Polydimethylsiloxane (PDMS) Coating onto Magnetic Nanoparticles Induced by Attractive Electrostatic Interaction
Abstract
:1. Introduction
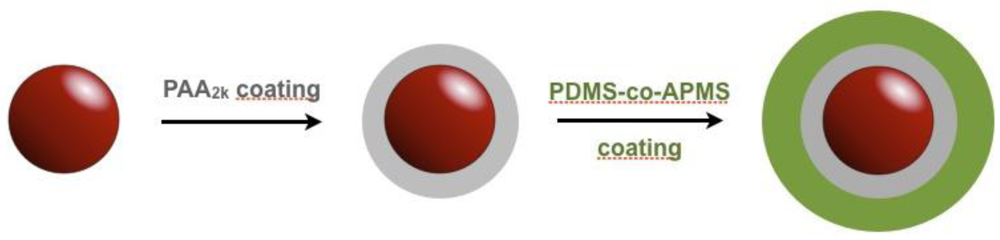
2. Experimental Section
2.1. Nanoparticle Synthesis and PAA Coating
2.2. Nanoparticle Coating with PDMS-co-APMS
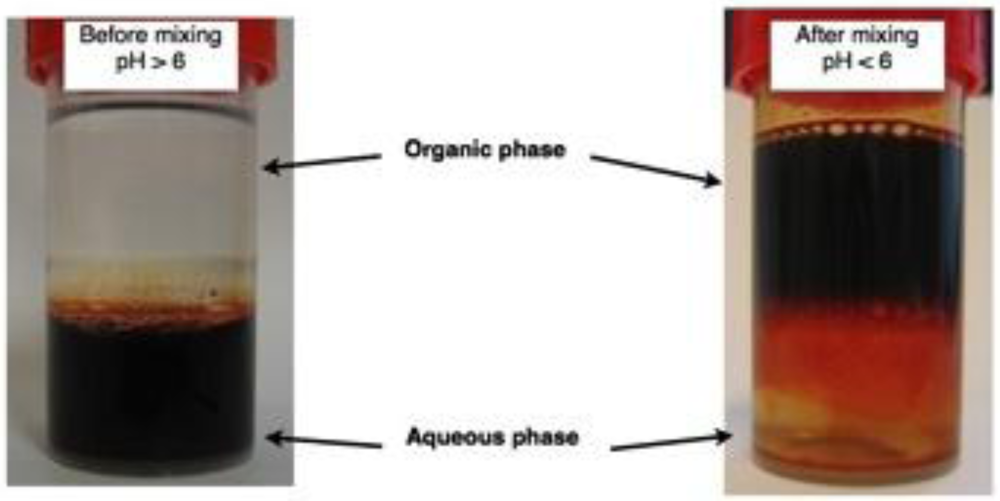
3. Results and Discussion
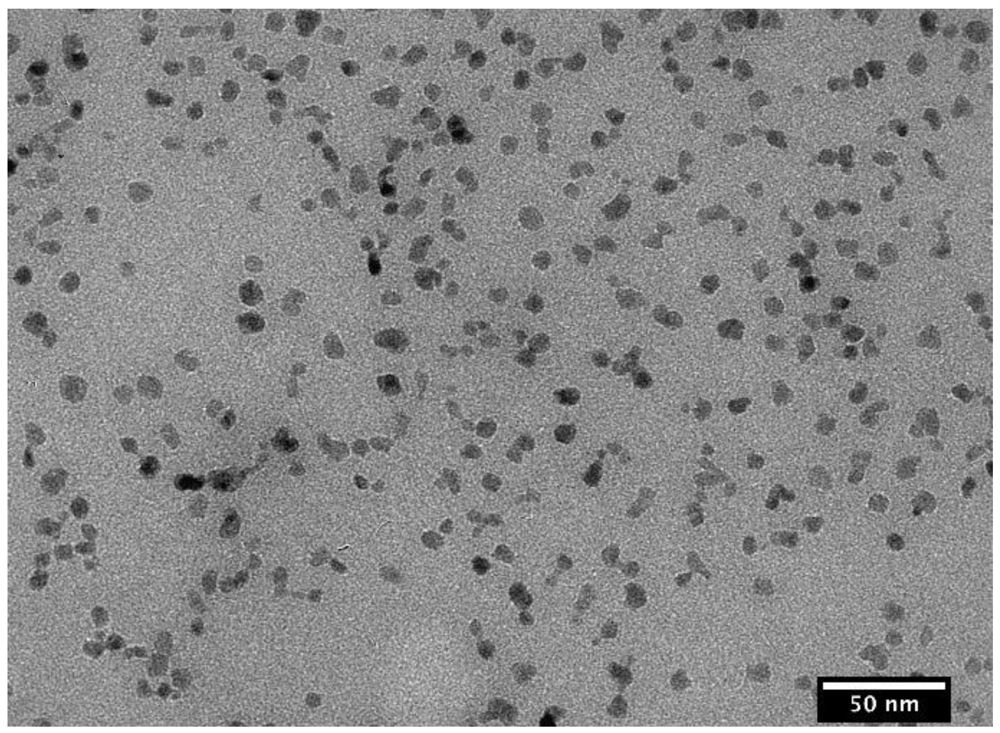
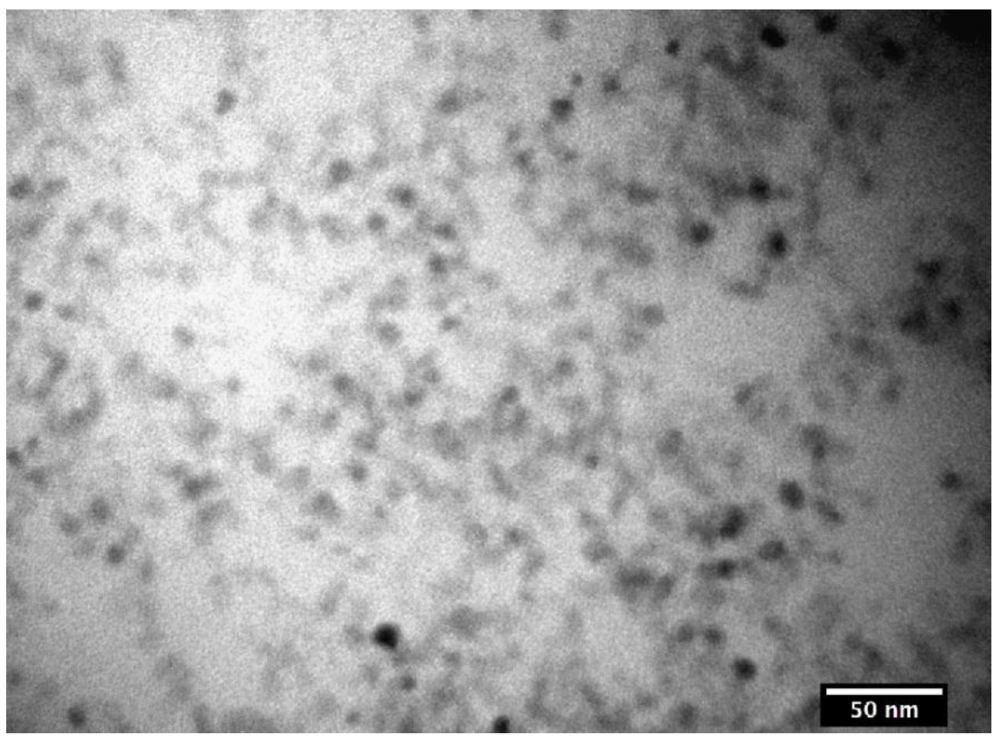
3.1. The Coatings of Nanoparticle and Their Characterization
| Bare nanoparticles | SC-PAA | SC-PAA-APMS | ||||||
| Amine content | Diameter [nm] | PDI | Diameter [nm] | PDI | Thickness of coating [nm] | Diameter [nm] | PDI | Thickness of coating [nm] |
| 2–3% | 11.6 | 0.16 | 20.5 | 0.17 | 4.5 | 41.6 | 0.14 | 10.6 |
| 4–5% | 11.6 | 0.16 | 20.5 | 0.17 | 4.5 | 84.2 | 0.28 | 32 |
| 6–7% | 11.6 | 0.16 | 20.5 | 0.17 | 4.5 | 66.9 | 0.27 | 23.2 |
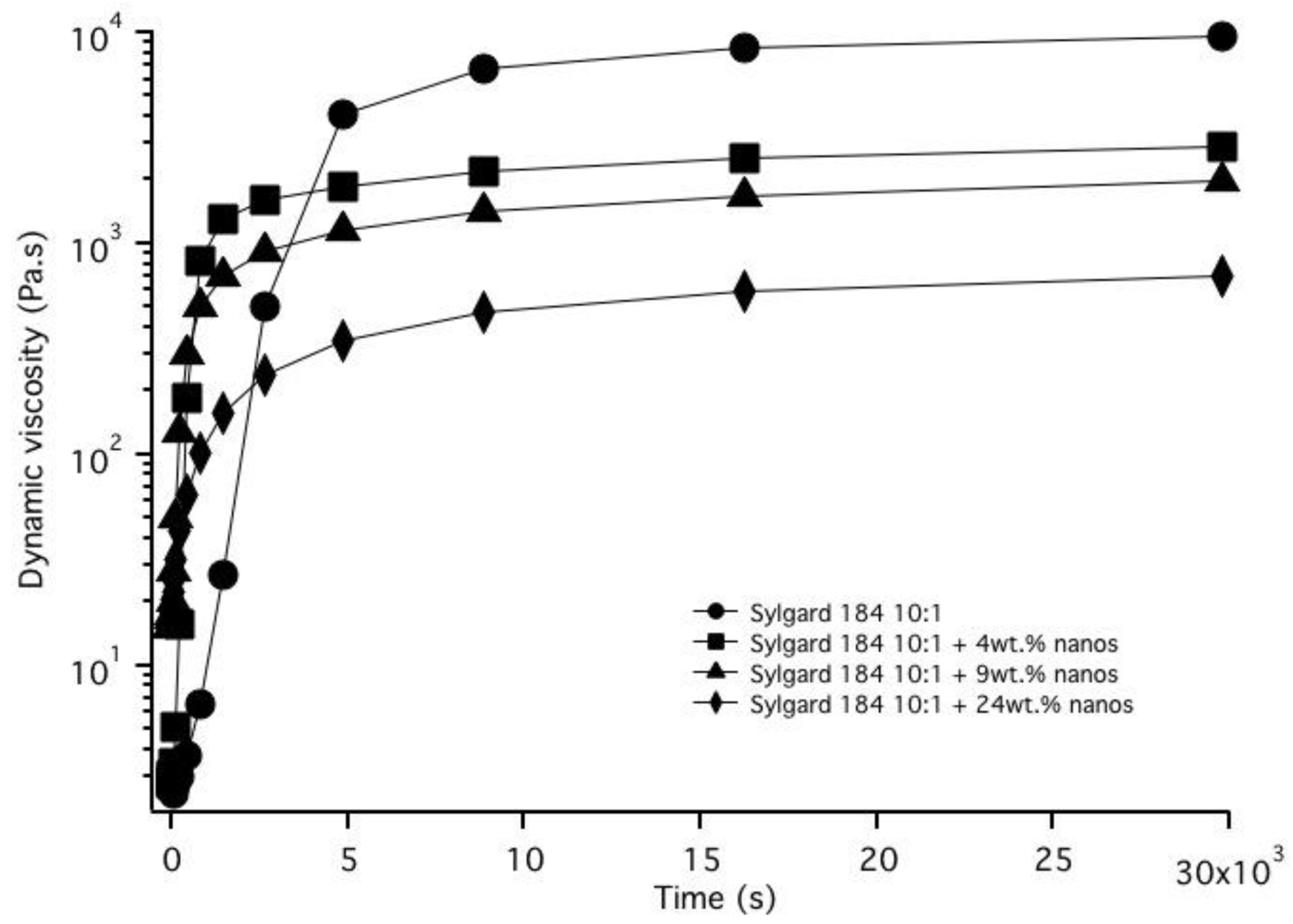
3.2. Nanoparticle Incorporation into PDMS Matrix
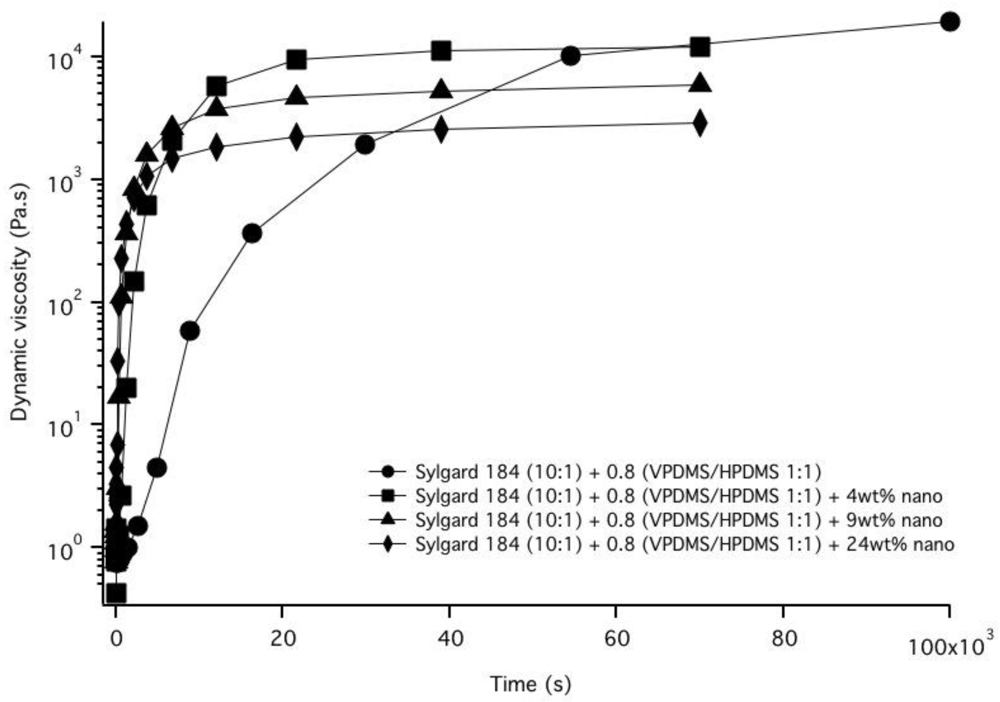
4. Conclusions
Acknowledgments
References
- Denver, H.; Heiman, T.; Martin, E.; Gupta, A.; Borca-Tasciuc, D.A. Fabrication of polydimethylsiloxane composites with nickel nanoparticle and nanowire fillers and study of their mechanical and magnetic properties. J. Appl. Phys. 2009, 106, 64909:1–64909:5. [Google Scholar]
- Antonel, P.S.; Jorge, G.; Perez, O.E.; Butera, A.; Leyva, A.G.; Negri, R.M. Magnetic and elastic properties of CoFe2O4− polydimethylsiloxane magnetically oriented elastomer nanocomposites. Macromol. Rapid Commun. 2011, 110, 43920:1–43920:8. [Google Scholar]
