Developmental Neurobiology of the Rat Attachment System and Its Modulation by Stress
Abstract
:1. Introduction
2. Odor Attachment Learning of the Infant Rat
3. Neural Encoding of the Maternal Odor
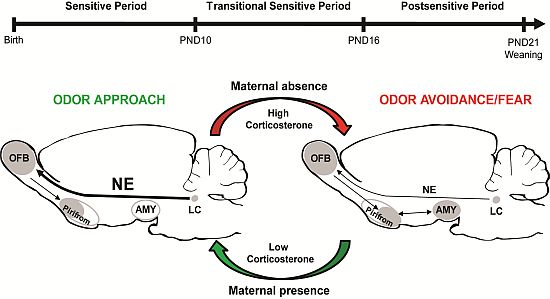
4. The Role of Corticosterone in the Termination of the Sensitive Period for Attachment Learning
5. Maternal Modulation of Pups’ Corticosterone Levels and Attachment Learning
6. Enduring Effects of Early Life Stress and Attachment
7. Summary and Conclusions
Acknowledgements
References
- Kim, J.J.; Diamond, D.M. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002, 3, 453–462. [Google Scholar] [CrossRef]
- McEwen, B.S. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging proces. Neurobiol. Aging 2002, 23, 921–939. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar]
- Heim, C.; Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry 2001, 49, 1023–1039. [Google Scholar]
- McEwen, B.S. Stressed or stressed out: What is the difference? J. Psychiatry Neurosci. 2005, 30, 315–318. [Google Scholar]
- Sandi, C.; Bisaz, R. A model for the involvement of neural cell adhesion molecules in stress-related mood disorders. Neuroendocrinology 2007, 85, 158–176. [Google Scholar] [CrossRef]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P. Developmental neurobiology of childhood stress and trauma. Psychiatr. Clin. North Am. 2002, 25, 397–426. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar]
- Sanchez, M.M.; Ladd, C.O.; Plotsky, P.M. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. Psychopathol. 2001, 13, 419–449. [Google Scholar] [CrossRef]
- Levine, S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 2005, 30, 939–946. [Google Scholar] [CrossRef]
- Bowlby, J. Attachment; Basic Books: New York, NY, USA, 1965. [Google Scholar]
- Bowlby, J. Attachment and Loss; Basic Books: New York, NY, USA, 1969; Volume 1. [Google Scholar]
- Rutter, M. Clinical implications of attachment concepts: Retrospect and prospect. J. Child Psychol. Psychiatry 1995, 36, 549–571. [Google Scholar]
- Hofer, M.A.; Sullivan, S.M. Towards a neurobiology of attachment. In Handbook of Developmental Cognitive Neuroscience; Nelson, C.A., Luciana, M., Eds.; MIT Press: Cambridge, MA, USA, 2001; pp. 599–616. [Google Scholar]
- Sullivan, R.M.; Holman, P.J. Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neurosci. Biobehav. Rev. 2010, 34, 835–844. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Toubas, P. Clinical usefulness of maternal odor in newborns: Soothing and feeding preparatory responses. Biol. Neonate 1998, 74, 402–408. [Google Scholar] [CrossRef]
- Pedersen, P.E.; Blass, E.M. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev. Psychobiol. 1982, 15, 349–355. [Google Scholar] [CrossRef]
- Hofer, M.A.; Shair, H.; Singh, P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiol. Behav. 1976, 17, 131–136. [Google Scholar] [CrossRef]
- Teicher, M.H.; Blass, E.M. First suckling response of the newborn albino rat: The roles of olfaction and amniotic fluid. Science 1977, 198, 635–636. [Google Scholar]
- Hill, D.L.; Almli, C.R. Olfactory bulbectomy in infant rats: Survival, growth and ingestive behaviors. Physiol. Behav. 1981, 27, 811–817. [Google Scholar] [CrossRef]
- Singh, P.J.; Tobach, E. Olfactory bulbectomy and nursing behavior in rat pups (Wistar DAB). Dev. Psychobiol. 1975, 8, 151–164. [Google Scholar] [CrossRef]
- Singh, P.J.; Tucker, A.M.; Hofer, M.A. Effects of nasal ZnSO4 irrigation and olfactory bulbectomy on rat pups. Physiol. Behav. 1976, 17, 373–382. [Google Scholar] [CrossRef]
- Leon, M.; Coopersmith, R.; Lee, S.; Sullivan, R.M.; Wilson, D.A.; Woo, C. Neural and Behavioral Plasticity Induced by Early Olfactory Learning; Academic Press: New York, NY, USA, 1987; p. 23. [Google Scholar]
- Lev, R.; Orlic, D. Protein absorption by the intestine of the fetal rat in utero. Science 1972, 177, 522–524. [Google Scholar]
- Narayanan, C.H.; Fox, M.W.; Hamburger, V. Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus). Behaviour 1971, 40, 100–134. [Google Scholar] [CrossRef]
- Hepper, P.G.; Cleland, J. Developmental aspects of kin recognition. Genetica 1998, 104, 199–205. [Google Scholar] [CrossRef]
- Smotherman, W.P. Odor aversion learning by the rat fetus. Physiol. Behav. 1982, 29, 769–771. [Google Scholar] [CrossRef]
- Smotherman, W.P.; Robinson, S.R. Prenatal expression of species-typical action patterns in the rat fetus (Rattus norvegicus). J. Comp. Psychol. 1987, 101, 190–196. [Google Scholar] [CrossRef]
- Smotherman, W.P.; Robinson, S.R. Prenatal influences on development: Behavior is not a trivial aspect of fetal life. J. Dev. Behav. Pediatr. 1987, 8, 171–176. [Google Scholar]
- Miller, S.S.; Spear, N.E. Olfactory learning in the rat immediately after birth: Unique salience of first odors. Dev. Psychobiol. 2009, 51, 488–504. [Google Scholar] [CrossRef]
- Blass, E.M.; Teicher, M.H. Suckling. Science 1980, 210, 15–22. [Google Scholar]
