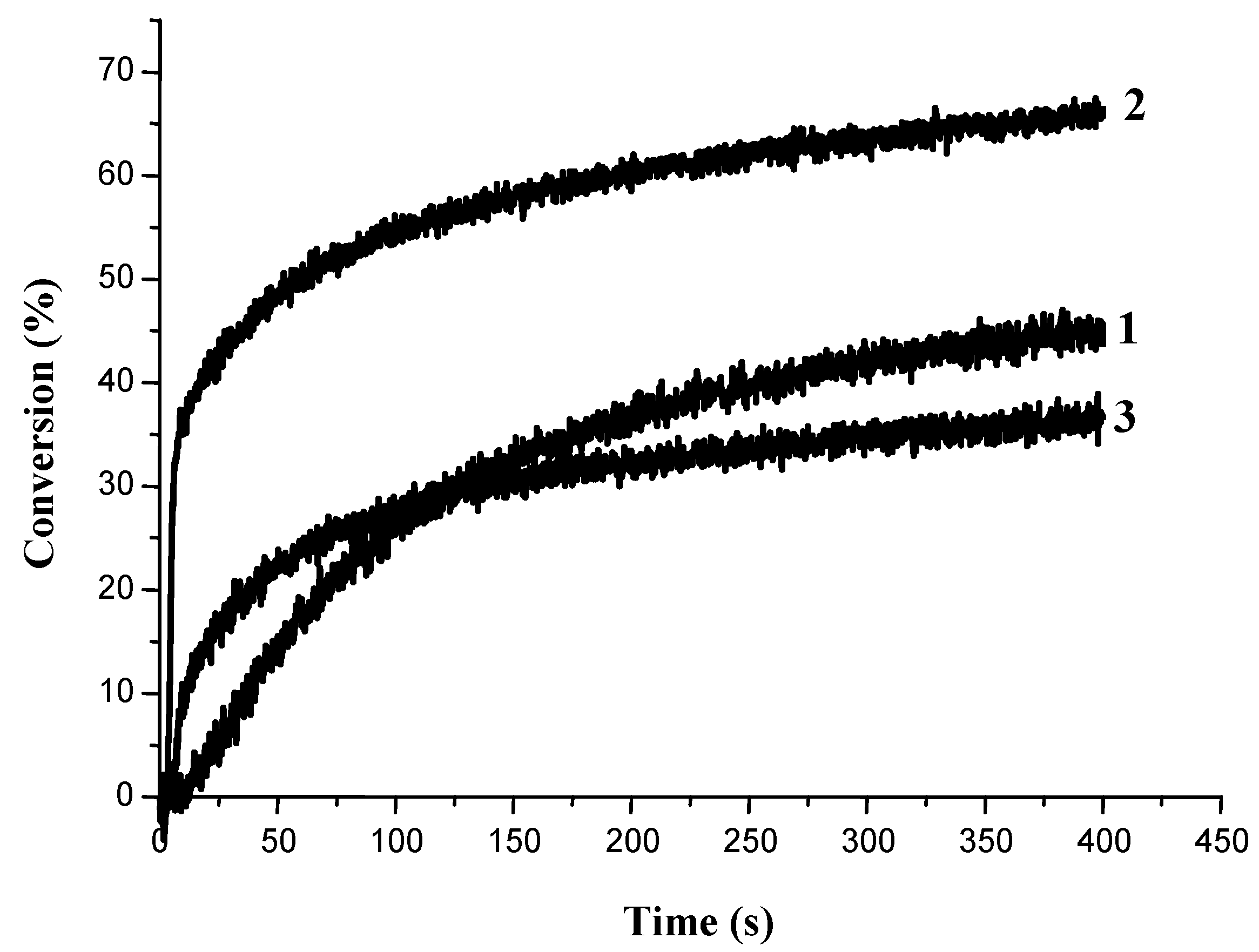
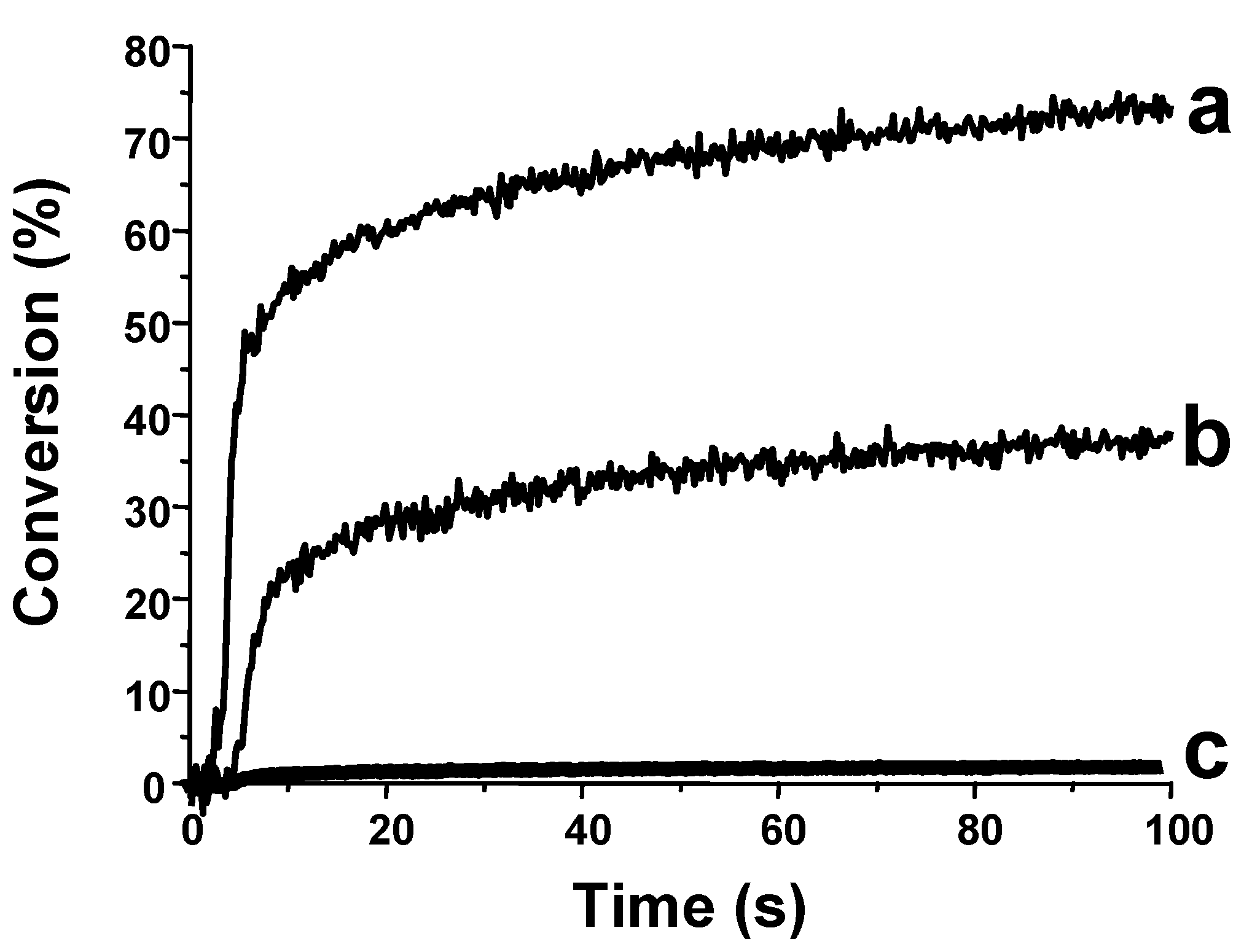
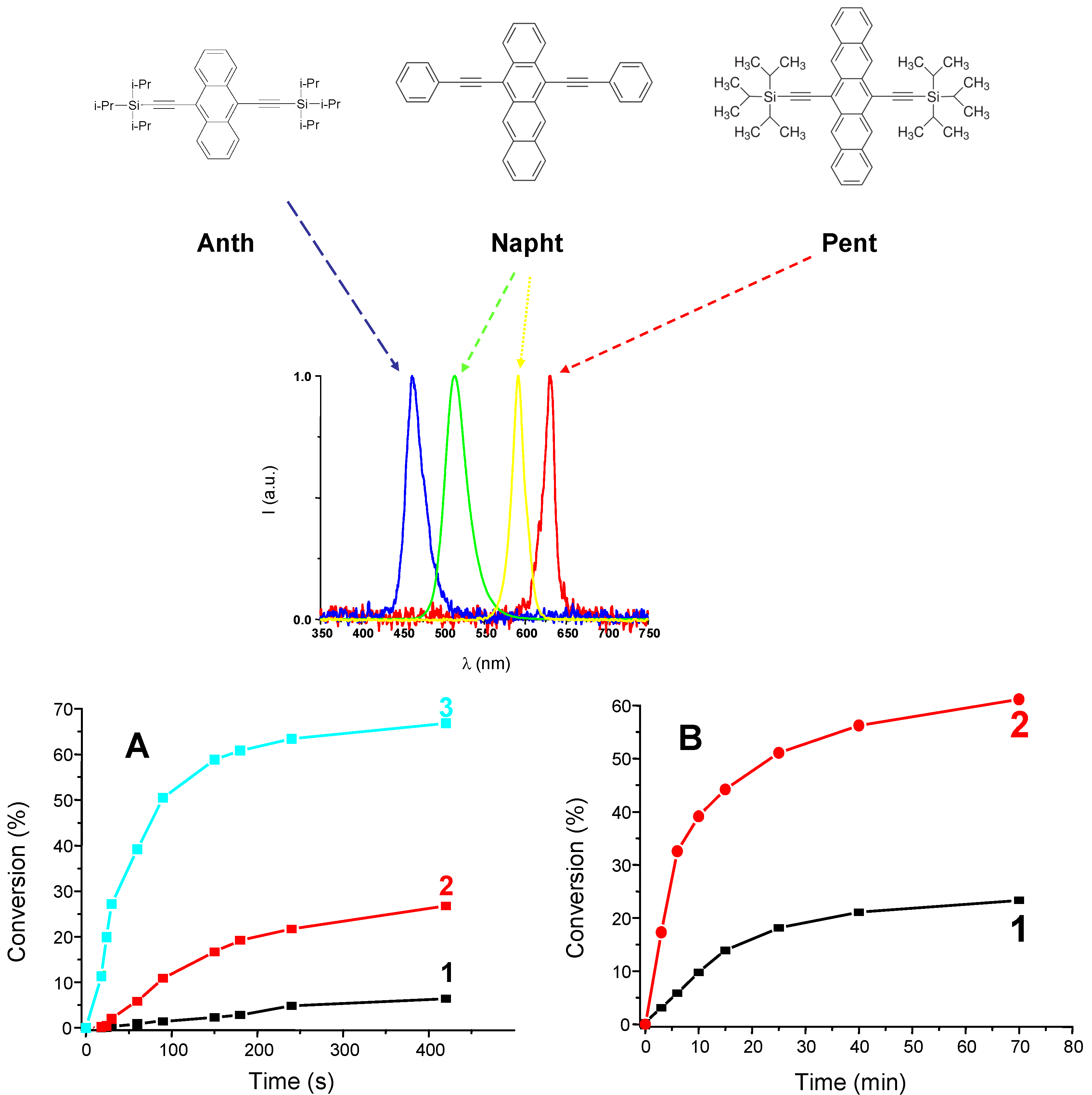
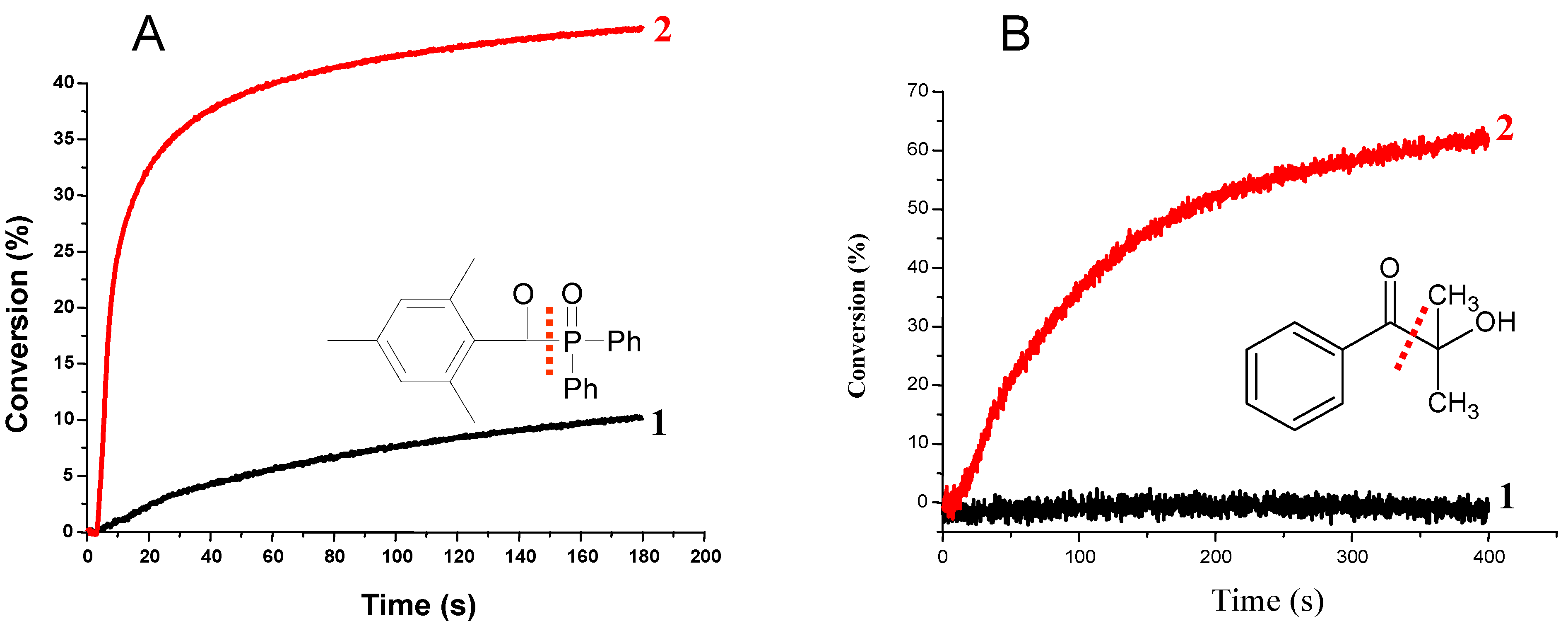
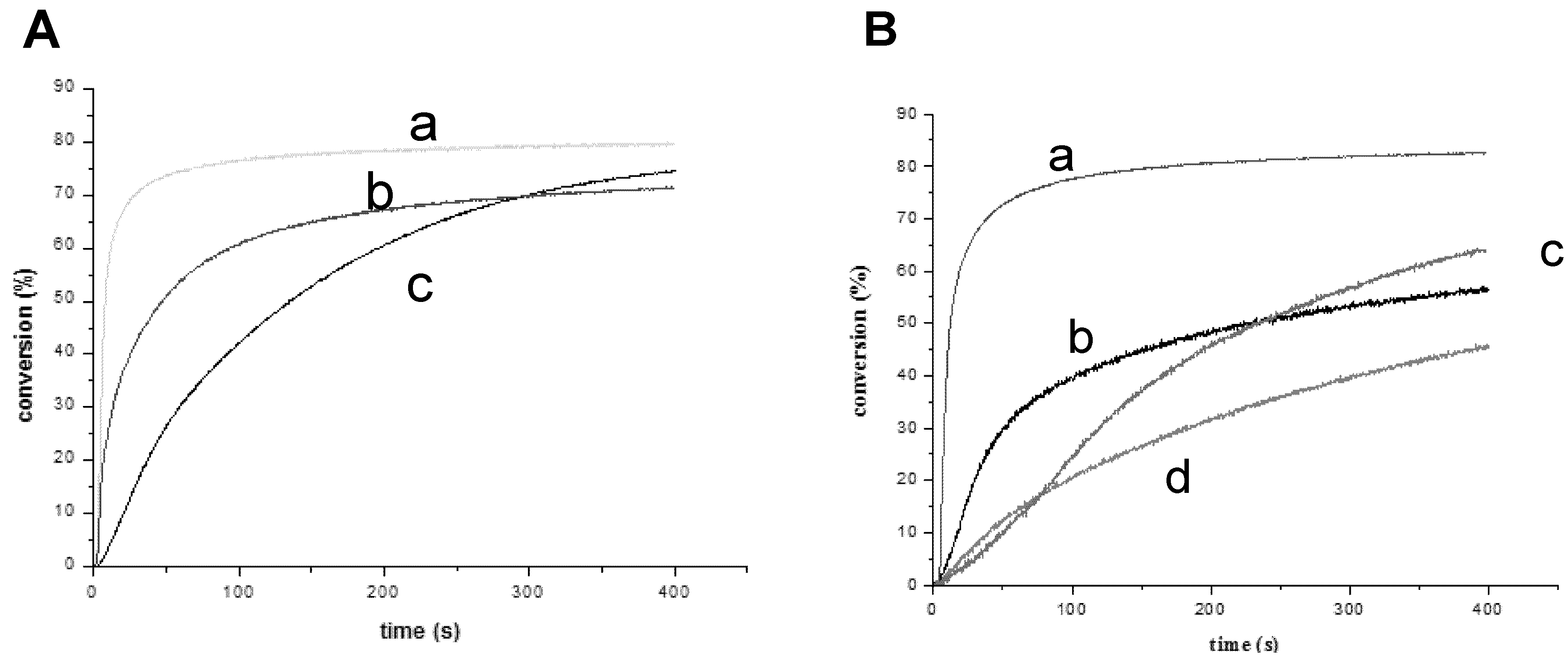
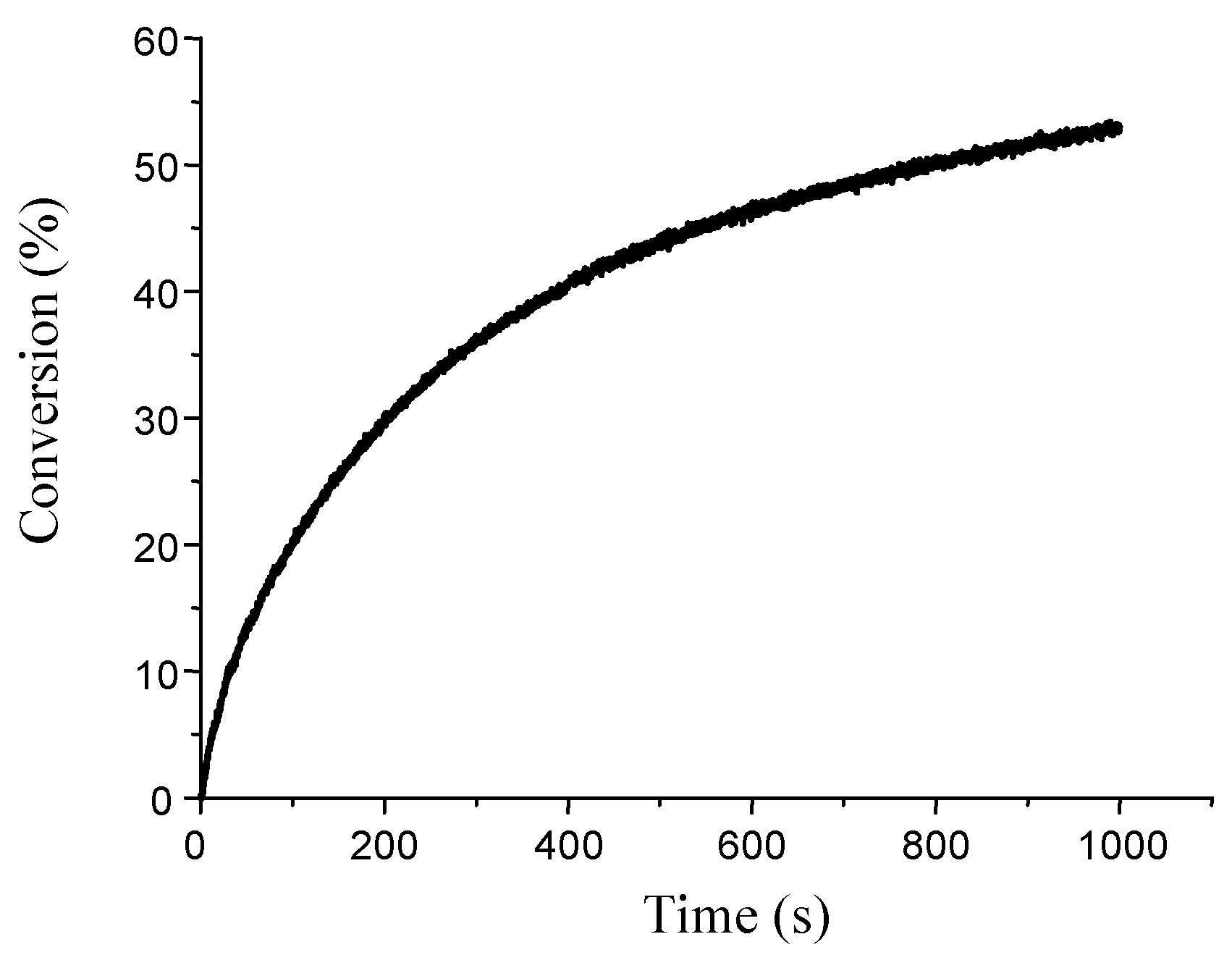
