Neuroinflammation and Neuromodulation in Neurological Diseases
Abstract
:1. Introduction
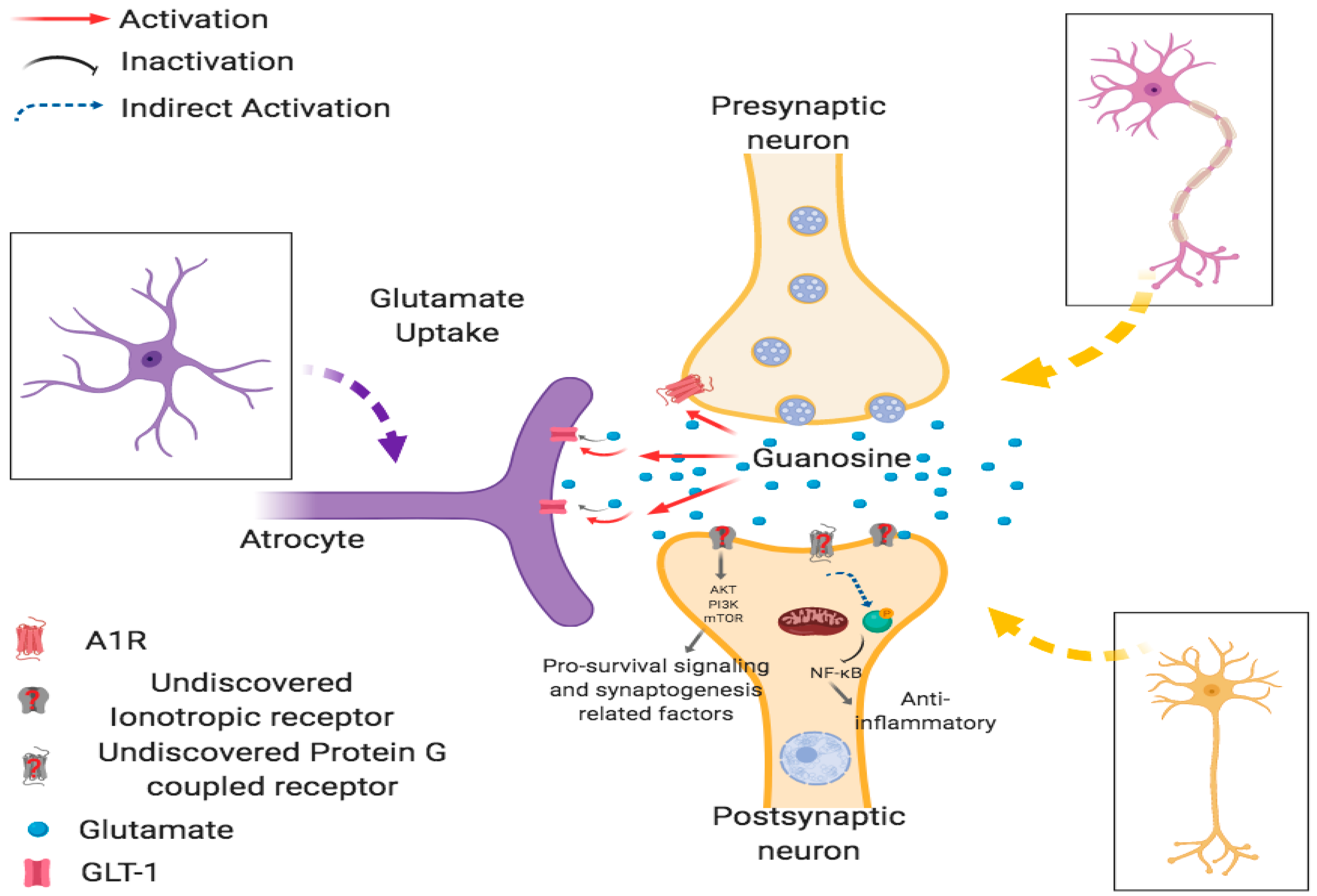
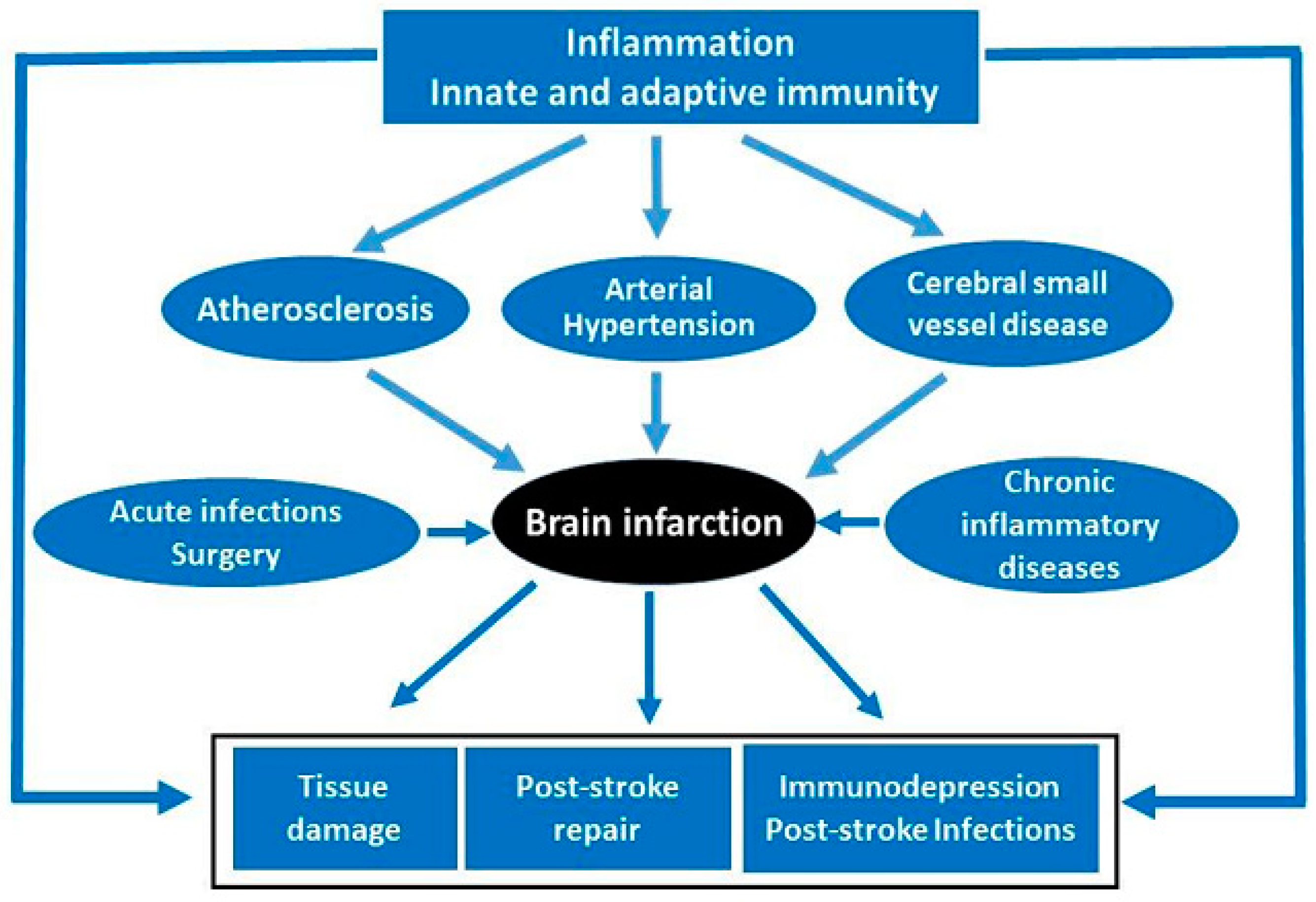
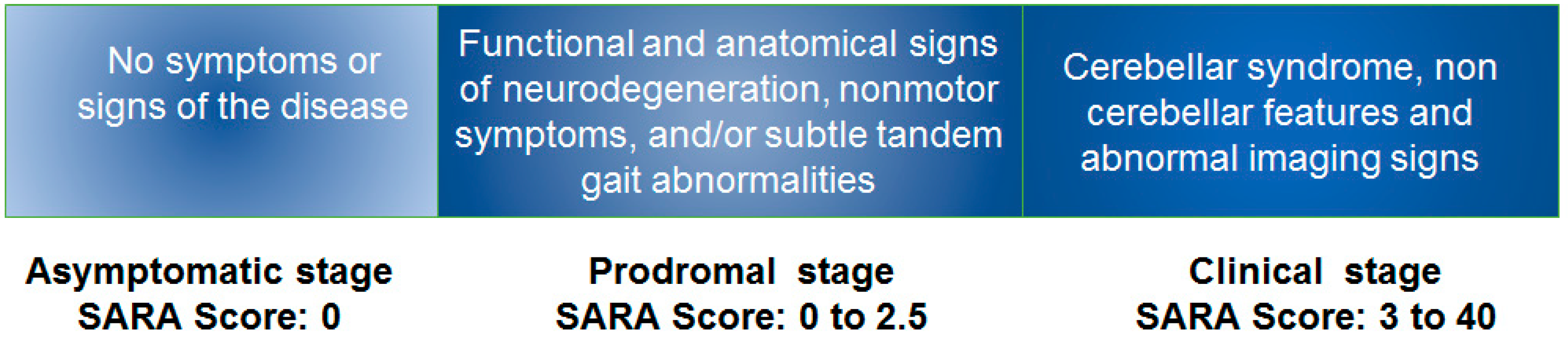
2. Lecture Highlights
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jácome, M.C.I.; Chacòn, L.M.M.; Cuesta, H.V.; Rizo, C.M.; Santiesteban, M.W.; Hernandez, L.R.; García, E.N.; Fraguela, M.E.G.; Verdecia, C.I.F.; Hurtado, Y.V.; et al. Peripheral inflammatory markers contributing to comorbidities in autism. Behav. Sci. 2016, 6, 29. [Google Scholar] [CrossRef]
- Noris-García, E.; Arce, S.; Nardin, P.; Lanigan, M.E.; Acuña, V.; Gutierrez, F.; Robinson-Agramonte, M.A.; Gonçalves, C.A. Peripheral levels of brain-derived neurotrophic factor and s100b in neuropsychiatric systemic lupus erythematous. Lupus 2018, 27, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Pérez, L.; Rodríguez-Labrada, R.; Laffita-Mesa, J.M. Prodromal spinocerebellar ataxia type 2: Prospects for early interventions and ethical challenges. Mov. Disord. 2017, 32, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, Q.; Anrather, J.; Shi, F.D. Immune interventions in stroke. Nat. Rev. Neurol. 2015, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Depino, A.M.; de los Angeles Robinson-Agramonte, M. Understanding on neuroimmunology in autism spectrum disorder. In Translational Aproaches in Autism Spectrum Disorders; Springer: Berlin/Heidelberg, Germany, 2015; pp. 155–180. [Google