- Youngentob, S.L.; Kent, P.F.; Sheehe, P.R.; Molina, J.C.; Spear, N.E.; Youngentob, L.M. Experience-induced fetal plasticity: The effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav. Neurosci. 2007, 121, 1293–1305. [Google Scholar] [CrossRef]
- Alberts, J.R.; May, B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Dev. Psychobiol. 1984, 17, 161–181. [Google Scholar] [CrossRef]
- Galef, B.G., Jr.; Kaner, H.C. Establishment and maintenance of preference for natural and artificial olfactory stimuli in juvenile rats. J. Comp. Physiol. Psychol. 1980, 94, 588–595. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Wilson, D.A.; Wong, R.; Correa, A.; Leon, M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res. Dev. Brain Res. 1990, 53, 243–247. [Google Scholar]
- Brake, S.C. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science 1981, 211, 506–508. [Google Scholar]
- Galef, B.G., Jr.; Sherry, D.F. Mother’s milk: A medium for transmission of cues reflecting the flavor of mother’s diet. J. Comp. Physiol. Psychol. 1973, 83, 374–378. [Google Scholar] [CrossRef]
- Johanson, I.B.; Teicher, M.H. Classical conditioning of an odor preference in 3-day-old rats. Behav. Neural Biol. 1980, 29, 132–136. [Google Scholar] [CrossRef]
- McLean, J.H.; Darby-King, A.; Sullivan, R.M.; King, S.R. Serotonergic influence on olfactory learning in the neonate rat. Behav. Neural Biol. 1993, 60, 152–162. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Wilson, D.A. Neural correlates of conditioned odor avoidance in infant rats. Behav. Neurosci. 1991, 105, 307–312. [Google Scholar] [CrossRef]
- Wilson, D.A.; Sullivan, R.M. Neurobiology of associative learning in the neonate: Early olfactory learning. Behav. Neural Biol. 1994, 61, 1–18. [Google Scholar] [CrossRef]
- Weldon, D.A.; Travis, M.L.; Kennedy, D.A. Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behav. Neurosci. 1991, 105, 450–458. [Google Scholar]
- Leon, M. Dietary control of maternal pheromone in the lactating rat. Physiol. Behav. 1975, 14, 311–319. [Google Scholar] [CrossRef]
- Leon, M. The neurobiology of filial learning. Annu. Rev. Psychol. 1992, 43, 377–398. [Google Scholar] [CrossRef]
- Hofer, M.; Sullivan, R. Towards a neurobiology of attachmen. In Handbook of Developmental Cognitive Neuroscience; Nelson, C.A., Luciana, M., Eds.; MIT Press: Cambridge, MA, USA, 2008; pp. 787–806. [Google Scholar]
- Campbell, B.A.; Spear, N.E. Ontogeny of memory. Psychol. Rev. 1972, 79, 215–236. [Google Scholar] [CrossRef]
- Rescorla, R.A. Behavioral studies of Pavlovian conditioning. Annu. Rev. Neurosci. 1988, 11, 329–352. [Google Scholar] [CrossRef]
- Rescorla, R.A. Inhibition of delay in Pavlovian fear conditioning. J. Comp. Physiol. Psychol. 1967, 64, 114–120. [Google Scholar] [CrossRef]
- Rush, A.N.; Robinette, B.L.; Stanton, M.E. Ontogenetic differences in the effects of unpaired stimulus preexposure on eyeblink conditioning in the rat. Dev. Psychobiol. 2001, 39, 8–18. [Google Scholar] [CrossRef]
- Stanton, M.E. Multiple memory systems, development and conditioning. Behav. Brain Res. 2000, 110, 25–37. [Google Scholar] [CrossRef]
- Stanton, M.E.; Fox, G.D.; Carter, C.S. Ontogeny of the conditioned eyeblink response in rats: Acquisition or expression? Neuropharmacology 1998, 37, 623–632. [Google Scholar] [CrossRef]
- Hoffmann, H.; Spear, N.E. Ontogenetic differences in conditioning of an aversion to a gustatory CS with a peripheral US. Behav. Neural Biol. 1988, 50, 16–23. [Google Scholar] [CrossRef]
- Raineki, C.; Shionoya, K.; Sander, K.; Sullivan, R.M. Ontogeny of odor-LiCl vs. odor-shock learning: Similar behaviors but divergent ages of functional amygdala emergence. Learn. Mem. 2009, 16, 114–121. [Google Scholar] [CrossRef]
- Camp, L.L.; Rudy, J.W. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev. Psychobiol. 1988, 21, 25–42. [Google Scholar] [CrossRef]
- Moriceau, S.; Wilson, D.A.; Levine, S.; Sullivan, R.M. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006, 26, 6737–6748. [Google Scholar]
- Sullivan, R.M.; Landers, M.; Yeaman, B.; Wilson, D.A. Good memories of bad events in infancy. Nature 2000, 407, 38–39. [Google Scholar]
- Sullivan, R.M.; Hofer, M.A.; Brake, S.C. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev. Psychobiol. 1986, 19, 615–623. [Google Scholar] [CrossRef]
- Raineki, C.; Pickenhagen, A.; Roth, T.L.; Babstock, D.M.; McLean, J.H.; Harley, C.W.; Lucion, A.B.; Sullivan, R.M. The neurobiology of infant maternal odor learning. Braz. J. Med. Biol. Res. 2010, 43, 914–919. [Google Scholar] [CrossRef] [Green Version]
- De Medeiros, C.B.; Fleming, A.S.; Johnston, C.C.; Walker, C.D. Artificial rearing of rat pups reveals the beneficial effects of mother care on neonatal inflammation and adult sensitivity to pain. Pediatr. Res. 2009, 66, 272–277. [Google Scholar] [CrossRef]
- Roth, T.L.; Sullivan, R.M. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry 2005, 57, 823–831. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Stackenwalt, G.; Nasr, F.; Lemon, C.; Wilson, D.A. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav. Neurosci. 2000, 114, 957–962. [Google Scholar] [CrossRef]