3.1.3. Development of PISs for Soft Irradiation Conditions
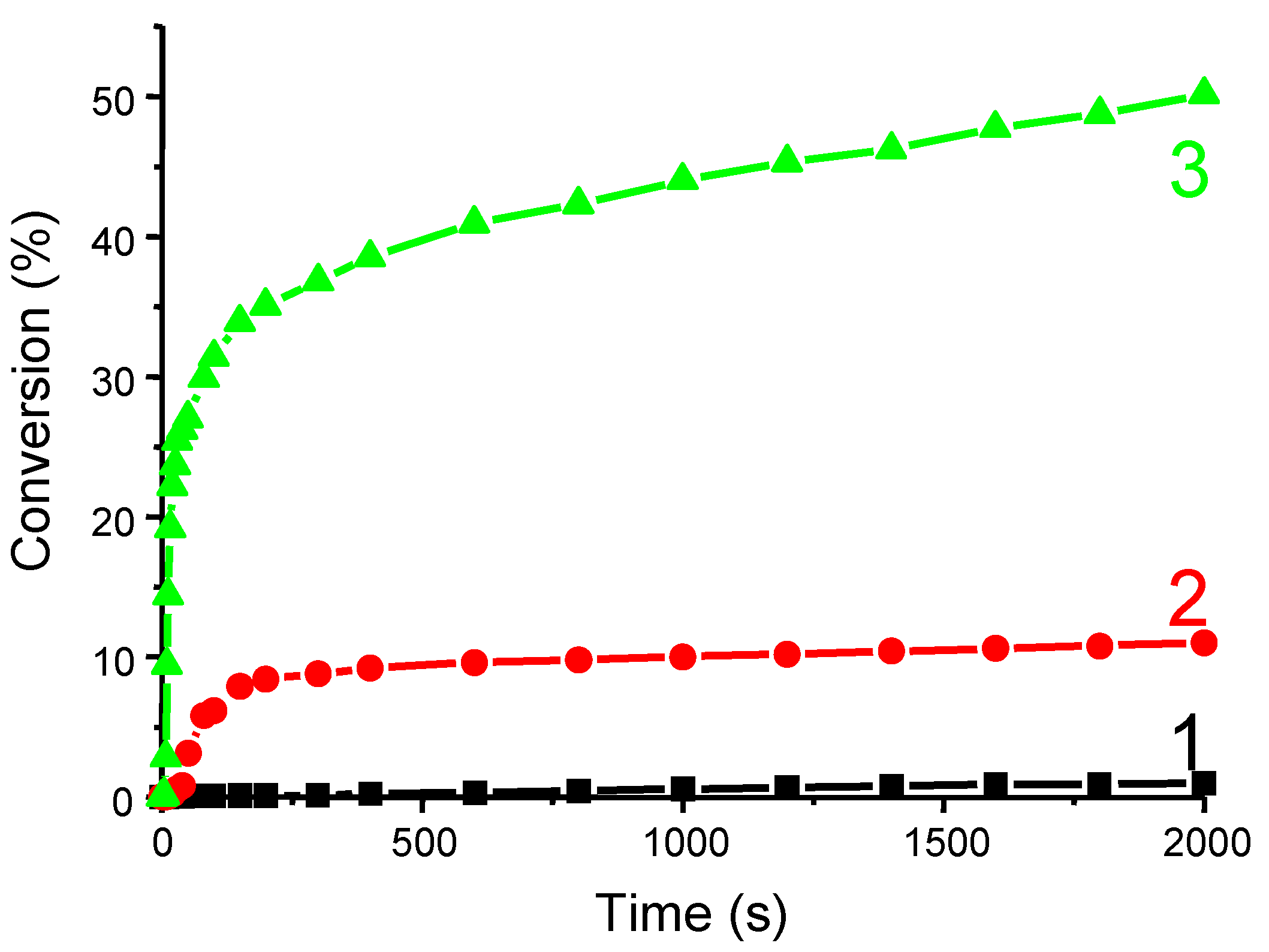

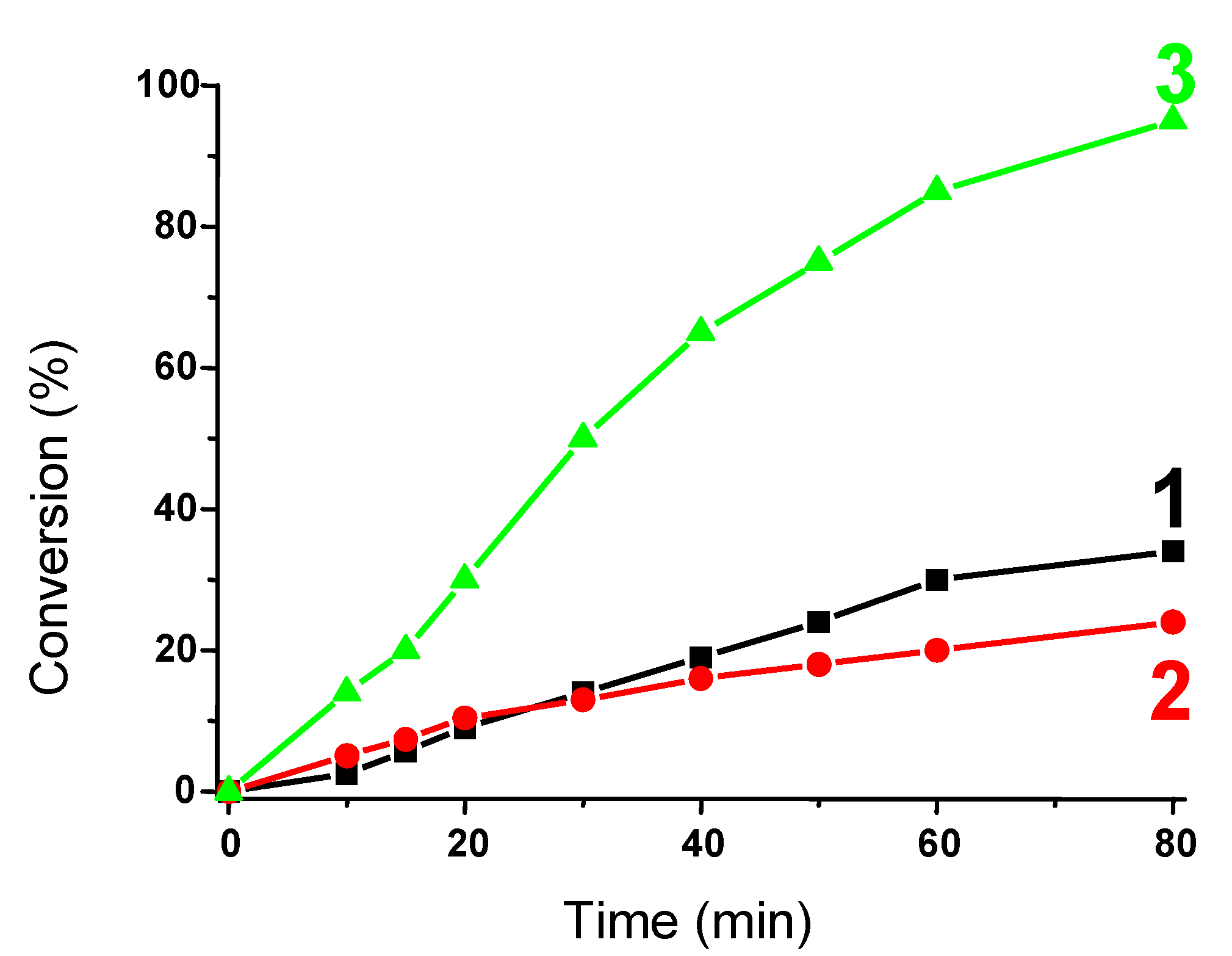
3.1.4. Use of Sunlight Irradiation
| Monomers | Rp/[M0] c (s−1) | Conversion |
|---|---|---|
| LDO a | 0.016 | 71.4% |
| ESO a | 0.002 | 21.5% |