Scholar]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012, 814, 23–45. [Google Scholar] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.C.; Tortorelli, L.S.; Galland, F.; Da Ré, C.; Negri, E.; Engelke, D.S.; Rodrigues, L.; Leite, M.C.; Gonçalves, C.A. Lipopolysaccharide modulates astrocytic s100b secretion: A study in cerebrospinal fluid and astrocyte cultures from rats. J. Neuroinflamm. 2011, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Furman, J.L.; Norris, C.M. Calcineurin and glial signaling: Neuroinflammation and beyond. J. Neuroinflamm. 2014, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.J.; Massie, A.; De Keyser, J. Immune players in the cns: The astrocyte. J. Neuroimmune Pharmacol. 2013, 8, 824–839. [Google Scholar] [CrossRef]
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013, 23, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Osipova, E.D.; Semyachkina-Glushkovskaya, O.V.; Morgun, A.V.; Pisareva, N.V.; Malinovskaya, N.A.; Boitsova, E.B.; Pozhilenkova, E.A.; Belova, O.A.; Salmin, V.V.; Taranushenko, T.E.; et al. Gliotransmitters and cytokines in the control of blood-brain barrier permeability. Rev. Neurosci. 2018, 29, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, D.G.; Almeida, R.F.; Souza, D.O.; Zimmer, E.R. The astrocyte biochemistry. Semin. Cell Dev. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.P.; Lara, D.R.; Souza, D.O. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol. Ther. 2007, 116, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Tasca, C.I.; Lanznaster, D.; Oliveira, K.A.; Fernandez-Duenas, V.; Ciruela, F. Neuromodulatory effects of guanine-based purines in health and disease. Front. Cell. Neurosci. 2018, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global burden of stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; De Silva, T.M.; Chen, J.; Faraci, F.M. Cerebral vascular disease and neurovascular injury in ischemic. Stroke Circ. Res. 2017, 120, 449–471. [Google Scholar]
- Macrez, R.; Ali, C.; Toutirais, O.; Le Mauff, B.; Defer, G.; Dirnagl, U.; Vivien, D. Stroke and the immune system: From pathophysiology to new therapeutic strategies. Lancet Neurol. 2011, 10, 471–480. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.; Li, H.; Zhao, R.; Huang, Q.; Liu, J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur. J. Med. Chem. 2019, 162, 132–146. [Google Scholar] [CrossRef]
- Auricchio, F.; Scavone, C.; Cimmaruta, D.; Di Mauro, G.; Capuano, A.; Sportiello, L.; Rafaniello, C. Drugs approved for the treatment of multiple sclerosis: Review of their safety profile. Expert Opin. Drug Saf. 2017, 16, 1359–1371. [Google Scholar] [CrossRef]
- National Multiple Sclerosis Society. Disease Modification: For-professionals/clinical-care/managing-ms/disease-modification. Accessed 10/19/18; owens gm. Am. J. Manag. Care 2013, 19, S307–S312. [Google Scholar]