- Blozovski, D.; Cudennec, A. Passive avoidence learning in the young rat. Dev. Psychobiol. 1980, 13, 513–518. [Google Scholar] [CrossRef]
- Collier, A.C.; Mast, J. Alleviation of avoidance deficits by approach alternatives in 10-day old rats. Physiol. Behav. 1979, 23, 615–618. [Google Scholar] [CrossRef]
- Myslivecek, J. Inhibitory learning and memory in newborn rats. Prog. Neurobiol. 1997, 53, 399–430. [Google Scholar] [CrossRef]
- Barr, G.A. Ontogeny of nociception and antinociception. NIDA Res. Monogr. 1995, 158, 172–201. [Google Scholar]
- Collier, A.C.; Bolles, R.C. The ontogenesis of defensive reactions to shock in preweanling rats. Dev. Psychobiol. 1980, 13, 141–150. [Google Scholar] [CrossRef]
- Emerich, D.F.; Scalzo, F.M.; Enters, E.K.; Spear, N.E.; Spear, L.P. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev. Psychobiol. 1985, 18, 215–227. [Google Scholar] [CrossRef]
- Fitzgerald, M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005, 6, 507–520. [Google Scholar] [CrossRef]
- Hess, E.H. Ethology: An approach to the complete analysis of behavior. In New Directions in Psychology; Brown, R., Galanter, E., Hess, E.H., Mendler, G., Eds.; Holt, Rinehart and Winston: New York, NY, USA, 1962; pp. 157–266. [Google Scholar]
- Salzen, E.A. Imprinting and environmental learning. In Development and Evolution of Behavior; Aronson, L.R., Tobach, E., Lehrman, D.S., Rosensbaltt, J., Eds.; W H Freeman: San Francisco, CA, USA, 1970; pp. 158–178. [Google Scholar]
- Rajecki, D.W.; Lamb, M.E.; Obmascher, P. Toward a general theory of infantile attachment: A comparative review of aspects of the social bond. Behav. Brain Sci. 1978, 3, 417–464. [Google Scholar]
- Harlow, H.F.; Harlow, M.K. The effect of rearing conditions on behavior. Int. J. Psychiatry 1965, 1, 43–51. [Google Scholar]
- Suomi, S.J. Gene-environment interactions and the neurobiology of social conflict. Ann. N. Y. Acad. Sci. 2003, 1008, 132–139. [Google Scholar] [CrossRef]
- Carlson, V.; Cicchetti, D.; Barnett, D.; Braunwald, K. Finding order in disorganization: Lessons from research on maltreated infants’ attachments to their caregivers. In Child Maltreatment: The Theory and Research on the Causes and Consequences of Child Abuse and Neglect; Cicchetti, D., Carlson, V., Eds.; Cambridge University Press: New York, NY, USA, 1990; pp. 494–528. [Google Scholar]
- Kojima, S.; Alberts, J.R. Maternal care can rapidly induce an odor-guided huddling preference in rat pups. Dev. Psychobiol. 2009, 51, 95–105. [Google Scholar] [CrossRef]
- Kojima, S.; Alberts, J.R. Warmth from skin-to-skin contact with mother is essential for the acquisition of filial huddling preference in preweanling rats. Dev. Psychobiol. 2011, 53, 813–827. [Google Scholar] [CrossRef]
- Panksepp, J.; De Eskinazi, F.G. Opiates and homing. J. Comp. Physiol. Psychol. 1980, 94, 650–663. [Google Scholar] [CrossRef]
- Sigling, H.O.; Wolterink-Donselaar, I.G.; Spruijt, B.M. Home seeking behavior in rat pups: Attachment vs. kin selection, oxytocin vs. vasopressin. Eur. J. Pharmacol. 2009, 612, 48–53. [Google Scholar] [CrossRef]
- Hoffman, C.M.; Flory, G.S.; Alberts, J.R. Neonatal thermotaxis improves reversal of a thermally reinforced operant response. Dev. Psychobiol. 1999, 34, 87–99. [Google Scholar] [CrossRef]
- Johnson, B.A.; Woo, C.C.; Duong, H.; Nguyen, V.; Leon, M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995, 699, 192–200. [Google Scholar] [CrossRef]
- Moriceau, S.; Sullivan, R.M. Unique neural circuitry for neonatal olfactory learning. J. Neurosci. 2004, 24, 1182–1189. [Google Scholar] [CrossRef]
- Moriceau, S.; Sullivan, R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006, 9, 1004–1006. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Leon, M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986, 392, 278–282. [Google Scholar]
- Wilson, D.A.; Leon, M. Early appearance of inhibition in the neonatal rat olfactory bulb. Brain Res. 1986, 391, 289–292. [Google Scholar]
- Wilson, D.A.; Sullivan, R.M. Olfactory associative conditioning in infant rats with brain stimulation as reward. I. Neurobehavioral consequences. Brain Res. Dev. Brain Res. 1990, 53, 215–221. [Google Scholar]
- Wilson, D.A.; Sullivan, R.M. Olfactory associative conditioning in infant rats with brain stimulation as reward: II. Norepinephrine mediates a specific component of the bulb response to reward. Behav. Neurosci. 1991, 105, 843–849. [Google Scholar] [CrossRef]
- Wilson, D.A.; Sullivan, R.M.; Leon, M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. J. Neurosci. 1987, 7, 3154–3162. [Google Scholar]
- Woo, C.C.; Coopersmith, R.; Leon, M. Localized changes in olfactory bulb morphology associated with early olfactory learning. J. Comp. Neurol. 1987, 263, 113–125. [Google Scholar] [CrossRef]
