Understanding the Impact of Intensive Horticulture Land-Use Practices on Surface Water Quality in Central Kenya
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area: Rivers in the Sub-Watersheds
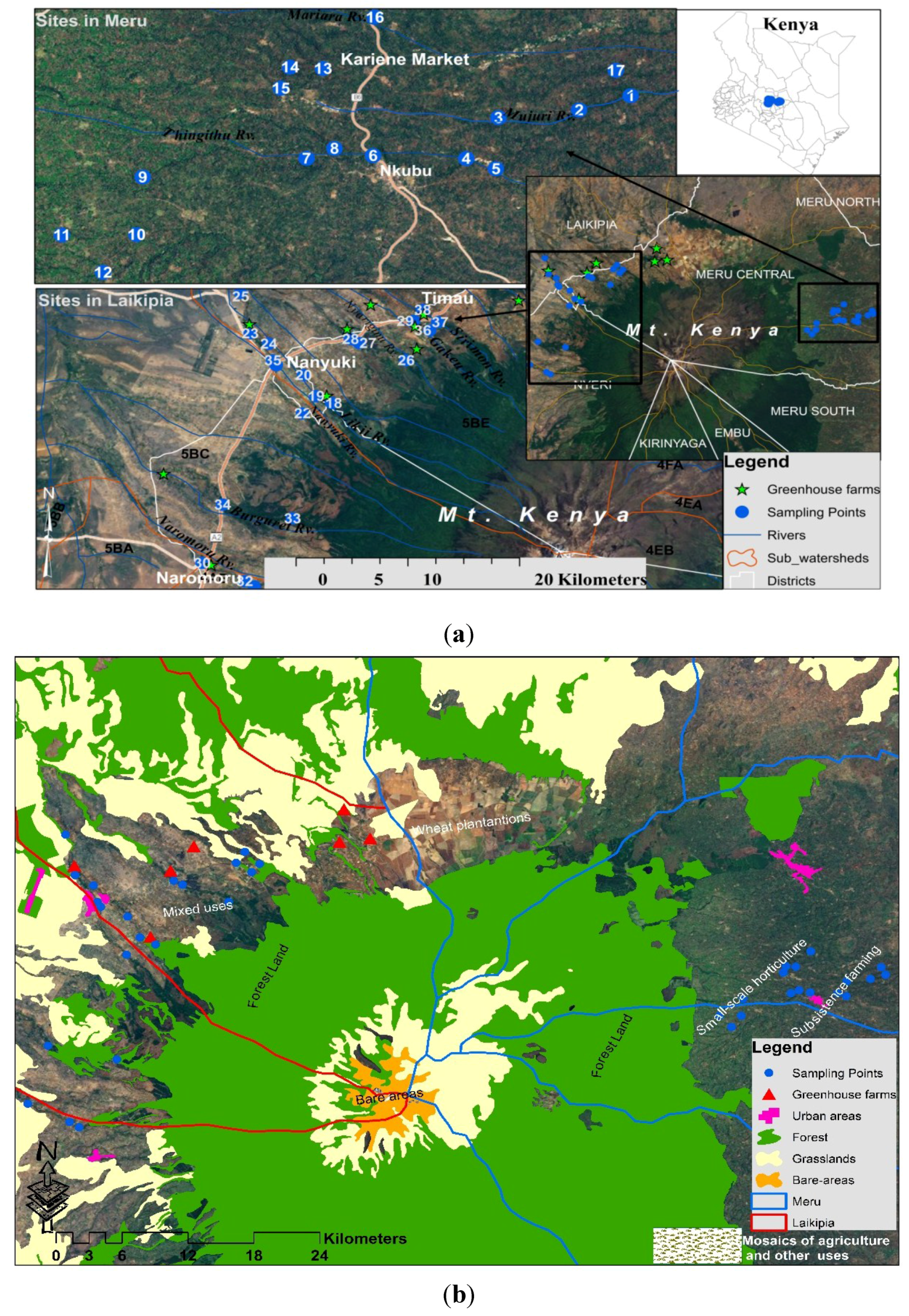
2.1.1. Field Sampling—Sites
| Site ID | Name | Site ID | Name |
|---|---|---|---|
| Site 1 | Mujuri Rv. (downstream) | Site 20 | Likii Rv. near Likii high sch.(midstream) |
| Site 2 | Mujuri Rv. (midstream) | Site 21 | Nanyuki Rv. below Bucaneer club (midstream) |
| Site 3 | Mujuri Rv. (upstream) | Site 22 | Nanyuki Rv. at Mt.Kenya Safariclub (upstream) |
| Site 4 | Thingithu Rv. (downstream; quary) | Site 23 | Nanyuki Rv. (downstream) |
| Site 5 | Thingithu Rv. Mujwa bridge (downstream) | Site 24 | Likii Rv. near Likii (downstream) |
| Site 6 | Thingithu Rv. at Nkubu bridge (midstream) | Site 25 | Likii Rv. and Nanyuki Rv. (downstream) |
