Ultrasonics Promoted Synthesis of 5-(Pyrazol-4-yl)-4,5-Dihydropyrazoles Derivatives
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus and Analysis
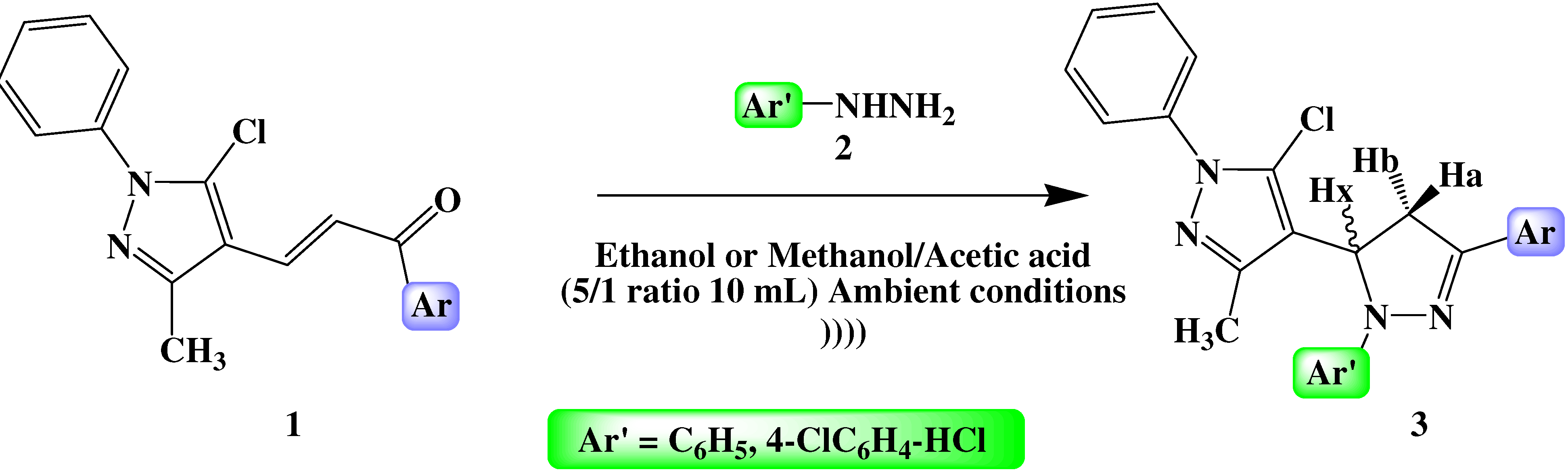
2.2. General Procedure for the Synthesis of 5-pyrazol-4,5-dihydropyrazoles Derivatives 3
2.2.1. Compound 3a
2.2.2. Compound 3b
2.2.3. Compound 3c
2.2.4. Compound 3d
2.2.5. Compound 3e
2.2.6. Compound 3f
2.2.7. Compound 3g
2.2.8. Compound 3h
2.2.9. Compound 3i
2.2.10. Compound 3j
3. Results and Discussion
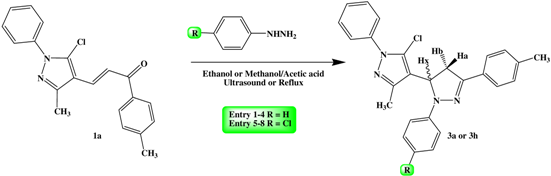 | |||
|---|---|---|---|
| Entry | Conditions | Time (min) | Yield b (%) |
| 1 | Ethanol or Methanol | 35 | 60 |
| 2 | Ethanol or Methanol/Acetic acid (10/1) | 20 | 75 |
| 3 | Ethanol or Methanol/Acetic acid (5/1) | 20 | 80 |
| 4 | Ethanol or Methanol/Acetic acid (10/3) | 20 | 75 |
| 5 | Ethanol or Methanol | 20 | 50 |
| 6 | Ethanol or Methanol/Acetic acid (10/1) | 15 | 65 |
| 7 | Ethanol or Methanol/Acetic acid (5/1) | 15 | 80 |
| 8 | Ethanol or Methanol/Acetic acid (10/3) | 15 | 75 |
| Compound 3 | Ar | Ar′ | Time reaction (min) | M.p. °C | Yield (%) |
|---|---|---|---|---|---|
| a | 4-H3CC6H4 | C6H5 | 20 | 133–135 | 80 |
| b | 4-BrC6H4 | C6H5 | 10 | 163–165 | 75 |
| c | 4-ClC6H4 | C6H5 | 15 | 153–155 | 70 |
| d | 4-O2NC6H4 | C6H5 | 20 | 178–180 | 80 |
| e | 4-H3COC6H4 | C6H5 | 3 | 130–132 | 80 |
| f | 3,4,5- tri-H3COC6H2 | C6H5 | 2 | 118–120 | 75 |
| g | 3,4-OCH2OC6H3 | C6H5 | 5 | 220–222 | 65 |
| h | 4-H3CC6H4 | 4-ClC6H4 | 15 | 158–160 | 80 |
| i | 4-ClC6H4 | 4-ClC6H4 | 10 | 150–152 | 70 |
| j | 4-H3COC6H4 | 4-ClC6H4 | 10 | 128–130 | 80 |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Yusuf, M.; Jain, P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab. J. Chem. 2011. [Google Scholar] [CrossRef]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.G.; Singh, S.; Alam, M.S. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur. J. Med. Chem. 2011, 46, 5763–5768. [Google Scholar]
- Khode, S.; Maddi, V.; Aragade, P.; Palkar, M.; Ronad, P.K.; Mamledesai, S.; Thippeswamy, A.H.M.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted)aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar] [CrossRef]
- Rathish, I.G.; Javed, K.; Ahmad, S.; Bano, S.; Alam, M.S.; Pillai, K.K.; Singh, S.; Bagchi, V. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg. Med. Chem. Lett. 2009, 19, 255–258. [Google Scholar]
- Schmidt, A.; Dreger, A. Recent advances in the chemistry of pyrazoles. Properties, biological activities, and syntheses. Curr. Org. Chem. 2011, 15, 1423–1463. [Google Scholar] [CrossRef]
- Abd-El Gawad, N.M.; Hassan, G.S.; Georgey, H.H. Design and synthesis of some pyrazole derivatives of expected anti-inflammatory and analgesic activities. Med. Chem. Res. 2012, 21, 983–994. [Google Scholar] [CrossRef]
- Insuasty, B.; Chamizo, L.; Muñoz, J.; Tigreros, A.; Quiroga, J.; Abonía, R.; Nogueras, M.; Cobo, J. Synthesis of 1-substituted 3-aryl-5-aryl(hetaryl)-2-pyrazolines and study of their antitumor activity. Arch. Pharm. 2012, 345, 275–286. [Google Scholar] [CrossRef]