- Woo, C.C.; Oshita, M.H.; Leon, M. A learned odor decreases the number of Fos-immunopositive granule cells in the olfactory bulb of young rats. Brain Res. 1996, 716, 149–156. [Google Scholar] [CrossRef]
- Raineki, C.; Moriceau, S.; Sullivan, R.M. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol. Psychiatry 2010, 67, 1137–1145. [Google Scholar] [CrossRef]
- Yuan, Q.; Harley, C.W.; Bruce, J.C.; Darby-King, A.; McLean, J.H. Isoproterenol increases CREB phosphorylation and olfactory nerve-evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor preference learning. Learn. Mem. 2000, 7, 413–421. [Google Scholar] [CrossRef]
- Yuan, Q.; Harley, C.W.; McLean, J.H.; Knopfel, T. Optical imaging of odor preference memory in the rat olfactory bulb. J. Neurophysiol. 2002, 87, 3156–3159. [Google Scholar]
- Sevelinges, Y.; Moriceau, S.; Holman, P.; Miner, C.; Muzny, K.; Gervais, R.; Mouly, A.M.; Sullivan, R.M. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry 2007, 62, 1070–1079. [Google Scholar] [CrossRef]
- Sevelinges, Y.; Sullivan, R.M.; Messaoudi, B.; Mouly, A.M. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn. Mem. 2008, 15, 649–656. [Google Scholar] [CrossRef]
- Sevelinges, Y.; Mouly, A.M.; Raineki, C.; Moriceau, S.; Forest, C.; Sullivan, R.M. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Dev. Cogn. Neurosci. 2011, 1, 77–87. [Google Scholar] [CrossRef]
- Langdon, P.E.; Harley, C.W.; McLean, J.H. Increased beta adrenoceptor activation overcomes conditioned olfactory learning deficits induced by serotonin depletion. Brain Res. Dev. Brain Res. 1997, 102, 291–293. [Google Scholar]
- Sullivan, R.M.; Wilson, D.A.; Lemon, C.; Gerhardt, G.A. Bilateral 6-OHDA lesions of the locus coeruleus impair associative olfactory learning in newborn rats. Brain Res. 1994, 643, 306–309. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Zyzak, D.R.; Skierkowski, P.; Wilson, D.A. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res. Dev. Brain Res. 1992, 70, 279–282. [Google Scholar]
- McLean, J.H.; Shipley, M.T. Postnatal development of the noradrenergic projection from locus coeruleus to the olfactory bulb in the rat. J. Comp. Neurol. 1991, 304, 467–477. [Google Scholar] [CrossRef]
- Nakamura, S.; Kimura, F.; Sakaguchi, T. Postnatal development of electrical activity in the locus ceruleus. J. Neurophysiol. 1987, 58, 510–524. [Google Scholar]
- Nakamura, S.; Sakaguchi, T. Development and plasticity of the locus coeruleus: A review of recent physiological and pharmacological experimentation. Prog. Neurobiol. 1990, 34, 505–526. [Google Scholar] [CrossRef]
- Wilson, D.A.; Sullivan, R.M.; Leon, M. Odor familiarity alters mitral cell response in the olfactory bulb of neonatal rats. Brain Res. 1985, 354, 314–317. [Google Scholar]
- Okutani, F.; Zhang, J.J.; Otsuka, T.; Yagi, F.; Kaba, H. Modulation of olfactory learning in young rats through intrabulbar GABA(B) receptors. Eur. J. Neurosci. 2003, 18, 2031–2036. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Wilson, D.A. Molecular biology of early olfactory memory. Learn. Mem. 2003, 10, 1–4. [Google Scholar] [CrossRef]
- Price, T.L.; Darby-King, A.; Harley, C.W.; McLean, J.H. Serotonin plays a permissive role in conditioned olfactory learning induced by norepinephrine in the neonate rat. Behav. Neurosci. 1998, 112, 1430–1437. [Google Scholar] [CrossRef]
- Okutani, F.; Zhang, J.J.; Yagi, F.; Kaba, H. Non-specific olfactory aversion induced by intrabulbar infusion of the GABA(A) receptor antagonist bicuculline in young rats. Neuroscience 2002, 112, 901–906. [Google Scholar] [CrossRef]
- Kehoe, P.; Blass, E.M. Behaviorally functional opioid systems in infant rats: I. Evidence for olfactory and gustatory classical conditioning. Behav. Neurosci. 1986, 100, 359–367. [Google Scholar] [CrossRef]
- Roth, T.L.; Sullivan, R.M. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Dev. Psychobiol. 2001, 39, 188–198. [Google Scholar] [CrossRef]
- Nelson, E.; Panksepp, J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behav. Neurosci. 1996, 110, 583–592. [Google Scholar] [CrossRef]
- Scheinin, M.; Lomasney, J.W.; Hayden-Hixson, D.M.; Schambra, U.B.; Caron, M.G.; Lefkowitz, R.J.; Fremeau, R.T., Jr. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res. Mol. Brain Res. 1994, 21, 133–149. [Google Scholar]
- Pieribone, V.A.; Nicholas, A.P.; Dagerlind, A.; Hokfelt, T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J. Neurosci. 1994, 14, 4252–4268. [Google Scholar]
- Rangel, S.; Leon, M. Early odor preference training increases olfactory bulb norepinephrine. Brain Res. Dev. Brain Res. 1995, 85, 187–191. [Google Scholar] [CrossRef]
- Moriceau, S.; Shionoya, K.; Jakubs, K.; Sullivan, R.M. Early-life stress disrupts attachment learning: The role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 2009, 29, 15745–15755. [Google Scholar] [CrossRef]
- Winzer-Serhan, U.H.; Raymon, H.K.; Broide, R.S.; Chen, Y.; Leslie, F.M. Expression of alpha 2 adrenoceptors during rat brain development—II. Alpha 2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience 1997, 76, 261–272. [Google Scholar]