| Site 7 | Kiuna Ndegwa Rv. (downstream) | Site 26 | Nyariginu Rv. (upstream) |
| Site 8 | Thingithu Rv.(upstream) | Site 27 | Nyariginu Rv. (midstream) |
| Site 9 | Kiuna Ndegwa Rv. (upstream) | Site 28 | Nyariginu Rv. (midstream) |
| Site 10 | Kithinu Rv. (downstream) | Site 29 | Gakeu stream (downstream) |
| Site 11 | Kithinu Rv. (upstream) | Site 30 | Narumoru Rv. (downstream) |
| Site 12 | Kirimbia Rv. (midstream) | Site 31 | Narumoru Rv. (upstream) |
| Site 13 | Gitauga Rv. (midstream) | Site 32 | Narumoru Rv. (midstream) |
| Site 14 | Gakuri Rv. (midstream) | Site 33 | Burguret Rv. (downstream) |
| Site 15 | Gatauga Rv. (midstream) | Site 34 | Burguret Rv. (upstream) |
| Site 16 | Mariara below bridge (midstream) | Site 35 | Nanyuki Rv. (midstream) |
| Site 17 | Njoe Rv. (downstream) | Site 36 | Gakeu stream (upstream) |
| Site 18 | Likii Rv. (upstream) | Site 37 | Sirimon Rv. (upstream) |
| Site 19 | Likii Rv. (midstream) | Site 38 | Sirimon Rv. (downstream) |
2.1.2. Field Measurement and Analysis
2.1.3. Statistical Analysis of Water Quality Data
Principal Component Analysis (PCA)
2.1.4. Discriminant Analysis (DA)
3. Results
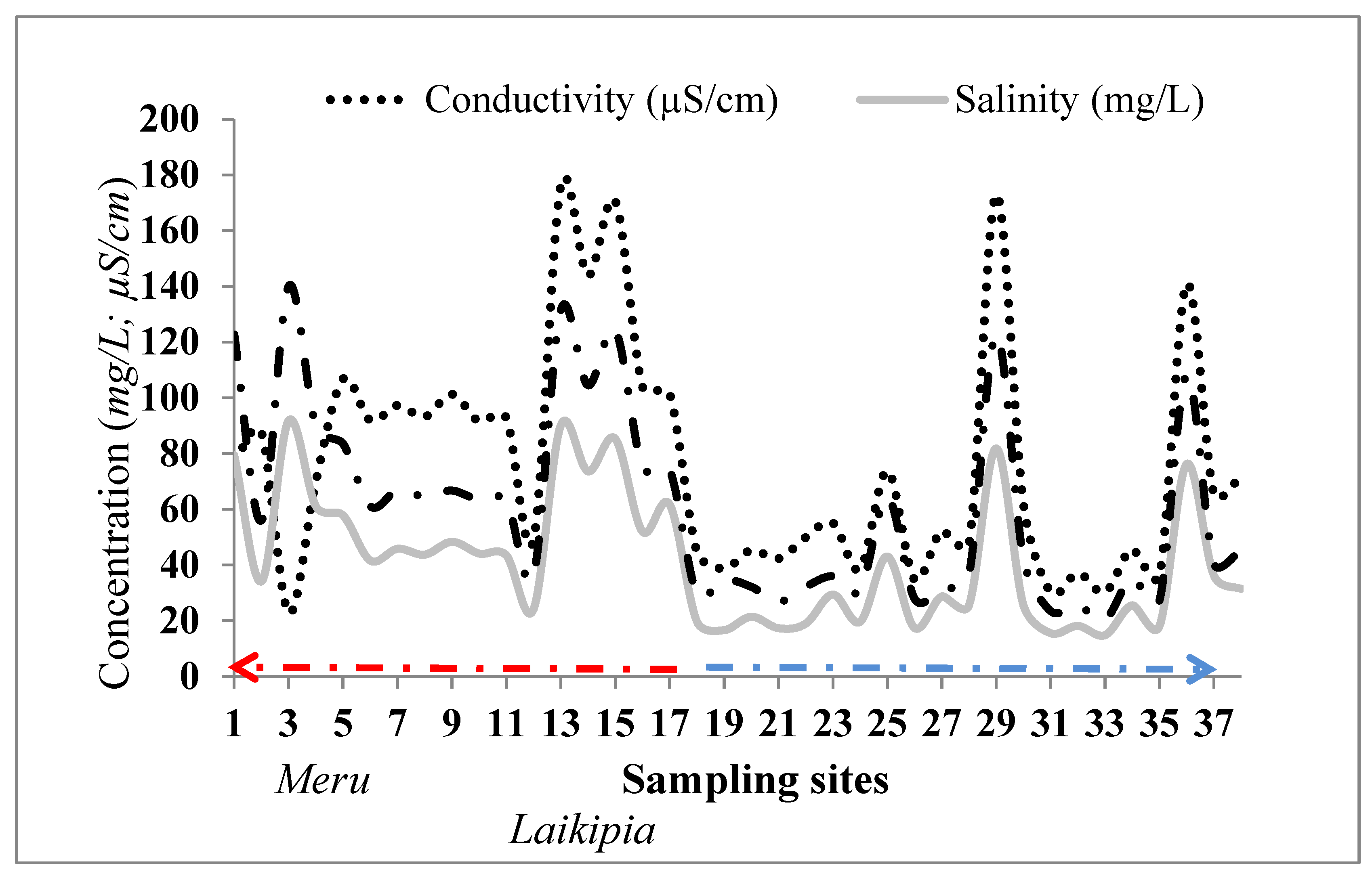
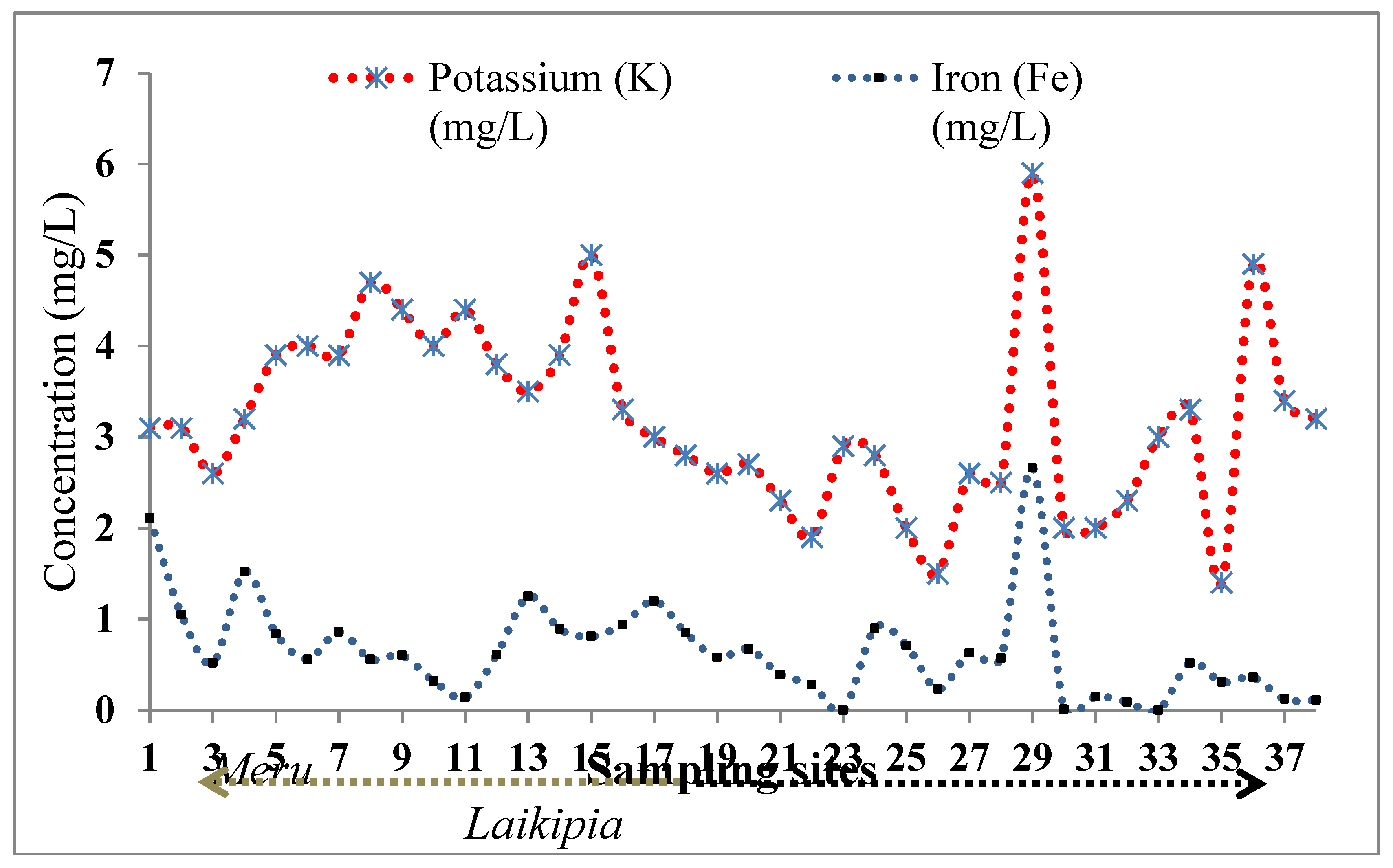
3.1. Field Data Description

3.2. PCA Results of Water Quality Data from Rivers Sampled in Laikipia and Meru
3.2.1. Principal Components (PCs)
| Variables | PC1 | PC2 | PC3 | PC4 | % Explained Variance |
|---|---|---|---|---|---|
| EC | 0.89 | 32.34 | |||
| Salinity | 0.88 | 17.01 | |||
| TDS | 0.87 | 11.53 | |||
| Potassium(K) | 0.84 | 8.68 | |||
| Sulfide (S2−) | −0.52 | 0.47 | 0.42 | 6.32 | |