- Mefford, O.T.; Carroll, M.R.J.; Vadala, M.L.; Goff, J.D.; Mejia-Ariza, R.; Saunders, M.; Woodward, R.C.; St Pierre, T.G.; Davis, R.M.; Riffle, J.S. Size analysis of PDMS-magnetite nanoparticle complexes: Experiment and theory. Chem. Mater. 2008, 20, 2184–2191. [Google Scholar]
- Mefford, O.T.; Vadala, M.L.; Goff, J.D.; Carroll, M.R.J.; Mejia-Ariza, R.; Caba, B.L.; St Pierre, T.G.; Woodward, R.C.; Davis, R.M.; Riffle, J.S. Stability of polydimethylsiloxane-magnetite nanoparticle dispersions against flocculation: Interparticle interactions of polydisperse materials. Langmuir 2008, 24, 5060–5069. [Google Scholar]
- Miles, W.C.; Goff, J.D.; Huffstetler, P.P.; Mefford, O.T.; Riffle, J.S.; Davis, R.M. The design of well-defined PDMS-Magnetite complexes. Polymer 2009, 51, 482–491. [Google Scholar]
- Wilson, K.S.; Goff, J.D.; Riffle, J.S.; Harris, L.A.; St Pierre, T.G. Polydimethylsiloxane-magnetite nanoparticle complexes and dispersions in polysiloxane carrier fluids. Polym. Advan. Technol. 2005, 16, 200–211. [Google Scholar] [CrossRef]
- Dutta, N.; Green, D. Nanoparticle stability in semidilute and concentrated polymer solutions. Langmuir 2008, 24, 5260–5269. [Google Scholar] [CrossRef]
- Aqil, A.; Vasseur, S.; Duguet, E.; Passirani, C.; Benoit, J.P.; Roch, A.; Muller, R.; Jerome, R.; Jerome, C. PEO coated magnetic nanoparticles for biomedical application. Eur. Polym. J. 2008, 44, 3191–3199. [Google Scholar] [CrossRef]