- Roozendaal, B.; Okuda, S.; van der Zee, E.A.; McGaugh, J.L. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 6741–6746. [Google Scholar]
- Ferry, B.; McGaugh, J.L. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol. Sin. 2000, 21, 481–493. [Google Scholar]
- McGaugh, J.L. Make mild moments memorable: Add a little arousal. Trends Cogn. Sci. 2006, 10, 345–347. [Google Scholar] [CrossRef]
- Haberly, L.B. Parallel-distributed processing in olfactory cortex: New insights from morphological and physiological analysis of neuronal circuitry. Chem. Senses 2001, 26, 551–576. [Google Scholar] [CrossRef]
- Schwob, J.E.; Price, J.L. The development of axonal connections in the central olfactory system of rats. J. Comp. Neurol. 1984, 223, 177–202. [Google Scholar] [CrossRef]
- Wilson, D.A.; Sullivan, R.M. Cortical processing of odor objects. Neuron 2011, 72, 506–519. [Google Scholar] [CrossRef]
- Zinyuk, L.E.; Datiche, F.; Cattarelli, M. Cell activity in the anterior piriform cortex during an olfactory learning in the rat. Behav. Brain Res. 2001, 124, 29–32. [Google Scholar] [CrossRef]
- Majak, K.; Ronkko, S.; Kemppainen, S.; Pitkanen, A. Projections from the amygdaloid complex to the piriform cortex: A PHA-L study in the rat. J. Comp. Neurol. 2004, 476, 414–428. [Google Scholar] [CrossRef]
- Swanson, L.W.; Petrovich, G.D. What is the amygdala? Trends Neurosci. 1998, 21, 323–331. [Google Scholar] [CrossRef]
- Wilson, D.A.; Stevenson, R.J. Olfactory perceptual learning: The critical role of memory in odor discrimination. Neurosci. Biobehav. Rev. 2003, 27, 307–328. [Google Scholar] [CrossRef]
- Davis, M.; Whalen, P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Wright, C.I.; Shin, L.M.; Kagan, J.; Whalen, P.J.; McMullin, K.G.; Rauch, S.L. Differential amygdalar response to novel versus newly familiar neutral faces: A functional MRI probe developed for studying inhibited temperament. Biol. Psychiatry 2003, 53, 854–862. [Google Scholar] [CrossRef]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef]
- Blanchard, D.C.; Blanchard, R.J. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 1972, 81, 281–290. [Google Scholar] [CrossRef]
- Maren, S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J. Neurosci. 1999, 19, 8696–8703. [Google Scholar]
- Sah, P.; Faber, E.S.; Lopez de Armentia, M.; Power, J. The amygdaloid complex: Anatomy and physiology. Physiol. Rev. 2003, 83, 803–834. [Google Scholar]
- Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef]
- Cahill, L.; Weinberger, N.M.; Roozendaal, B.; McGaugh, J.L. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron 1999, 23, 227–228. [Google Scholar] [CrossRef]
- Debiec, J.; LeDoux, J.E. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Ann. N. Y. Acad. Sci. 1071, 521–524. [Google Scholar]
- Fanselow, M.S.; Gale, G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003, 985, 125–134. [Google Scholar] [CrossRef]
- Fanselow, M.S.; LeDoux, J.E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 1999, 23, 229–232. [Google Scholar] [CrossRef]
- Goosens, K.A.; Maren, S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn. Mem. 2001, 8, 148–155. [Google Scholar] [CrossRef]
- Maren, S. The amygdala, synaptic plasticity, and fear memory. Ann. N. Y. Acad. Sci. 2003, 985, 106–113. [Google Scholar] [CrossRef]
- Sigurdsson, T.; Doyere, V.; Cain, C.K.; LeDoux, J.E. Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology 2007, 52, 215–227. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef]
- Sullivan, R.M. Developing a sense of safety: The neurobiology of neonatal attachment. Ann. N. Y. Acad. Sci. 1008, 122–131. [Google Scholar]
- Sullivan, R.M.; Wilson, D.A. Role of the amygdala complex in early olfactory associative learning. Behav. Neurosci. 1993, 107, 254–263. [Google Scholar] [CrossRef]
- Thompson, J.; Sullivan, R.M.; Wilson, D.A. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 2008, 1200, 58–65. [Google Scholar]
- Zhang, J.H.; Sato, M.; Tohyama, M. Region-specific expression of the mRNAs encoding beta subunits (beta 1, beta 2, and beta 3) of GABAA receptor in the rat brain. J. Comp. Neurol. 1991, 303, 637–657. [Google Scholar] [CrossRef]
- Stork, O.; Ji, F.Y.; Kaneko, K.; Stork, S.; Yoshinobu, Y.; Moriya, T.; Shibata, S.; Obata, K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000, 865, 45–58. [Google Scholar] [CrossRef]
- Duvarci, S.; Pare, D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J. Neurosci. 2007, 27, 4482–4491. [Google Scholar] [CrossRef]
- Barr, G.A.; Moriceau, S.; Shionoya, K.; Muzny, K.; Gao, P.; Wang, S.; Sullivan, R.M. Transitions in infant learning are modulated by dopamine in the amygdala. Nat. Neurosci. 2009, 12, 1367–1369. [Google Scholar]
- Walker, C.D.; Perrin, M.; Vale, W.; Rivier, C. Ontogeny of the stress response in the rat: Role of the pituitary and the hypothalamus. Endocrinology 1986, 118, 1445–1451. [Google Scholar] [CrossRef]