| ELO a | 0.0002 | 7.0% |
| EFA a | 0.001 | 9.0% |
| LDO b | 0.05 | 81.4% |
| ESO b | 0.006 | 43.1% |
| ELO b | 0.0006 | 29.7% |
| EFA b | 0.001 | 29.0% |
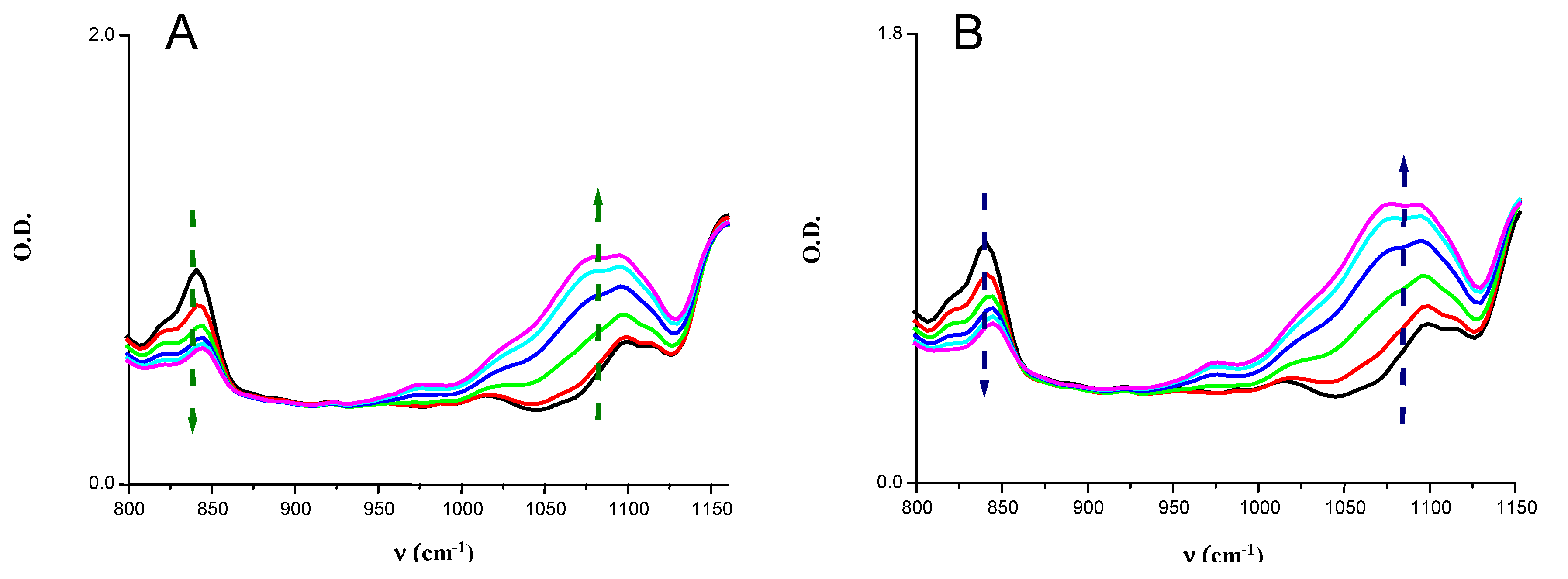
| Monomer | Tackfree |
|---|---|
| LDO a | 48 min |
| ESO a | * |
| ELO a | 50 min |
| EFA a | * |
| LDO b | 12 min |
| ESO b | 45 min |
| ELO b | 9 min |
| EFA b | * |
4. Conclusions
References
- Radiation Curing of Polymeric Materials; Hoyle, C.E.; Kinstle, J.F. (Eds.) American Chemical Society: Washington, DC, USA, 1990.