| Cadmium (Cd) | −0.44 | −0.43 | 6.22 | ||
| Iron (Fe) | 0.44 | 4.91 | |||
| DO | −0.78 | 3.59 | |||
| Nitrate (NO3−) | 0.75 | 3.37 | |||
| Water temp. (C°) | 0.50 | 0.73 | 2.75 | ||
| Zinc (Zn 2+) | 0.77 | 1.56 | |||
| pH | −0.42 | 0.60 | 0.96 | ||
| Phosphate (PO43−) | 0.58 | 0.67 | |||
| Copper (Cu 2+) | −0.81 | 0.09 | |||
| Eigen value | 4.53 | 2.38 | 1.61 | 1.22 | |
| Explained variance | 0.30 | 0.18 | 0.12 | 0.10 |
3.2.2. PCA Biplots—Loadings and Scores

3.2.3. Results of Discriminant Analysis (DA)
 = Forest;
= Forest;  = Urban;
= Urban;  = LSHORT;
= LSHORT;  = SSHORT; and
= SSHORT; and  = Mixed agriculture land uses respectively.
= Mixed agriculture land uses respectively.
 = Forest;
= Forest;  = Urban;
= Urban;  = LSHORT;
= LSHORT;  = SSHORT; and
= SSHORT; and  = Mixed agriculture land uses respectively.
= Mixed agriculture land uses respectively.