- Rosenfeld, P.; Ekstrand, J.; Olson, E.; Suchecki, D.; Levine, S. Maternal regulation of adrenocortical activity in the infant rat: Effects of feeding. Dev. Psychobiol. 1993, 26, 261–277. [Google Scholar] [CrossRef]
- Walker, C.D.; Scribner, K.A.; Cascio, C.S.; Dallman, M.F. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 1991, 128, 1385–1395. [Google Scholar] [CrossRef]
- Dallman, M.F. Moments in time—The neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology 2000, 141, 1590–1592. [Google Scholar] [CrossRef]
- Levine, S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol. Behav. 2001, 73, 255–260. [Google Scholar] [CrossRef]
- Rosenfeld, P.; Suchecki, D.; Levine, S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci. Biobehav. Rev. 1992, 16, 553–568. [Google Scholar] [CrossRef]
- Grino, M.; Paulmyer-Lacroix, O.; Faudon, M.; Renard, M.; Anglade, G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology 1994, 135, 2549–2557. [Google Scholar] [CrossRef]
- Gould, E.; Cameron, H.A. Early NMDA receptor blockade impairs defensive behavior and increases cell proliferation in the dentate gyrus of developing rats. Behav. Neurosci. 1997, 111, 49–56. [Google Scholar] [CrossRef]
- Takahashi, L.K. Stimulus control of behavioral inhibition in the preweanling rat. Physiol. Behav. 1994, 55, 717–721. [Google Scholar] [CrossRef]
- Wiedenmayer, C.P.; Barr, G.A. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behav. Brain Res. 2001, 126, 147–157. [Google Scholar] [CrossRef]
- Wiedenmayer, C.P.; Magarinos, A.M.; McEwen, B.S.; Barr, G.A. Age-specific threats induce CRF expression in the paraventricular nucleus of the hypothalamus and hippocampus of young rats. Horm. Behav. 2005, 47, 139–150. [Google Scholar] [CrossRef]
- Upton, K.J.; Sullivan, R.M. Defining age limits of the sensitive period for attachment learning in rat pups. Dev. Psychobiol. 2010, 52, 453–464. [Google Scholar] [CrossRef]
- Takahashi, L.K.; Rubin, W.W. Corticosteroid induction of threat-induced behavioral inhibition in preweanling rats. Behav. Neurosci. 1993, 107, 860–866. [Google Scholar] [CrossRef]
- Rosenfeld, P.; van Eekelen, J.A.; Levine, S.; de Kloet, E.R. Ontogeny of corticosteroid receptors in the brain. Cell. Mol. Neurobiol. 1993, 13, 295–319. [Google Scholar] [CrossRef]
- Diorio, D.; Viau, V.; Meaney, M.J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993, 13, 3839–3847. [Google Scholar]
- Alexis, M.N.; Kitraki, E.; Spanou, K.; Stylianopoulou, F.; Sekeris, C.E. Ontogeny of the glucocorticoid receptor in the rat brain. Adv. Exp. Med. Biol. 1990, 265, 269–276. [Google Scholar]
- Kitraki, E.; Alexis, M.N.; Papalopoulou, M.; Stylianopoulou, F. Glucocorticoid receptor gene expression in the embryonic rat brain. Neuroendocrinology 1996, 63, 305–317. [Google Scholar] [CrossRef]
- Stutzmann, G.E.; McEwen, B.S.; LeDoux, J.E. Serotonin modulation of sensory inputs to the lateral amygdala: Dependency on corticosterone. J. Neurosci. 1998, 18, 9529–9538. [Google Scholar]
- McGaugh, J.L.; Roozendaal, B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 2002, 12, 205–210. [Google Scholar] [CrossRef]
- Wiedenmayer, C.P.; Magarinos, A.M.; McEwen, B.S.; Barr, G.A. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann. N. Y. Acad. Sci. 2003, 1008, 304–307. [Google Scholar]
- Stanton, M.E.; Levine, S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Dev. Psychobiol. 1990, 23, 411–426. [Google Scholar] [CrossRef]
- Suchecki, D.; Nelson, D.Y.; van Oers, H.; Levine, S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: Effects of maternal deprivation. Psychoneuroendocrinology 1995, 20, 169–182. [Google Scholar] [CrossRef]
- Shionoya, K.; Moriceau, S.; Bradstock, P.; Sullivan, R.M. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm. Behav. 2007, 52, 391–400. [Google Scholar] [CrossRef]
- DeVries, A.C.; Glasper, E.R.; Detillion, C.E. Social modulation of stress responses. Physiol. Behav. 2003, 79, 399–407. [Google Scholar] [CrossRef]
- Kikusui, T.; Winslow, J.T.; Mori, Y. Social buffering: Relief from stress and anxiety. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 2215–2228. [Google Scholar]
- Kirschbaum, C.; Prussner, J.C.; Stone, A.A.; Federenko, I.; Gaab, J.; Lintz, D.; Schommer, N.; Hellhammer, D.H. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom. Med. 1995, 57, 468–474. [Google Scholar]
- Gregg, M.E.; James, J.E.; Matyas, T.A.; Thorsteinsson, E.B. Hemodynamic profile of stress-induced anticipation and recovery. Int. J. Psychophysiol. 1999, 34, 147–162. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Nigh, C.K.; Sims, M.L.; Long, S.J. Plasma cortisol and vocalization responses of postweaning age guinea pigs to maternal and sibling separation: Evidence for filial attachment after weaning. Dev. Psychobiol. 1995, 28, 103–115. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Maken, D.S.; Graves, F.C. Presence of mother and unfamiliar female alters levels of testosterone, progesterone, cortisol, adrenocorticotropin, and behavior in maturing Guinea pigs. Horm. Behav. 2002, 42, 42–52. [Google Scholar] [CrossRef]