- Velázquez-Perez, L.; Rodríguez-Labrada, R.; Canales-Ochoa, N.; Montero, J.M.; Sánchez-Cruz, G.; Aguilera-Rodríguez, R.; Almaguer-Mederos, L.E.; Laffita-Mesa, J.M. Progression of early features of spinocerebellar ataxia type 2 in individuals at risk: A longitudinal study. Lancet Neurol. 2014, 13, 482–489. [Google Scholar] [CrossRef]
- Velázquez-Pérez, L.C.; Rodríguez-Labrada, R.; Fernandez-Ruiz, J. Spinocerebellar ataxia type 2: Clinicogenetic aspects, mechanistic insights, and management approaches. Front. Neurol. 2017, 8, 472. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson-Agramonte, M.d.l.A.; Gonçalves, C.-A.; Farina de Almeida, R.; González Quevedo, A.; Chow, S.; Velázquez Pérez, L.; Díaz de la Fé, A.; Sesterheim, P.; Souza, D.O.G. Neuroinflammation and Neuromodulation in Neurological Diseases. Behav. Sci. 2019, 9, 99. https://doi.org/10.3390/bs9090099
Robinson-Agramonte MdlA, Gonçalves C-A, Farina de Almeida R, González Quevedo A, Chow S, Velázquez Pérez L, Díaz de la Fé A, Sesterheim P, Souza DOG. Neuroinflammation and Neuromodulation in Neurological Diseases. Behavioral Sciences. 2019; 9(9):99. https://doi.org/10.3390/bs9090099
Chicago/Turabian StyleRobinson-Agramonte, Maria de los Angeles, Carlos-Alberto Gonçalves, Roberto Farina de Almeida, Alina González Quevedo, Sandra Chow, Luis Velázquez Pérez, Amado Díaz de la Fé, Patricia Sesterheim, and Diogo Onofre Gomes Souza. 2019. "Neuroinflammation and Neuromodulation in Neurological Diseases" Behavioral Sciences 9, no. 9: 99. https://doi.org/10.3390/bs9090099