- Lasers in Polymer Science and Technology: Applications; Fouassier, J.P.; Rabek, J.F. (Eds.) CRC Press: Boca Raton, FL, USA, 1990.
- Pappas, S.P. UV-Curing: Science and Technology; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Radiation Curing in Polymer Science and Technology; Fouassier, J.P.; Rabek, J.F. (Eds.) Chapman & Hall: London, UK, 1993.
- Photoresponsive Polymers; Krongauz, V.; Trifunac, A. (Eds.) Chapman and Hall: New York, NY, USA, 1994.
- Reiser, A. Photoreactive Polymers: The Science and Technology of Resists; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Fouassier, J.P. Photoinitiation, Photopolymerization, Photocuring: Fundamentals and Applications; Carl Hanser GmbH: Munich, Germany, 1995. [Google Scholar]
- Davidson, S. Exploring the Science, Technology and Application of UV and EB Curing; SITA Technology Ltd.: London, UK, 1999. [Google Scholar]
- Neckers, D.C. UV and EB at the Millenium; SITA Technology Ltd.: London, UK, 1999. [Google Scholar]
- Crivello, J.V.; Dietliker, K. Photoinitiators for Free Radical, Cationic and Anionic Photopolymerization; Bradley, G., Ed.; Surface Coatings Technology Series; Wiley: New York, NY, USA, 1999; Volume III. [Google Scholar]
- Dietliker, K. A Compilation of Photoinitiators Commercially Available for UV Today; SITA Technology Ltd.: London, UK, 2002. [Google Scholar]
- Belfied, K.D.; Crivello, J.V. Photoinitiated Polymerization; ACS Symp. Ser. 847; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Photochemistry and UV Curing; Fouassier, J.P. (Ed.) Research Signpost: Trivandrum, India, 2006.
- 14. Photochemistry and Photophysics of Polymer Materials; Allen, N.S. (Ed.) Wiley: Hoboken, NJ, USA, 2010.
- Basics of Photopolymerization Reactions; Fouassier, J.P.; Allonas, X. (Eds.) Research Signpost: Trivandrum, India, 2010.
- Green, W.A. Industrial Photoinitiators; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Peiffer, R.W. Photopolymerization: Fundamentals and Applications; Scranton, A.B., Bowman, A., Eds.; ACS Symp. Ser. 673; Americal Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Handbook of Vinyl Polymers; Mishra, M.K.; Yagci, Y. (Eds.) CRC Press: Boca Raton, FL, USA, 2009.
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley VCH: Weinheim, Germany, 2012. [Google Scholar]
- Esposito Corcione, C.; Previderio, A.; Frigione, M. Kinetics characterization of a novel photopolymerizable siloxane-modified acrylic resin. Thermochim. Acta 2010, 509, 56–61. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Greco, A.; Maffezzoli, A. Photopolymerization kinetics of an epoxy-based resin for stereolithography. J. Appl. Polym. Sci. 2004, 92, 3484–3491. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Greco, A.; Maffezzoli, A. Time–temperature and time-irradiation intensity superposition for photopolymerization of an epoxy based resin. Polymer 2005, 46, 8018–8027. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Greco, A.; Maffezzoli, A. Temperature evolution during stereolithography building with a commercial epoxy resin. Polym. Eng. Sci. 2006, 46, 493–502. [Google Scholar] [CrossRef]
- Greco, A.; Esposito Corcione, C.; Cavallo, A.; Maffezzoli, A. Influence of stone particles on the rheological behavior of a novel photopolymerizable siloxane-modified acrylic resin. J. Appl. Polym. Sci. 2011, 122, 942–947. [Google Scholar] [CrossRef]
- Frigione, M.; Esposito Corcione, C. Rheological and kinetic characterization of UV photopolymerizable formulations as a function of the boehmite nanoparticle content. Open Mater. Sci. J. 2012, 6, 68–76. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Frigione, M. Factors influencing photo curing kinetics of novel UV-cured siloxane-modified acrylic coatings: Oxygen inhibition and composition. Thermochim. Acta 2012, 534, 21–27. [Google Scholar] [CrossRef]
- Yilmaz, G.; Iskin, B.; Yilmaz, F.; Yagci, Y. Mesoporous graphitic carbon nitride as a heterogeneous visible light photoinitiator for radical polymerization. ACS Macro Lett. 2012, 1, 1212–1215. [Google Scholar] [CrossRef]
- Karaka-Balta, D.; Temel, G.; Okal, N.; Arsu, N. Thioxanthone–diphenyl anthracene: Visible light photoinitiator. Macromolecules 2012, 45, 119–125. [Google Scholar] [CrossRef]
- Yilmaz, G.; Acik, G.; Yagci, Y. Counteranion sensitization spproach to photoinitiated free radical polymerization. Macromolecules 2012, 45, 2219–2224. [Google Scholar] [CrossRef]
- Kumbaraci, V.; Aydogan, B.; Talinli, N.; Yagci, Y. Naphthodioxinone-1,3-benzodioxole as photochemically masked one-component Type II photoinitiator for free radical polymerization. J. Polym. Sci. Part A 2012, 50, 2612–2618. [Google Scholar]
- Temel, G.; Enginol, B.; Aydin, M.; Karaca Balta, D.; Arsu, N. Photopolymerization and photophysical properties of amine linked benzophenone photoinitiator for free radical polymerization. J. Photochem. Photobiol. A 2011, 219, 26–31. [Google Scholar]
- Keskin Dogruyol, S.; Dogruyol, Z.; Arsu, N. A thioxanthone-based visible photoinitiator. J. Polym. Sci. Part A 2011, 49, 4037–4040. [Google Scholar]
- Sevinc Esen, D.; Karasu, F.; Arsu, N. The investigation of photoinitiated polymerization of multifunctional acrylates with TX-BT by Photo-DSC and RT-FTIR. Progr. Org. Coat. 2011, 70, 102–107. [Google Scholar]
- Korkut, S.E.; Temel, G.; Karaca Balta, D.; Arsu, N.; Kasım Şener, M. Type II photoinitiator substituted zinc phthalocyanine: Synthesis, photophysical and photopolymerization studies. J. Luminescence 2013, 136, 389–394. [Google Scholar]
- Asvos, X.; Siskos, M.G.; Zarkadis, A.K.; Hermann, R.; Brede, O. The 2-benzoyl xanthone/triethylamine system as a type II photoinitiator: A laser flash photolysis and computational study. J. Photochem. Photobiol. A 2011, 219, 255–264. [Google Scholar] [CrossRef]
- Santos, W.G.; Schmitt, C.C.; Neumann, M.G. Polymerization of HEMA photoinitiated by the Safranine/diphenylborinate system. J. Photochem. Photobiol. A 2013, 252, 124–130. [Google Scholar]
- Kitano, H.; Ramachandran, K.; Bowden, N.B.; Scranton, A.B. Unexpected visible-light-induced free radical photopolymerization at low light intensity and high viscosity using a titanocene photoinitiator. J. Appl. Polym. Sci. 2013, 128, 611–618. [Google Scholar] [CrossRef]
- Han, J.; Wang, J.; Shen, K.; Wang, G.; Li, Y.; Zhao, D. Synthesis of novel photochromic spiropyran dyes containing quaternary ammonium salt or cinnamoyl moiety and their properties as photoinitiators. J. Appl. Polym. Sci. 2012, 126, 30–37. [Google Scholar] [CrossRef]
- Ivan, M.G.; Scaiano, J.C. Photoimaging and Photolithographic Process in Polymers. In Handbook on Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 479–508. [Google Scholar]
- Muftuogli, A.E.; Tasdelen, M.A.; Yagci, Y. Photografting of Polymeric Materials. In Handbook on Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 509–540. [Google Scholar]
- Yang, J.; Shi, S.; Xu, F.; Nie, J. Synthesis and photopolymerization kinetics of benzophenone sesamol one-component photoinitiator. Photochem. Photobiol. Sci. 2013, 12, 323–329. [Google Scholar] [CrossRef]