- Yeh, K.Y. Corticosterone concentrations in the serum and milk of lactating rats: Parallel changes after induced stress. Endocrinology 1984, 115, 1364–1370. [Google Scholar] [CrossRef]
- Levine, S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science 1962, 135, 795–796. [Google Scholar]
- Van Oers, H.J.; de Kloet, E.R.; Li, C.; Levine, S. The ontogeny of glucocorticoid negative feedback: Influence of maternal deprivation. Endocrinology 1998, 139, 2838–2846. [Google Scholar]
- Ivy, A.S.; Brunson, K.L.; Sandman, C.; Baram, T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience 2008, 154, 1132–1142. [Google Scholar] [CrossRef]
- Gilles, E.E.; Schultz, L.; Baram, T.Z. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr. Neurol. 1996, 15, 114–119. [Google Scholar] [CrossRef]
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900. [Google Scholar] [CrossRef]
- Avishai-Eliner, S.; Gilles, E.E.; Eghbal-Ahmadi, M.; Bar-El, Y.; Baram, T.Z. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J. Neuroendocrinol. 2001, 13, 799–807. [Google Scholar]
- Moriceau, S.; Raineki, C.; Holman, J.D.; Holman, J.G.; Sullivan, R.M. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front. Behav. Neurosci. 2009, 3. [Google Scholar] [CrossRef]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef]
- Avishai-Eliner, S.; Yi, S.J.; Newth, C.J.; Baram, T.Z. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci. Lett. 1995, 192, 49–52. [Google Scholar] [CrossRef]
- Bale, T.L.; Baram, T.Z.; Brown, A.S.; Goldstein, J.M.; Insel, T.R.; McCarthy, M.M.; Nemeroff, C.B.; Reyes, T.M.; Simerly, R.B.; Susser, E.S.; et al. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 2010, 68, 314–319. [Google Scholar]
- Brunson, K.L.; Chen, Y.; Avishai-Eliner, S.; Baram, T.Z. Stress and the developing hippocampus: A double-edged sword? Mol. Neurobiol. 2003, 27, 121–136. [Google Scholar] [CrossRef]
- Franklin, T.B.; Mansuy, I.M. The prevalence of epigenetic mechanisms in the regulation of cognitive functions and behaviour. Curr. Opin. Neurobiol. 2010, 20, 441–449. [Google Scholar] [CrossRef]
- Denenberg, V.H. Early experience and emotional development. Sci. Am. 1963, 208, 138–146. [Google Scholar] [CrossRef]
- Denenberg, V.H.; Carlson, P.V.; Stephens, M.W. Effects of infantile shock upon emotionality at weaning. J. Comp. Physiol. Psychol. 1962, 55, 819–820. [Google Scholar] [CrossRef]
- Harlow, H.F.; Harlow, M.K. The affectional systems. In Behavior of Nonhuman Primates; Schrier, A., Harlow, H.F., Stollnitz, F., Eds.; Academic Press: New York, NY, USA, 1965; Volume 2, pp. 287–344. [Google Scholar]
- Levine, S. The pituitary-adrenal system and the developing brain. Prog. Brain Res. 1970, 32, 79–85. [Google Scholar] [CrossRef]
- Levine, S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 1967, 156, 258–260. [Google Scholar]
- Rosenzweig, M.R.; Bennett, E.L.; Diamond, M.C.; Wu, S.Y.; Slagle, R.W.; Saffran, E. Influences of environmental complexity and visual stimulation on development of occipital cortex in rat. Brain Res. 1969, 14, 427–445. [Google Scholar] [CrossRef]
- Jacobson-Pick, S.; Richter-Levin, G. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav. Brain Res. 2010, 214, 268–276. [Google Scholar] [CrossRef]
- Vermetten, E.; Bremner, J.D. Olfaction as a traumatic reminder in posttraumatic stress disorder: Case reports and review. J. Clin. Psychiatry 2003, 64, 202–207. [Google Scholar] [CrossRef]
- Kaufman, J.; Plotsky, P.M.; Nemeroff, C.B.; Charney, D.S. Effects of early adverse experiences on brain structure and function: Clinical implications. Biol. Psychiatry 2000, 48, 778–790. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry 2004, 65, 18–28. [Google Scholar]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P.; Kim, D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003, 27, 33–44. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Gracci, F.; Santucci, D.; Alleva, E. Birth spacing in the mouse communal nest shapes adult emotional and social behavior. Physiol. Behav. 2009, 96, 532–539. [Google Scholar] [CrossRef]
- Coe, C.L.; Glass, J.C.; Wiener, S.G.; Levine, S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology 1983, 8, 401–409. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Cameron, J.L. Translating research findings on early experience to prevention: Animal and human evidence on early attachment relationships. Am. J. Prev. Med. 2006, 31, S175–S181. [Google Scholar] [CrossRef]
- Suomi, S.J. Early determinants of behaviour: Evidence from primate studies. Br. Med. Bull. 1997, 53, 170–184. [Google Scholar] [CrossRef]
- Gunnar, M.; Quevedo, K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007, 58, 145–173. [Google Scholar] [CrossRef]
- Kaffman, A.; Meaney, M.J. Neurodevelopmental sequelae of postnatal maternal care in rodents: Clinical and research implications of molecular insights. J. Child Psychol. Psychiatry 2007, 48, 224–244. [Google Scholar] [CrossRef]