| Results | Function 1 | Function 2 | Function 3 | Function 4 |
|---|---|---|---|---|
| Eigen value | 11.18 | 2.03 | 0.50 | 0.20 |
| % variance | 80.36 | 14.59 | 3.61 | 1.45 |
| Canonenical correlation % of variance | 0.96 | 0.82 | 0.58 | 0.41 |
| Wilks‘ Lambda | 0.02 | 0.18 | 0.55 | 0.83 |
| Land Use Groups | Function | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Forest | −3.99 | 0.64 | −1.70 | 0.04 |
| Urban | −0.45 | −2.55 | 0.04 | −0.53 |
| LSHORT | −2.08 | −0.06 | 0.49 | 0.40 |
| SSHORT | 0.52 | 1.81 | 0.34 | −0.53 |
| MAG | 5.93 | −0.14 | −0.37 | 0.29 |
| Forest | Urban | LSHORT | SSHORT | MAG | Total | ||
|---|---|---|---|---|---|---|---|
| Count | Forest | 4 | 0 | 0 | 0 | 0 | 4 |
| Urban | 0 | 5 | 1 | 0 | 0 | 6 | |
| LSHORT | 1 | 1 | 11 | 0 | 0 | 13 | |
| SSHORT | 0 | 1 | 0 | 7 | 0 | 8 | |
| MAG | 0 | 0 | 0 | 0 | 7 | 7 | |
| % | Forest | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Urban | 0.0 | 83.3 | 16.7 | 0.0 | 0.0 | 100.0 | |
| LSHORT | 7.7 | 7.7 | 84.6 | 0.0 | 0.0 | 100.0 | |
| SSHORT | 0.0 | 12.5 | 0.0 | 87.5 | 0.0 | 100.0 | |
| MAG | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 |
4. Discussion
4.1. Occurrence and Variability of Common Elements in Surface Water
4.1.1. Electrical Conductivity (EC), Total Dissolved Solids (TDS), and Salinity
4.1.2. Potassium and Iron
4.1.3. Water Temperature, Dissolved Oxygen (DO) and pH
4.2. Discussion of Results from Principal Component Analysis (PCA)
4.2.1. Principal Components (PCs)
4.2.2. Contributions of Different Land Uses on Stream Water Quality as Revealed by DA
Forest Use and Mixed Agriculture (MAG) Land Use
Large-Scale Horticulture (LSHORT) Land Use
Small-Scale Intensive Horticulture (SSHORT)
Urban Use
5. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Thoen, R.; Jaffee, S.; Dolan, C. Equatorial rose: The Kenyan-European cut flower supply chain. Supply Chain Development in Emerging Markets: Case Studies of Supportive Public Policy; World Bank: Washington, DC, USA, 2000. [Google Scholar]
- Dolan, C.; Humphrey, J. Changing governance patterns in the trade in fresh vegetables between Africa and the United Kingdom. Environ. Plan. 2004, 36, 491–509. [Google Scholar] [CrossRef]
- Dever, J. Case study #6–5. Small-farm access to high-value horticultural markets in Kenya. In Food Policy for Developing Countries: Case Studies; Cornell University library: Ithaca, NY, USA, 2007. [Google Scholar]
- Weinberger, K.; Lumpkin, T.A. Diversification into horticulture and poverty reduction: A research agenda. World Dev. 2007, 35, 1464–1480. [Google Scholar] [CrossRef]
- Ulrich, A. Export-oriented horticultural production in Laikipia, Kenya: Assessing the implications for rural livelihoods. Sustainability 2014, 6, 336–347. [Google Scholar] [CrossRef]
- Kibichii, S.; Shivoga, W.A.; Muchiri, M.; Miller, S.N. Macroinvertebrate assemblages along a land-use gradient in the upper River Njoro watershed of Lake Nakuru drainage basin, Kenya. Lakes Reservoirs Res. Manag. 2007, 12, 107–117. [Google Scholar] [CrossRef]
- Owiti, G.E.; Oswe, I.A. Human impact on Lake ecosystems: The case of Lake Naivasha, Kenya. Afr. J. Aquat. Sci. 2007, 32, 79–88. [Google Scholar] [CrossRef]
- Nyakundi, W.O.; Magoma, G.; Ochora, J.; Myende, A.B. A Survey of Pesticide Use and Application Patterns among Farmers: A Case Study from Selected Horticultural Farms in Rift Valley and Central Provinces, Kenya. Available online: http://elearning.jkuat.ac.ke/journals/ojs/index.php/jscp/article/view/744 (accessed on 29 October 2015).