- Borrell, M.; Leal, L.G. Interfacial activity of polymer-coated gold nanoparticles. Langmuir 2007, 23, 12497–12502. [Google Scholar] [CrossRef]
- Vadala, M.L.; Rutnakornpituk, M.; Zalich, M.A.; St Pierre, T.G.; Riffle, J.S. Block copolysiloxanes and their complexation with cobalt nanoparticles. Polymer 2004, 45, 7449–7461. [Google Scholar]
- Ditsch, A.; Laibinis, P.E.; Wang, D.I.C.; Hatton, T.A. Controlled clustering and enhanced stability of polymer-coated magnetic nanoparticles. Langmuir 2005, 21, 6006–6018. [Google Scholar]
- Galicia, J.A.; Sandre, O.; Cousin, F.; Guemghar, D.; Menager, C.; Cabuil, V. Designing magnetic composite materials using aqueous magnetic fluids. J. Phys.-Condens. Matter. 2003, 15, S1379–S1402. [Google Scholar]
- Denver, H.; Heiman, T.; Martin, E.; Gupta, A.; Borca-Tasciuc, D.A. Mechanical Characterization of Nickel Nanoparticles Elastomer Composites. In Nsti Nanotech 2008; Annaheim, Canada, 2008; pp. 301–303. [Google Scholar]
- Evans, B.A.; Shields, A.R.; Carroll, R.L.; Washburn, S.; Falvo, M.R.; Superfine, R. Magnetically actuated nanorod arrays as biomimetic cilia. Nano Lett. 2007, 7, 1428–1434. [Google Scholar]
- Goyal, A.; Kumar, A.; Patra, P.K.; Mahendra, S.; Tabatabaei, S.; Alvarez, P.J.J.; John, G.; Ajayan, P.M. In Situ synthesis of metal nanoparticle embedded free standing multifunctional PDMS films. Macromol. Rapid Commun. 2009, 30, 1116–1122. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Functionalization of monodisperse magnetic nanoparticles. Langmuir 2007, 23, 2158–2168. [Google Scholar] [CrossRef]