- Rosspeintner, A.; Griesser, M.; Pucher, N.; Iskra, K.; Liska, R.; Gescheidt, G. Toward the photoinduced reactivity of 1,5-diphenylpenta-1,4-diyn-3-one (DPD): Real-time investigations by magnetic resonance. Macromolecules 2009, 42, 8034–8038. [Google Scholar] [CrossRef]
- Moszner, N.; Lamparth, I.; Angermann, J.; Fischer, U.K.; Zeuner, F.; Bock, T.; Liska, R.; Rheinberger, V. Synthesis of bis(3-{[2-(allyloxy)ethoxy]methyl}-2,4,6-trimethylbenzoyl)(phenyl)phosphine oxide—A tailor-made photoinitiator for dental adhesives. Beilstein J. Org. Chem. 2010, 6, 1–9. [Google Scholar]
- Dworak, C.; Kopeinig, S.; Hoffmann, H.; Liska, R. Photoinitiating monomers based on di- and triacryloylated hydroxylamine derivatives. J. Polym. Sci. Part A 2009, 47, 392–403. [Google Scholar] [CrossRef]
- Li, Z.; Siklos, M.; Pucher, N.; Cicha, K.; Ajami, A.; Husinsky, W.; Rosspeintner, A.; Vauthey, E.; Gescheidt, G.; Stampfl, J.; et al. Synthesis and structure-activity relationship of several aromatic ketone-based two-photon initiators. J. Polym. Sci. Part A 2011, 49, 3688–3699. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar]
- Kabatc, J.; Jurek, K. Free radical formation in three-component photoinitiating systems. Polymer 2012, 53, 1973–1980. [Google Scholar]
- Czech, Z.; Butwin, A.; Kabatc, J. Photoreactive s-triazine as crosslinking agents for UV-crosslinkable acrylic pressure-sensitive adhesives. J. Appl. Polym. Sci. 2011, 120, 3621–3627. [Google Scholar] [CrossRef]
- Jedrzejewska, B.; Urbanski, S. Studies on an argon laser-induced photopolymerization employing both mono- and bischromophoric hemicyanine dye-borate complex as a photoinitiator. Part III. J. Appl. Polym. Sci. 2010, 118, 1395–1405. [Google Scholar]
- Kabatc, J.; Krzyzanowska, E.; Jedrzejewska, B.; Pietrzak, M.; Paczkowski, J. Novel N-ethyl-2-styrylquinolinum iodides as sensitizers in photoinitiated free radical polymerization of trimethylolopropane triacrylate (TMPTA). J. Appl. Polym. Sci. 2010, 118, 165–172. [Google Scholar]
- Lalevée, J.; Tehfe, M.A.; Allonas, X.; Foussier, J.P. Photoinitiating systems based on unusual radicals. In Polymer Initiators; Ackrine, W.J., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; chapter 8. [Google Scholar]
- Lalevée, J.; Tehfe, M.A.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.P. Redox or photoinduced ring opening polymerization: Initiating systems based on organosilanes bearing a Si–Si bond. In Radical Polymerization: New Developments; Pauloskas, I.O., Urbonas, L.A., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; chapter 7. [Google Scholar]
- Fouassier, J.P.; Lalevée, J. Three-component photoinitiating systems: Towards innovative tailor made high performance combinations. Adv. Chem. 2012, 2, 2621–2629. [Google Scholar]
- Lalevée, J.; Fouassier, J.P. Overview of Radical Initiation. In In Encyclopedia of Radicals in Chemistry, Biology and Materials; Studer, A., Chatgilialoglou, C., Eds.; Wiley: New York, NY, USA, 2012; Volume 1, chapter 2. [Google Scholar]
- Lalevee, J.; EL Roz, M.; Allonas, X.; Fouassier, J.P. On the silyl radical chemistry in photopolymerization reactions. In Organosilanes: Properties, Performance and Applications; Wyman, E., Skief, M.C., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2009; chapter 6. [Google Scholar]
- Fouassier, J.P.; Lalevée, J. Design of Chromophores for Photoinitiators of Polymerization: Brief Survey and Recent Achievements. In New Developments in Chromophore Research; Nova Science Publishers: Hauppauge, NY, USA, in press.
- Lalevée, J.; Tehfe, M.A.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Green light induced cationic ring opening polymerization reactions: Perylene bis-dicarboximides as efficient photosensitizers. Macromol Chem. Phys. 2013. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Clément, J.L.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P.; Lalevée, J. A new cleavable photoinitiator architecture with huge molar extinction coefficients for polymerization in the 340–420 nm range. Macromolecules 2013, 46, 736–746. [Google Scholar]
- Lalevée, J.; El Roz, M.; Tehfe, M.A.; Allonas, X.; Fouassier, J.P. Long wavelength cationic photopolymerization in aerated media: A remarkable titanocene/tris(trimethylsilyl)silane/onium salt photoinitiating system. Macromolecules 2009, 42, 8669–8674. [Google Scholar]
- Lalevée, J.; Blanchard, N.; Fries, C.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. New thioxanthone and xanthone derivative based on silyl radical chemistry. Polym. Chem. 2011, 2, 1077–1084. [Google Scholar] [CrossRef]
- Tarzi, O.I.; Allonas, X.; Ley, C.; Fouassier, J.P. Pyrromethene derivatives in three-component photoinitiating systems for free radical photopolymerization. J. Polym. Sci. Part A 2010, 48, 2594–2603. [Google Scholar]
- Tehfe, M.A.; Dumur, F.; Contal, E.; Graff, B.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P.; Lalevée, J. New insights in radical and cationic polymerization upon visible light exposure: Role of novel photoinitiator systems based on the pyrene chromophore. Polym. Chem. 2013, 4, 1625–1634. [Google Scholar]
- Telitel, S.; Blanchard, N.; Schweitzer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Bodipy derivatives and boranil as new photoinitiating systems of cationic polymerization exhibiting a tunable absorption in the 400–600 nm spectral range. Polymer 2013. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Trifunctional photoinitiators based on a triazine skeleton for visible light sources and UV LED induced polymerizations. Macromolecules 2012, 45, 8639–8647. [Google Scholar]
- Telitel, S.; Lalevée, J.; Blanchard, N.; Kavalli, T.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Photopolymerization of cationic monomers and acrylate/divinylether blends under visible lights using pyrromethene dyes. Macromolecules 2012, 45, 6864–6868. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Zein-Fakih, A.; Lalevée, J.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P. Pyridinium salts: New systems for photopolymerization reactions upon visible light exposure. Eur. Polym. J. 2013, 49, 567–574. [Google Scholar]
- Tehfe, M.A.; Lalevée, J.; Dumur, F.; Zein-Fakih, A.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P. Dye photosensitized cationic ring-opening polymerization: search for new dye skeletons. Polymer 2012, 53, 4947–4954. [Google Scholar]
- Tehfe, M.A.; Lalevée, J.; Fouassier, J.P. A breakthrough for long wavelength absorbing photoinitiating systems under soft irradiation conditions based on violanthrone derivatives. Macromolecules 2011, 44, 8374–8379. [Google Scholar]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Cationic photosensitive formulations based on the silyl radical chemistry for green and red diode laser exposure. Polym. Chem. 2012, 3, 1899–1902. [Google Scholar]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. Household LED irradiation under air: Cationic polymerization using iridium or ruthenium complex photocatalysts. Polym. Bull. 2012, 68, 341–347. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.A.; Morlet-Savary, F.; Telitel, S.; Graff, B.; Fouassier, J.P. Photopolymerization of N-vinylcarbazole using visible-light harvesting iridium complexes as photoinitiators. Macromolecules 2012. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Telitel, S.; Sun, J.; Zhao, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Iridium complexes incorporating coumarin moiety as photocatalysts for ring opening photopolymerizations. Polymer 2012, 53, 2803–2808. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Design of new Type I & Type II photoinitiators possessing highly coupled pyrene-ketone moieties. Polym. Chem. 2013, 4, 2313–2324. [Google Scholar]