- Al-Saadi, M.S.; Rostom, S.A.F.; Faidallah, H.M. 3-Methyl-2-(4-substitutedphenyl)-4,5-dihydronaphtho[1,2-c]-pyrazoles: Synthesis and in vitro biological evaluation as antitumour agents. Arch. Pharm. 2008, 341, 181–190. [Google Scholar] [CrossRef]
- Insuasty, B.; García, A.; Quiroga, J.; Abonía, R.; Ortiz, A.; Nogueras, M.; Cobo, J. Efficient microwave-assisted synthesis and antitumor activity of novel 4,4'-methylenebis[2-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)phenols]. Eur. J. Med. Chem. 2011, 46, 2436–2440. [Google Scholar] [CrossRef]
- Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonía, R.; Nogueras, M.; Sánchez, A.; Cobo, J. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar]
- Deng, H.; Yu, Z.Y.; Shi, G.Y.; Chen, M.J.; Tao, K.; Hou, T.P. Synthesis and in vitro antifungal evaluation of 1,3,5-trisubstituted-2-pyrazoline derivatives. Chem. Biol. Drug Des. 2012, 79, 279–289. [Google Scholar] [CrossRef]
- Parashar, B.; Jain, A.; Bharadwaj, S.; Sharma, V.K. Synthesis and pharmacological properties of some novel pyrazolidine and pyrazole derivatives. Med. Chem. Res. 2012, 21, 1692–1699. [Google Scholar] [CrossRef]
- Gupta, M.; Paul, S.; Gupta, R. Efficient and novel one-pot synthesis of antifungal active 1-substituted-8-aryl-3-alkyl/aryl-4H-pyrazolo[4,5-f][1,2,4]triazolo[4,3-b][1,2,4]triazepines using solid support. Eur. J. Med. Chem. 2011, 46, 631–635. [Google Scholar] [CrossRef]
- Keter, F.K.; Darkwa, J. Perspective: The potential of pyrazole-based compounds in medicine. Biometals 2012, 25, 9–21. [Google Scholar] [CrossRef]
- Agrawal, M.; Sonar, P.K.; Saraf, S.K. Synthesis of 1,3,5-trisubstituted pyrazoline nucleus containing compounds and screening for antimicrobial activity. Med. Chem. Res. 2012, 21, 3376–3381. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Xie, Y.-S.; Zhao, B.-X.; Lv, H.-S.; Liu, W.-Y. The synthesis, x-ray crystal structure and optical properties of novel 5-aryl-3-ferrocenyl-1-pyridazinyl-pyrazoline derivatives. J. Fluoresc. 2011, 21, 355–364. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Zhao, B.-X.; Liu, W.-Y.; Lv, H.-S. A new highly selective “turn on” fluorescent sensor for zinc ion based on a pyrazoline derivative. J. Photochem. Photobiol. A 2011, 218, 6–10. [Google Scholar]
- Liu, W.-Y.; Xie, Y.-S.; Zhao, B.-X.; Wang, B.-S.; Lv, H.-S.; Gong, Z.-L.; Song, L.; Zheng, L.-W. The synthesis, X-ray crystal structure and optical properties of novel 5-aryl-1-arylthiazolyl-3-ferrocenyl-pyrazoline derivatives. J. Photochem. Photobiol. A 2010, 214, 135–144. [Google Scholar] [CrossRef]
- Bian, B.S.; Ji, S.; Shi, H. Synthesis and fluorescent property of some novel bischromophore compounds containing pyrazoline and naphthalimide groups. Dye Pigment. 2008, 76, 348–352. [Google Scholar] [CrossRef]
- Tao, Y.T.; Balasubramaniam, E. Organic light-emitting diodes based on variously substituted pyrazoloquinolines as emitting material. J. Matr. Chem. 2001, 13, 1207–1212. [Google Scholar]