- Yang, T.I.; Brown, R.N.C.; Ckempel, L.; Kofinas, P. Controlled synthesis of core-shell iron-silica nanoparticles and their magneto-dielectric properties in polymer composites. Nanotechnology 2011, 22, 105601:1–105601:8. [Google Scholar]
- Zhang, L.; He, R.; Gu, H.C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar]
- Bell, N.S.; Frischknecht, A.L.; Piech, M. Grafted low molecular weight polymers as steric stabilizers of commercial titania nanoparticles in polydimethylsiloxane fluids. J. Disper. Sci. Tech. 2010, 32, 128–140. [Google Scholar] [CrossRef]
- Prucker, O.; Ruhe, J. Synthesis of poly(styrene) monolayers attached to high surface area silica gels through self-assembled monolayers of azo initiators. Macromolecules 1998, 31, 592–601. [Google Scholar] [CrossRef]
- Valentine, M.T.; Perlman, Z.E.; Gardel, M.L.; Shin, J.H.; Matsudaira, P.; Mitchison, T.J.; Weitz, D.A. Colloid surface chemistry critically affects multiple particle tracking measurements of biomaterials. Biophys. J. 2004, 86, 4004–4014. [Google Scholar] [CrossRef]
- Sehgal, A.; Lalatonne, Y.; Berret, J.-F.; Morvan, M. Precipitation-redispersion of cerium oxide nanoparticles with poly(acrylic acid): Toward stable dispersions. Langmuir 2005, 21, 9359–9364. [Google Scholar]
- Chanteau, B.; Fresnais, J.; Berret, J.F. Electrosteric enhanced stability of functional sub-10 nm cerium and iron oxide particles in cell culture medium. Langmuir 2009, 25, 9064–9070. [Google Scholar]