- Tehfe, M.A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Light harvesting organic photoinitiators of polymerization. Macromol. Rapid Comm. 2013, 34, 239–245. [Google Scholar]
- Tehfe, M.A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Blanchard, N.; Fouassier, J.P. Organic photocatalyst for polymerization reactions: 9,10-bis[(triisopropylsilyl)ethynyl] anthracene. ACS Macro Lett. 2012, 1, 198–203. [Google Scholar]
- Tehfe, M.A.; Lalevée, J.; Telitel, S.; Contal, E.; Dumur, F.; Gigmes, D.; Bertin, D.; Nechab, M.; Bertrand, M.; Morlet-Savary, F.; et al. Polyaromatic structures as organophotocatalysts for efficient dual radical/cationic photopolymerizations under visible lights and interpenetrated polymer networks synthesis. Macromolecules 2012, 45, 4454–4460. [Google Scholar]
- Lalevée, J.; Fouassier, J.P. Recent advances in sunlight induced polymerization: Role of new photoinitiating systems based on the silyl chemistry. Polym. Chem. 2011, 2, 1107–1113. [Google Scholar] [CrossRef]
- Eiselé, G.; Fouassier, J.-P.; Reeb, R. Kinetics of photocrosslinking reactions of a DCPA/EA matrix in the presence of thiols and acrylates. J. Polym. Sci. Part A 1997, 35, 2333–2345. [Google Scholar]
- Bibaut-Renauld, C.; Burget, D.; Fouassier, J.-P.; Varelas, C.-G.; Thomatos, J.; Tsagaropoulos, G.; Ryrfors, L.O.; Karlsson, O.J. Use of α-diketones as visible photoinitiators for the photocrosslinking of waterborne latex paints. J. Polym. Sci. Part A 2002, 40, 3171–3181. [Google Scholar] [CrossRef]
- Decker, C.; Bendaikha, T. Interpenetrating polymer networks. II. Sunlight-induced polymerization of multifunctional acrylates. J. Appl. Polym. Sci. 1998, 70, 2269–2282. [Google Scholar] [CrossRef]
- Chiang, W.; Lin, W.T. Syntheses and cured films properties of UV-autocurable BTDA-based multiacrylate resins. J. Appl. Polym. Sci. 1994, 51, 1901–1909. [Google Scholar] [CrossRef]
- Chiang, W.; Ding, F.C. Synthesis and properties of ultraviolet-curable resins via a thio–ene (thiol and allyl) addition reaction. J. Appl. Polym. Sci. 2002, 86, 1878–1885. [Google Scholar] [CrossRef]
- Zang, H.L.; Massingilland, J.L.; Woo, J.T. Zero VOC sunlight curable coatings. J. Coat. Technol. 2000, 72, 79–81. [Google Scholar] [CrossRef]
- Decker, C.; Zahouily, K.; Decker, D.; Nguyen Thi Viet, T. Performance analysis of acylphosphine oxides in photoinitiated polymerization. Polymer 2001, 42, 7551–7560. [Google Scholar] [CrossRef]
- Paczkowska, B.; Strzelec, S.; Linden, L.A.; Packowski, D. Photochemical preparation of polymer-clay composites. J. Appl. Clay Sci. 2004, 25, 221–227. [Google Scholar]
- Ledwith, A. Possibilities for promoting cationic polymerization by common sources of free radicals. Polymer 1978, 19, 1217–1219. [Google Scholar] [CrossRef]
- Baumann, H.; Timpe, H.J. Lichtinitiierte polymer- und polymerisationsreaktionen. photoinduzierte zersetzung von diaryliodonium- und triarylsulfoniumsalzen durch benzoinderivate und benzilketale. Z. Chem. 1984, 24, 18–19. [Google Scholar] [CrossRef]
- Yagci, Y.; Schnabel, W. Acylphosphine oxides as free radical promoters in cationic polymerizations. Makromol. Chem. Rapid Commun. 1987, 8, 209–213. [Google Scholar]
- Yagci, Y.; Kminek, I.; Schnabel, W. Long wavelength photoinitiated cationic polymerization using diphenyliodonium salt and catena-poly (phenyl-4-phenylphenylsilicon). Polymer 1993, 34, 426–428. [Google Scholar]
- Yagci, Y.; Ledwith, A. Mechanistic and kinetic studies on the photoinitiated polymerization of tetrahydrofuran. J. Polym. Sci. Part A 1988, 26, 1911–1918. [Google Scholar]
- Crivello, J.V.; Sangermano, M. Visible and long-wavelength photoinitiated cationic polymerization. J. Polym. Sci. Part A 2001, 39, 343–356. [Google Scholar] [CrossRef]
- Dursun, C.; Degirmenci, M.; Yagci, Y.; Jockusch, S.; Turro, N.J. Free radical promoted cationic polymerization by using bisacylphosphine oxide photoinitiators: Substituent effect on the reactivity of phosphinoyl radicals. Polymer 2003, 44, 7389–7396. [Google Scholar]
- Crivello, J.V. Radical-promoted visible light photoinitiated cationic polymerization of epoxides. J. Macromol. Sci. Part A 2009, 46, 474–483. [Google Scholar]
- Yagci, Y.; Denizligil, S. Photoinitiated cationic polymerization using O-phthaldehyde and pyridinium salt. J. Polym. Sci. Part A 1995, 33, 1461–1464. [Google Scholar] [CrossRef]
- Yagci, Y.; Reetz, I. Externally stimulated initiator systems for cationic polymerization. Prog. Polym. Sci. 1998, 23, 1485–1538. [Google Scholar] [CrossRef]
- Bulut, U.; Gunbas, G.E.; Topare, L. A quinoxaline derivative as a long wavelength photosensitizer for diaryliodonium salts. J. Polym. Sci. Part A 2010, 48, 209–213. [Google Scholar] [CrossRef]
- Crivello, J.V. A new visible light sensitive photoinitiator system for the cationic polymerization of epoxides. J. Polym. Sci. Part A 2009, 47, 866–875. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Curcumin: A naturally occurring long-wavelength photosensitizer for diaryliodonium salts. J. Polym. Sci. Part A 2005, 43, 5217–5321. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Chany, A.C.; Morlet-Savary, F.; Fouassier, J.P. Green bulb lamp induced cationic photopolymerization reactions under air. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J.P. Oxygen mediated and wavelength tunable cationic photopolymerization reactions under air and low Intensity: A new concept. Progr. Organ. Coat. 2011, 70, 23–31. [Google Scholar]
- Tehfe, M.A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: sunlight induced cationic polymerization of renewable epoxy monomer under air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Blanchard, N.; Fries, C.; Lalevée, J.; Allonas, X.; Fouassier, J.P. Bis(germyl)ketones: Toward a new class of type I photoinitiating systems under visible light irradiation. Macromol. Rapid Comm. 2010, 31, 473–478. [Google Scholar] [CrossRef]