- Wang, P.; Onozawa-Komatsuzaki, N.; Himeda, Y.; Sugihara, H.; Arakawa, H.; Kasuga, K. 3-(2-Pyridyl)-2-pyrazoline derivatives: Novel fluorescent probes for Zn2+ ion. Tetrahedron Lett. 2001, 42, 9199–9201. [Google Scholar]
- Gok, S.; Demet, M.M.; Özdemir, A.; Turan-Zitouni, G. Evaluation of antidepressant-like effect of 2-pyrazoline derivatives. Med. Chem. Res. 2010, 19, 94–101. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Özdemir, A.; Turan-Zitouni, G.; Altintop, M.D.; Can, O.D. New pyrazoline derivatives and their antidepressant activity. Eur. J. Med. Chem. 2010, 45, 4383–4387. [Google Scholar] [CrossRef]
- Amnerkar, N.D.; Bhusari, K.P. Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. Eur. J. Med. Chem. 2010, 45, 149–159. [Google Scholar] [CrossRef]
- Siddiqui, N.; Alam, P.; Ahsan, W. Design, synthesis, and in-vivo pharmacological screening of N,3-(substituteddiphenyl)-5-phenyl-1H-pyrazoline-1-carbothioamide derivatives. Arch. Pharm. 2009, 342, 173–181. [Google Scholar] [CrossRef]
- De Los Santos, J.M.; López, Y.; Aparicio, D.; Palacios, F. A convenient synthesis of substituted pyrazolidines and azaproline derivatives through highly regio- and diastereoselective reduction of 2-pyrazolines. J. Org. Chem. 2008, 73, 550–557. [Google Scholar]
- Jung, M.E.; Min, S.-J.; Houk, K.N.; Ess, D. Synthesis and relative stability of 3,5-diacyl-4,5-dihydro-1H-pyrazoles prepared by dipolar cycloaddition of enones and R-diazoketones. J. Org. Chem. 2004, 69, 9085–9089. [Google Scholar]
- Suga, H.; Furihata, Y.; Sakamoto, A.; Itoh, K.; Okumura, Y.; Tsuchida, T.; Kakehi, A.; Baba, T. Asymmetric cycloaddition reactions of diazoesters with 2-alkenoic acid derivatives catalyzed by binaphthyldiimine-Ni(II) complexes. J. Org. Chem. 2011, 76, 7377–7387. [Google Scholar] [CrossRef]
- Alex, K.; Tillack, A.; Schwarz, N.; Beller, M. Zinc-catalyzed synthesis of pyrazolines and pyrazoles via hydrohydrazination. Org. Lett. 2008, 10, 2377–2379. [Google Scholar]
- Cui, S.-L.; Wang, J.; Wang, Y.-G. Facile access to pyrazolines via domino reaction of the Huisgen zwitterions with aziridines. Org. Lett. 2008, 10, 13–16. [Google Scholar]
- Wang, Y.; Hu, W.J.; Song, W.; Lim, R.K.V.; Lin, Q. Discovery of long-wavelength photoactivatable diaryltetrazoles for bioorthogonal 1,3-dipolar cycloaddition reactions. Org. Lett. 2008, 10, 3725–3728. [Google Scholar] [CrossRef]
- Li, F.; Xie, Z.F.; Liu, F.M. Syntheses and fluorescent property of 5-(2-Phenyl-1,2,3-triazoly)-3- aryl pyrazoline derivatives. Chem. J. Chin. U 2006, 26, 1058–1061. (in Chinese). [Google Scholar]
- Fischer, E.; Knovenagel, O. Ueber die verbindungen des phenylhydrazins mit acroleïn, mesityloxyd und allylbromid. Ann. Chem. 1887, 239, 194–206. [Google Scholar] [CrossRef]
- Azarifar, D.; Maleki, B. Silica-supported synthesis of some 1,3,5-trisubstituted 2-pyrazolines under solvent-free and microwave irradiation conditions. J. Heterocycl. Chem. 2005, 42, 157–159. [Google Scholar]