- Fresnais, J.; Lavelle, C.; Berret, J.F. Nanoparticle aggregation controlled by desalting kinetics. J. Phys. Chem. 2009, 113, 16371–16379. [Google Scholar]
- Fresnais, J.; Berret, J.F.; Frka-Petesic, B.; Sandre, O.; Perzynski, R. Electrostatic co-assembly of iron oxide nanoparticles and polymers: Towards the generation of highly persistent superparamagnetic nanorods. Advan. Mater. 2008, 20, 3877–3881. [Google Scholar] [CrossRef]
- Frka-Petesic, B.; Fresnais, J.; Berret, J.F.; Dupuis, V.; Perzynski, R.; Sandre, O. Stabilization and controlled association of superparamagnetic nanoparticles using block copolymers. J. Magn. Magn. Mater. 2009, 321, 667–670. [Google Scholar] [CrossRef]
- Bee, A.; Massart, R.; Neveu, S. Synthesis of very fine maghemite particles. J. Magn. Magn. Mat. 1995, 149, 6–9. [Google Scholar] [CrossRef]
- Massart, R.; Dubois, E.; Cabuil, V.; Hasmonay, E. Preparation and properties of monodisperse magnetic fluids. J. Magn. Magn. Mat. 1995, 149, 1–5. [Google Scholar] [CrossRef]
- Mériguet, G.; Dubois, E.; Perzynski, R. Liquid-liquid phase-transfer of magnetic nanoparticles in organic solvents. J. Coll. Int. Sci. 2003, 267, 78–85. [Google Scholar] [CrossRef]
- Machunsky, S.; Grimm, P.; Schmid, H.J.; Peuker, U.A. Liquid-liquid phase transfer of magnetite nanoparticles. Colloid. Surface. A. 2009, 348, 186–190. [Google Scholar] [CrossRef]