- Telitel, S.; Schweitzer, S.; Morlet-Savary, F.; Graff, B.; Tschamber, T.; Blanchard, N.; Fouassier, J.P.; Lelli, M.; Lacôte, E.; Lalevée, J. Soft photopolymerizations initiated by dye-sensitized formation of NHC-boryl radicals under visible lights. Macromolecules 2013, 46, 43–48. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Zein-Fakih, A.; Ball, B.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. N-vinylcarbazole: an additive for free radical promoted cationic polymerization upon visible-light. ACS Macro Lett. 2012, 1, 802–806. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. On the use of bis(cyclopentadienyl)titanium(IV) dichloride in visible light induced ring opening photopolymerization. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.-P. Iridium photocatalysts in free radical polymerization under visible lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Blanchard, N.; Fouassier, J.-P. Tunable Organophotocatalysts for polymerization reactions under visible lights, macromolecules. Macromolecules 2012, 45, 1746–1752. [Google Scholar]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Shih, H.-W.; Vander Wal, M.N.; Grange, R.L.; MacMillan, D.W.C. Enantioselective α-benzylation of aldehydes via photoredox organocatalysis. J. Am. Chem. Soc. 2010, 132, 13600–13603. [Google Scholar]
- Zeitler, K. Photoredox catalysis with visible light. Angew. Chem. Int. Ed. 2009, 48, 9785–9789. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar]
- Dai, C.; Narayanam, J.M.R.; Stephenson, C.R.J. Visible-light-mediated conversion of alcohols to halides. Nat. Chem. 2011, 3, 140–145. [Google Scholar]
- Ischay, M.A.; Lu, Z.; Yoon, T.P. [2+2] cycloadditions by oxidative visible light photocatalysis. J. Am. Chem. Soc. 2010, 132, 8572–8574. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Larraufie, M.H.; Pellet, R.; Fensterbank, L.; Goddard, J.P.; Lacôte, E.; Malacria, M.; Ollivier, C. Visible-light-induced photoreductive generation of radicals from epoxides and aziridines. Angew. Chem. Int. Ed. 2011, 50, 4463–4466. [Google Scholar]
- Courant, T.; Masson, G. Photoredox-initiated α-alkylation of imines through a three-component radical/cationic reaction. Chem. Eur. J. 2012, 18, 423–427. [Google Scholar] [CrossRef]
- Zhang, G.; Song, I.Y.; Ahn, K.H.; Park, T.; Choi, W. Free radical polymerization initiated and controlled by visible light photocatalysis at ambient temperature. Macromolecules 2011, 44, 7594–7599. [Google Scholar] [CrossRef]
- Pelletier, H.; Belgacem, N.; Gandini, A. Acrylated vegetable oils as photocrosslinkable materials. J. Appl. Polym. Sci. 2006, 99, 3218–3221. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Karagoz, B.; Bicak, N.; Yagci, Y. Synthesis of block copolymers by combination of ATRP and photoiniferter processes. Polym. Intern. 2008, 57, 1182–1187. [Google Scholar] [CrossRef]
- Dworak, C.; Koch, T.; Varga, F.; Liska, R. Photopolymerization of biocompatible phosphorus-containing vinyl esters and vinyl carbamates. J. Polym. Sci. Part A 2010, 48, 2916–2924. [Google Scholar] [CrossRef]
- Nagata, M.; Inaki, K. Biodegradable and photocurable multiblock copolymers with shape-memory properties from poly(ε-caprolactone) diol, poly(ethylene glycol), and 5-cinnamoyloxyisophthalic acid. J. Appl. Polym. Sci. 2011, 120, 3556–3564. [Google Scholar] [CrossRef]
- Jabbari, E.; He, X. Synthesis and characterization of bioresorbable in situ crosslinkable ultra low molecular weight poly(lactide) macromer. J. Mater. Sci. Mater. Med. 2008, 19, 311–318. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Kayaman-Apohan, N.; Vezir Kahraman, M.; Karadenizli, S.; Erdem Kuruca, S.; Güngör, A. Preparation of bow tie–type methacrylated poly(caprolactone-co-lactic acid) scaffolds: Effect of collagen modification on cell growth. Polym. Adv. Techn. 2012, 23, 1403–1413. [Google Scholar] [CrossRef]
- Schuster, M.; Turecek, C.; Weigel, G.; Saf, R.; Stampfl, J.; Varga, F.; Liska, R. Vinyl esters: Low cytotoxicity monomers for the fabrication of biocompatible 3D scaffolds by lithography based additive manufacturing. J. Polym. Sci. Part A 2009, 47, 7078–7089. [Google Scholar] [CrossRef]
- Hedin, J.; Oestlund, A.; Nyden, M. UV induced cross-linking of starch modified with glycidyl methacrylate. Carbohydr. Polym. 2010, 79, 606–613. [Google Scholar] [CrossRef]
- Barrett, D.G.; Merkel, T.J.; Luft, J.C.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar]
- Jiratumnukul, N.; Itarat, R. Ultraviolet curable epoxidized sunflower oil/organo clay nanocomposite coatings. J. Appl. Polym. Sci. 2008, 110, 2164–2167. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Someya, Y.; Suzuki, S. Bio-based nanocomposites composed of photo-cured epoxidized soybean oil and supramolecular hydroxystearic acid nanofibers. J. Polym. Sci. Part B 2009, 47, 669–673. [Google Scholar]
- Gupta, M.K.; Singh, R.P. Cationic Initiators in Photocuring Applications. In Basics of Photopolymerization Reactions; Fouassier, J.P., Allonas, X., Eds.; Research Signpost: Trivandrum, India, 2010; Volume 1, pp. 23–35. [Google Scholar]
- Acosta Ortiz, R.; Prieto López, D.; Guillén Cisneros, M.L.; Rico Valverde, J.C.; Crivello, J.V. A kinetic study of the acceleration effect of substituted benzyl alcohol on the cationic photopolymerization rate of epoxidized natural oils. Polymer 2005, 46, 1535–1541. [Google Scholar]
- Decker, C.; Nguyen Thi Viet, T.; Le Xuan, H. Photoréticulation de caoutchoucs fonctionnalisés: Polymérisation cationique de caoutchoucs époxydés. Eur. Polym. J. 1996, 32, 1319–1326. [Google Scholar]
- Crivello, J.V.; Narajan, R.; Sternstein, S.S. Fabrication and mechanical characterization of glass fiber reinforced UV-cured composites from epoxidized vegetable oils. J. Appl Polym Sci. 1997, 64, 2073–2087. [Google Scholar]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of biobased polyols by thiol−ene coupling from vegetable oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar]
- Raj Mahendran, A.; Wuzella, G.; Aust, N.; Kandelbauer, A. Photocrosslinkable modified vegetable oil based resin for wood surface coating application. Progr. Org. Coatings 2012, 74, 697–704. [Google Scholar]
- Kollbe Ahn, B.; Sung, J.; Kim, N.; Kraft, S.; Susan Sun, X. UV-curable pressure-sensitive adhesives derived from functionalized soybean oils and rosin ester. Polym. Int. 2012. [Google Scholar] [CrossRef]
- Thanamongkollit, N.; Miller, K.R.; Soucek, M.D. Route to co-acrylic modified alkyd resins via a controlled polymerization technique. Progr. Org. Coat. 2012, 73, 425–434. [Google Scholar]
- Teck Chye Ang, D.; Neon Gan, S. Novel approach to enhance film properties of environmentally friendly UV-curable alkyd coating using epoxidised natural rubber. Progr. Org. Coat. 2012, 73, 409–414. [Google Scholar]
- Teck Chye Ang, D.; Neon Gan, S. Environment friendly UV-curable resins from palm stearin alkyds. J. Appl. Polym. Sci. 2012, 125, 306–313. [Google Scholar]
- Bao, Y.; He, J.; Li, Y. Facile and efficient synthesis of hyperbranched polyesters based on renewable castor oil. Polym. Int. 2012. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490-514. https://doi.org/10.3390/app3020490
Tehfe MA, Louradour F, Lalevée J, Fouassier J-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Applied Sciences. 2013; 3(2):490-514. https://doi.org/10.3390/app3020490
Chicago/Turabian StyleTehfe, Mohamad Ali, Fanny Louradour, Jacques Lalevée, and Jean-Pierre Fouassier. 2013. "Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry" Applied Sciences 3, no. 2: 490-514. https://doi.org/10.3390/app3020490