- Korosi, A.; Baram, T.Z. The pathways from mother’s love to baby’s future. Front. Behav. Neurosci. 2009, 3. [Google Scholar] [CrossRef]
- Pryce, C.R.; Feldon, J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 2003, 27, 57–71. [Google Scholar] [CrossRef]
- Sanchez, M.M. The impact of early adverse care on HPA axis development: Nonhuman primate models. Horm. Behav. 2006, 50, 623–631. [Google Scholar] [CrossRef]
- Tang, A.C.; Akers, K.G.; Reeb, B.C.; Romeo, R.D.; McEwen, B.S. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc. Natl. Acad. Sci. USA 2006, 103, 15716–15721. [Google Scholar]
- Tang, A.C.; Reeb-Sutherland, B.C.; Yang, Z.; Romeo, R.D.; McEwen, B.S. Neonatal novelty-induced persistent enhancement in offspring spatial memory and the modulatory role of maternal self-stress regulation. J. Neurosci. 2011, 31, 5348–5352. [Google Scholar]
- Rosenfeld, P.; Wetmore, J.B.; Levine, S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol. Behav. 1992, 52, 787–791. [Google Scholar] [CrossRef]
- Plotsky, P.M.; Meaney, M.J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol Brain Res. 1993, 18, 195–200. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Mothering style and methylation. Nat. Neurosci. 2004, 7, 791–792. [Google Scholar] [CrossRef]
- Meerlo, P.; Horvath, K.M.; Nagy, G.M.; Bohus, B.; Koolhaas, J.M. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J. Neuroendocrinol. 1999, 11, 925–933. [Google Scholar]
- Levine, S. The influence of social factors on the response to stress. Psychother. Psychosom. 1993, 60, 33–38. [Google Scholar] [CrossRef]
- Levine, S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann. N. Y. Acad. Sci. 1994, 746, 275–288, discussion 289–293. [Google Scholar] [CrossRef]
- Meaney, M.J.; Bhatnagar, S.; Diorio, J.; Larocque, S.; Francis, D.; O’Donnell, D.; Shanks, N.; Sharma, S.; Smythe, J.; Viau, V. Molecular basis for the development of individual differences in the hypothalamic-pituitary-adrenal stress response. Cell. Mol. Neurobiol. 1993, 13, 321–347. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann. N. Y. Acad. Sci. 1994, 746, 294–304, discussion 304–297. [Google Scholar] [CrossRef]
- Fenoglio, K.A.; Chen, Y.; Baram, T.Z. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J. Neurosci. 2006, 26, 2434–2442. [Google Scholar] [CrossRef]
- Andersen, S.L.; Lyss, P.J.; Dumont, N.L.; Teicher, M.H. Enduring neurochemical effects of early maternal separation on limbic structures. Ann. N. Y. Acad. Sci. 1999, 877, 756–759. [Google Scholar] [CrossRef]
- Caldji, C.; Diorio, J.; Meaney, M.J. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology 2003, 28, 1950–1959. [Google Scholar] [CrossRef]
- Cirulli, F.; Berry, A.; Alleva, E. Early disruption of the mother-infant relationship: Effects on brain plasticity and implications for psychopathology. Neurosci. Biobehav. Rev. 2003, 27, 73–82. [Google Scholar] [CrossRef]
- Hall, F.S.; Wilkinson, L.S.; Humby, T.; Robbins, T.W. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse 1999, 32, 37–43. [Google Scholar] [CrossRef]
- Higley, J.D.; Hasert, M.F.; Suomi, S.J.; Linnoila, M. Nonhuman primate model of alcohol abuse: Effects of early experience, personality, and stress on alcohol consumption. Proc. Natl. Acad. Sci. USA 1991, 88, 7261–7265. [Google Scholar]
- Ladd, C.O.; Huot, R.L.; Thrivikraman, K.V.; Nemeroff, C.B.; Meaney, M.J.; Plotsky, P.M. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000, 122, 81–103. [Google Scholar]
- Liu, D.; Diorio, J.; Day, J.C.; Francis, D.D.; Meaney, M.J. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000, 3, 799–806. [Google Scholar] [CrossRef]
- Tyler, K.; Moriceau, S.; Sullivan, R.M.; Greenwood-van Meerveld, B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol. Motil. 2007, 19, 761–768. [Google Scholar] [CrossRef]
- Tanaka, M. Emotional stress and characteristics of brain noradrenaline release in the rat. Ind. Health 1999, 37, 143–156. [Google Scholar] [CrossRef]
- Tsuda, A.; Ida, Y.; Satoh, H.; Tsujimaru, S.; Tanaka, M. Stressor predictability and rat brain noradrenaline metabolism. Pharmacol. Biochem. Behav. 1989, 32, 569–572. [Google Scholar] [CrossRef]
- Raineki, C.; Rincon Cortes, M.; Belnoue, L.; Sullivan, R.M. Effects of early life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 2012, in press. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bisaz, R.; Sullivan, R.M. Developmental Neurobiology of the Rat Attachment System and Its Modulation by Stress. Behav. Sci. 2012, 2, 79-102. https://doi.org/10.3390/bs2020079
Bisaz R, Sullivan RM. Developmental Neurobiology of the Rat Attachment System and Its Modulation by Stress. Behavioral Sciences. 2012; 2(2):79-102. https://doi.org/10.3390/bs2020079
Chicago/Turabian StyleBisaz, Reto, and Regina M. Sullivan. 2012. "Developmental Neurobiology of the Rat Attachment System and Its Modulation by Stress" Behavioral Sciences 2, no. 2: 79-102. https://doi.org/10.3390/bs2020079