- Levai, A. Synthesis of heterocyclic compounds by the reactions of exocyclic α,β-unsaturated ketones. J. Heterocycl. Chem. 2004, 41, 299–310. [Google Scholar] [CrossRef]
- Levai, A. Synthesis of 2-pyrazolines by the reactions of α,β-unsaturated aldehydes, ketones, and esters with diazoalkanes, nitrile imines, and hydrazines. J. Heterocycl. Chem. 2002, 39, 1–13. [Google Scholar] [CrossRef]
- Joshi, M.J.; Vekariya, P.B.; Dodiya, B.L.; Ghetiya, R.M.; Joshi, H.S. Synthesis and biological study of some new chalcones and oxopyrimidines containing imidazo[1,2-a]pyridine nucleus. J. Heterocycl. Chem. 2012, 49, 130–134. [Google Scholar]
- Trilleras, J.; de la Torre, P.; Pacheco, D.J.; Quiroga, J.; Nogueras, M.; Cobo, J. Solvent-free microwave-assisted synthesis o substituted pyridines using NH4OAc as nitrogen source. Lett. Org. Chem. 2011, 8, 652–655. [Google Scholar] [CrossRef]
- Rostom, S.A.F.; Badr, M.H.; El Razik, H.A.A.; Ashour, H.M.A.; Abdel Wahab, A.E. Synthesis of some pyrazolines and pyrimidines derived from polymethoxy chalcones as anticancer and antimicrobial agents. Arch. Pharm. 2011, 344, 572–587. [Google Scholar] [CrossRef]
- Foroumadi1, A.; Emami, S.; Sorkhi, M.; Nakhjiri, S.; Nazarian, Z.; Heydari, S.; Ardestani, S.K.; Poorrajab, F.; Shafiee1, A. Chromene-based synthetic chalcones as potent antileishmanial agents: Synthesis and biological activity. Chem. Biol. Drug Des. 2010, 75, 590–596. [Google Scholar]
- Sivakumar, P.M.; Ganesan, S.; Veluchamy, P.; Doble, M. Novel chalcones and 1,3,5-triphenyl-2-pyrazoline derivatives as antibacterial agents. Chem. Biol. Drug Des. 2010, 76, 407–411. [Google Scholar] [CrossRef]
- Sreevidya, T.V.; Narayana, B.; Yathirajan, H.S. Synthesis and characterization of some chalcones and their cyclohexenone derivatives. Cent. Eur. J. Chem. 2010, 8, 174–181. [Google Scholar]
- Voskienė, A.; Mickevičius, V. Cyclization of chalcones to isoxazole and pyrazole derivatives. Chem. Heterocycl. Compd. 2009, 45, 1485–1488. [Google Scholar] [CrossRef]
- Cella, R.; Stefani, H.A. Ultrasound in heterocycles chemistry. Tetrahedron 2009, 65, 2619–2641. [Google Scholar] [CrossRef]
- Li, J.-T.; Zhang, X.-H.; Lin, Z.-P. An improved synthesis of 1,3,5-triaryl-2-pyrazolines in acetic acid aqueous solution under ultrasound irradiation. Beilstein J. Org. Chem. 2007, 3, 1–4. [Google Scholar] [CrossRef]
- Li, J.-T.; Yang, W.-Z.; Wang, S.-X.; Li, S.-H.; Li, T.-S. Improved synthesis of chalcones under ultrasound irradiation. Ultrason. Sonochem 2002, 9, 237–239. [Google Scholar] [CrossRef]
- Zare, L.; Mahmoodi, N.O.; Yahyazadeh, A.; Nikpassand, M. Ultrasound-promoted regio and chemoselective synthesis of pyridazinones and phthalazinones catalyzed by ionic liquid [bmim]Br/AlCl3. Ultrason. Sonochem 2012, 19, 740–744. [Google Scholar] [CrossRef]
- Saleh, T.S.; El-Rahman, N.M.A.; Elkateb, A.A.; Shaker, N.O.; Mahmoud, N.A.; Gabal, S.A. Ultrasound promoted synthesis of some novel fused pyrans. Ultrason. Sonochem 2012, 19, 491–497. [Google Scholar]