- Zhu, W.; Joseph, G.; Karen, S. Land-use impact on water pollution: Elevated pollutant input and reduced pollutant retention. J. Contemp. Water Res. Edu. 2008, 138, 15–21. [Google Scholar] [CrossRef]
- West, P.C.; Narisma, G.T.; Barford, C.C.; Kucharik, C.J.; Foley, J.A. An alternative approach for quantifying climate regulation by ecosystems. Front. Ecol. Environ. 2010, 9, 126–133. [Google Scholar] [CrossRef]
- Wan, R.; Cai, S.; Li, H.; Yang, G.; Li, Z.; Nie, X. Inferring land use and land cover impact on stream water quality using a bayesian hierarchical modeling approach in the Xitiaoxi River watershed, China. J. Environ. Manag. 2014, 133, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saoke, P. Kenya pops situation report: DDT, pesticides and Polychlorinated Biphenyls. Available online: http://www.ipen.org/ipepweb1/library/ipep_pdf_reports/1ken%20kenya%20country%20situation%20report.pdf (accessed on 29 October 2015).
- Kithiia, S.M. Effects of Sediments Loads on Water Quality within the Nairobi River Basins, Kenya. Available online: http://www.ij-ep.org/paperInfo.aspx?ID=85 (accessed on 29 October 2015).
- Liniger, H.; Gikonyo, J.; Kiteme, B.; Wiesmann, U. Assessing and managing scarce tropical mountain water resources. Mt. Res. Dev. 2005, 25, 163–173. [Google Scholar] [CrossRef]
- Nziguheba, G.; Smolders, E. Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci. Total Environ. 2008, 390, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Heimann, K.; Ridd, M.; Negri, A.P. Pesticide contamination and phytotoxicity of sediment interstitial water to tropical benthic microalgae. Water Res. 2013, 47, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Arunakumara, K.K.I.U.; Walpola, B.; Yoon, M.H. Current status of heavy metal contamination in Asia’s rice lands. Rev. Environ. Sci. Biotechnol. 2013, 12, 355–377. [Google Scholar] [CrossRef]
- Singh, B.P. Nontraditional crop production in Africa for export. In Trends in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2011. [Google Scholar]
- Otero, N.; Vitòria, L.; Soler, A.; Canals, A. Fertiliser characterisation: Major, trace and rare earth elements. Appl. Geochem. 2005, 20, 1473–1488. [Google Scholar] [CrossRef]
- Lugon-Moulin, N.; Ryan, L.; Donini, P.; Rossi, L. Cadmium content of phosphate fertilizers used for tobacco production. Agrono. Sustain. Dev. 2006, 26, 151–155. [Google Scholar] [CrossRef]
- Chanda, A.; Akhand, A.; Das, A.; Hazra, S. Cr, Pb and Hg contamination on agricultural soil and paddy grain after irrigation using metropolitan sewage effluent. J. Appl. Environ. Biol. Sci. 2011, 1, 464–469. [Google Scholar]
- Justus, F.; Yu, D. Spatial distribution of greenhouse commercial horticulture in Kenya and the role of demographic, infrastructure and topo-edaphic factors. ISPRS Int. J. Geo-Inf. 2014, 3, 274–296. [Google Scholar] [CrossRef]
- Vega, M.; Pardo, R.; Barrado, E.; Debán, L. Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 1998, 32, 3581–3592. [Google Scholar] [CrossRef]
- Tong, S.; Chen, W. Modeling the relationship between land use and surface water quality. J. Environ. Manag. 2002, 66, 377–393. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Singh, D.S.; Singh, A.K. Water potential of microorganisms. Acta. Hortic. 2010, 93, 155–167. [Google Scholar]
- Arrigo, J.S. Using cooperative water quality data for a holistic understanding of Rivers and streams: Quality of the Chhoti Gandak River using principal component analysis, Ganga Plain, India. J. Earth Syst. Sci. 2011, 119, 117–127. [Google Scholar]
- Otero, N.; Tolosana-Delgado, R.; Soler, A.; Pawlowsky-Glahn, V.; Canals, A. Relative vs. Absolute statistical analysis of compositions: A comparative study of surface waters of a Mediterranean River. Water Res. 2005, 39, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.L.; Chappell, R.W.; Loftis, J.C. Water quality sample collection, data treatment and results presentation for principal components analysis—Literature review and Illinois River Watershed case study. Water Res. 2012, 46, 3110–3122. [Google Scholar] [CrossRef] [PubMed]