- Abiman, P.; Wildgoose, G.G.; Crossley, A.; Jones, J.H.; Compton, R.G. Contrasting pKa of protonated bis(3-aminopropyl)-terminated polyethylene glycol “Jeffamine” and the associated thermodynamic parameters in solution and covalently attached to graphite durfaces. Chem. Eur. J. 2007, 13, 9663–9667. [Google Scholar] [CrossRef]
- Xiangli, F.J.; Chen, Y.W.; Jin, W.Q.; Xu, N.P. Polydimethylsiloxane (PDMS)/ceramic composite membrane with high flux for pervaporation of ethanol-water mixtures. Ind. Eng. Chem. Res. 2007, 46, 2224–2230. [Google Scholar] [CrossRef]
- Douadi-Masrouki, S.; Frka-Petesic, B.; Sandre, O.; Cousin, F.; Dupuis, V.; Perzynski, R.; Cabuil, V. Neutron reflectivity on polymer multilayers doped with magnetic nanoparticles. In Magnetism and Magnetic Materials; Perov, N., Ed.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2009; Volume 152–153, pp. 194–197. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sötebier, C.; Michel, A.; Fresnais, J. Polydimethylsiloxane (PDMS) Coating onto Magnetic Nanoparticles Induced by Attractive Electrostatic Interaction. Appl. Sci. 2012, 2, 485-495. https://doi.org/10.3390/app2020485
Sötebier C, Michel A, Fresnais J. Polydimethylsiloxane (PDMS) Coating onto Magnetic Nanoparticles Induced by Attractive Electrostatic Interaction. Applied Sciences. 2012; 2(2):485-495. https://doi.org/10.3390/app2020485
Chicago/Turabian StyleSötebier, Carina, Aude Michel, and Jérôme Fresnais. 2012. "Polydimethylsiloxane (PDMS) Coating onto Magnetic Nanoparticles Induced by Attractive Electrostatic Interaction" Applied Sciences 2, no. 2: 485-495. https://doi.org/10.3390/app2020485