- Zhang, Z.-H.; Li, J.-J.; Li, T.-S. Ultrasound-assisted synthesis of pyrroles catalyzed by zirconium chloride under solvent-free conditions. Ultrason. Sonochem 2012, 19, 264–269. [Google Scholar]
- Nagargoje, D.; Mandhane, P.; Shingote, S.; Badadhe, P.; Gill, C. Ultrasound assisted one pot synthesis of imidazole derivatives using diethyl bromophosphate as an oxidant. Ultrason. Sonochem 2012, 19, 94–96. [Google Scholar] [CrossRef]
- Dabiri, M.; Noroozi Tisseh, Z.; Bahramnejad, M.; Bazgir, A. Sonochemical multi-component synthesis of spirooxindoles. Ultrason. Sonochem 2011, 18, 1153–1159. [Google Scholar]
- Trilleras, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Síntesis y estudio de la actividad antitumoral y antituberculosa de análogos heterocíclicos enónicos derivados del pirazol. Revista Ciencias 2008, 12, 123–140. [Google Scholar]
- Trilleras, J.; Quiroga, J.; Cobo, J.; Low, J.N.; Glidewelld, C. Hydrogen-bonded chains in 3-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-1-(4-methoxyphenyl)-propenone and 3-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-1-(3,4,5-trimethoxyphenyl)propenone. Acta Cryst. 2005, C61, 414–416. [Google Scholar]
- Trilleras, J.; Quiroga, J.; Cobo, J.; Low, J.N.; Glidewelld, C. 5-Chloro-3-methyl-1-phenyl-1H-pyrazole-4-carbaldehyde: Sheets built from C—H---O and C—H---π(arene) hydrogen bonds. Acta Cryst. 2005, E61, 1055–1057. [Google Scholar]
- Xie, Z.; Moa, Z.; Liu, G.; Liu, F. Crystal structure of 5-pyrazol-4,5-dihydropyrazoles derivatives. J. Heterocycl. Chem. 2008, 45, 1485–1488. [Google Scholar] [CrossRef]
- Ziarati, A.; Safaei-Ghomi, J.; Rohani, S. Sonochemically synthesis of pyrazolones using reusable catalyst CuI nanoparticles that was prepared by sonication. Ultrason. Sonochem 2013, 20, 1069–1075. [Google Scholar] [CrossRef]
- Shekouhy, M.; Hasaninejad, A. Ultrasound-promoted catalyst-free one-pot four component synthesis of 2H-indazolo[2,1-b]phthalazine-triones in neutral ionic liquid 1-butyl-3-methylimidazolium bromide. Ultrason. Sonochem 2012, 19, 307–313. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry sonoprocessing: The link the trends and (pobably) the future. Ultrason. Sonochem 2003, 10, 175–179. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Trilleras, J.; Polo, E.; Quiroga, J.; Cobo, J.; Nogueras, M. Ultrasonics Promoted Synthesis of 5-(Pyrazol-4-yl)-4,5-Dihydropyrazoles Derivatives. Appl. Sci. 2013, 3, 457-468. https://doi.org/10.3390/app3020457
Trilleras J, Polo E, Quiroga J, Cobo J, Nogueras M. Ultrasonics Promoted Synthesis of 5-(Pyrazol-4-yl)-4,5-Dihydropyrazoles Derivatives. Applied Sciences. 2013; 3(2):457-468. https://doi.org/10.3390/app3020457
Chicago/Turabian StyleTrilleras, Jorge, Efraín Polo, Jairo Quiroga, Justo Cobo, and Manuel Nogueras. 2013. "Ultrasonics Promoted Synthesis of 5-(Pyrazol-4-yl)-4,5-Dihydropyrazoles Derivatives" Applied Sciences 3, no. 2: 457-468. https://doi.org/10.3390/app3020457