- Selle, B.; Schwientek, M.; Lischeid, G. Understanding processes governing water quality in catchments using principal component scores. J. Hydrol. 2013, 486, 31–38. [Google Scholar] [CrossRef]
- Notter, B. Rainfall-Runoff Modeling of Meso-Scale Catchments in the Upper Ewaso Ng’iro Basin, Kenya. Master’ Thesis, University of Berne, Bern, Switzerland, 2003. [Google Scholar]
- Dougall, H.W.; Glover, P.E. On the chemical composition of Themeda Triandra and cynodondactylon. Afr. J. Ecol. 1964, 2, 67–70. [Google Scholar] [CrossRef]
- Shisanya, C.A.; Mucheru, M.W.; Mugendi, D.N.; Kung’u, J.B. Effect of organic and inorganic nutrient sources on soil mineral nitrogen and maize yields in Central highlands of Kenya. Soil Tillage Res. 2009, 103, 239–246. [Google Scholar] [CrossRef]
- Murage, E.W.; Karanja, N.K.; Smithson, P.C.; Woomer, P.L. Diagnostic indicators of soil quality in productive and non-productive smallholders’ fields of Kenya’s Central highlands. Agric. Ecosyst. Environ. 2000, 79, 1–8. [Google Scholar] [CrossRef]
- Okoba, B.O.; Sterk, G. Farmers’ identification of erosion indicators and related erosion damage in the Central highlands of Kenya. CATENA 2006, 65, 292–301. [Google Scholar] [CrossRef]
- Gicheru, P.T.; Kiome, R.M. Reconnaissance Soil Survey of Chuka-Nkubu Area. Available online: http://library.wur.nl/isric/fulltext/isricu_i23135_001.pdf (accessed on 29 October 2015).
- WRI. World Resources Institute (WRI). Download Kenya GIS Data. Available online: http://www.Wri.Org (accessed on 29 October 2015).
- Eyre, B.D.; Pepperell, P. A spatially intensive approach to water quality monitoring in the Rous River catchment, NSW, Australia. J. Environ. Manag. 1999, 56, 97–118. [Google Scholar] [CrossRef]
- Wang, X. Integrating water-quality management and land-use planning in a watershed context. J. Environ. Manag. 2001, 61, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Geotech. Smart3 Colorimeter Operator’s Manual. 2011. Available online: http://www.geotechenv.com/Manuals/LaMotte_Manuals/smart3_colorimeter_operators_manual.pdf (accessed on 29 October 2015).
- Reimann, C.; Filzmoser, P.; Garrett, R.G.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Filzmoser, P.; Hron, K.; Reimann, C. The bivariate statistical analysis of environmental (compositional) data. Sci. Total Environ. 2010, 408, 4230–4238. [Google Scholar] [CrossRef] [PubMed]
- Filzmoser, P.; Hron, K.; Reimann, C. Interpretation of multivariate outliers for compositional data. Comput. Geosci. 2012, 39, 77–85. [Google Scholar] [CrossRef]
- Shrestha, S.; Futab, K.; Takashi, N. Use of principal component analysis, factor analysis and discriminant analysis to evaluate spatial and temporal variations in water quality of the Mekong River. J. Hydroinform. 2008, 10, 43–56. [Google Scholar] [CrossRef]
- Gonçalves, C.; Esteves da Silva, J.C.G.; Alpendurada, M.F. Chemometric interpretation of pesticide occurence in soil samples from an intensive horticulture area in North Portugal. Analytica. Chimica. Acta. 2006, 560, 164–171. [Google Scholar] [CrossRef]
- Chambers, J.M.; Cleveland, W.S.; Kleiner, B.; Tukey, P.A. Graphical Methods for Data Analysis; Wadsworth Publishing: Belmont, CA, USA, 1983. [Google Scholar]
- Kasangaki, A.; Chapman, L.J.; Balirwa, J. Land use and the ecology of benthic macroinvertebrate assemblages of high-altitude rainforest streams in Uganda. Freshw. Biol. 2008, 53, 681–697. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Mishra, G.; Ball, K.; Arbuckle, J.; Crawford, D. Dietary patterns of Australian adults and their association with socioeconomic status: Results from the 1995 national nutrition survey. Eur. J. Clin. Nutr. 2002, 56, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Palomino, A.; Santamarina, C. Fabric map for kaolinite: Effects of pH and ionic concentration on behavior. Clays Clay Miner. 2005, 53, 211–223. [Google Scholar] [CrossRef]
- Rolfe, R.F.; Miller, R.F.; McQueen, I.S. Dispersion Characteristics of Montmorillonite, Kaolinite, and Hike Clays in Waters of Varying Quality, and Their Control with Phosphate Dispersants. Available online: https://pubs.er.usgs.gov/publication/pp (accessed on 29 October 2015).
- Razmkhah, H.; Abrishamchi, A.; Torkian, A. Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: A case study on Jajrood River (Tehran, Iran). J. Environ. Manag. 2010, 91, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Nyakeya, K.; Raburu, P.O.; Masese, F.O.; Gichuki, J. Assessment of pollution impacts on the ecological integrity of the Kisian and Kisat Rivers in Lake Victoria drainage basin, Kenya. Afr. J. Sci. Technol. 2009, 3, 97–107. [Google Scholar]
- 1. Kanga, J.; Lee, S.W.; Cho, K.H.; Ki, S.J.; Cha, S.M.; Kim, J.H. Linking land-use type and stream water quality using spatial data of fecal indicator bacteria and heavy metals in the Yeongsan River Basin. Water Res. 2010, 44, 4143–4157. [Google Scholar] [CrossRef] [PubMed]
- Kipkoech, A.K.; Okalebo, J.R.; Kifuko-Koech, M.K.; Ndungu, K.W.; Nekesa, A.O. Assessing Factors Influencing Types, Rate of Application and Timing of Fertilizer Use among Small-Scale Farmers of Western Kenya. Available online: http://repository.ruforum.org/documents/assessing-factors-influencing-types-rate-application-and-timing-fertilizer-use-among-small (accessed on 29 October 2015).
- Espinoza, L.; Norman, R.; Salton, N.; Daniels, M. The Nitrogen and Phosphorous Cycle in Soils. Available online: https://www.uaex.edu/publications/pdf/FSA-2148.pdf (accessed on 29 October 2015).
- Silberbush, M.; Lieth, J.H. Nitrate and potassium uptake by greenhouse roses (rosa hybrida) along successive flower-cut cycles: A model and its calibration. Sci. Hortic. 2004, 101, 127–141. [Google Scholar] [CrossRef]
- Gaw, S.K.; Wilkins, A.L.; Kim, N.D.; Palmer, G.T.; Robinson, P. Trace element and ΣDDT concentrations in horticultural soils from the Tasman, Waikato and Auckland regions of New Zealand. Sci. Total Environ. 2006, 355, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Better Farming. Greenhouse Wastewater Discharges Provoke Legislation Debate. Available online: http://www.betterfarming.com/online-news/greenhouse-wastewater-discharges-provoke-legislation-debate 5435 (accessed on 29 October 2015).
- Atkinson, J.D.; Chamberlain, E.E.; Dingley, J.M.; Reid, W.H.; Brien, R.M.; Cottier, W.; Jack, H.; Taylor, G.G. Plant protection in New Zealand; New Zealand Department of Education: Wellington, New Zealand, 1956. [Google Scholar]
- Alsanius, B.W.; Jung, V.; Hultberg, M.; Khalil, S.; Gustafsson, A.K.; Burleigh, S. Sustainable greenhouse systems—The potential of microorganisms. Acta. Hortic. 2011, 893, 155–167. [Google Scholar] [CrossRef]
- Grasselly, D.; Merlin, G.; Sédilot, C.; Vanel, F.; Dufour, G.; Rosso, L. Denitrification of soilless tomato crops run-off water by horizontal subsurface constructed wetlands. Acta. Hortic. 2005, 691, 329–332. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muriithi, F.K.; Yu, D. Understanding the Impact of Intensive Horticulture Land-Use Practices on Surface Water Quality in Central Kenya. Environments 2015, 2, 521-545. https://doi.org/10.3390/environments2040521
Muriithi FK, Yu D. Understanding the Impact of Intensive Horticulture Land-Use Practices on Surface Water Quality in Central Kenya. Environments. 2015; 2(4):521-545. https://doi.org/10.3390/environments2040521
Chicago/Turabian StyleMuriithi, Faith K., and Danlin Yu. 2015. "Understanding the Impact of Intensive Horticulture Land-Use Practices on Surface Water Quality in Central Kenya" Environments 2, no. 4: 521-545. https://doi.org/10.3390/environments2040521





