Behavioral Studies and Genetic Alterations in Corticotropin-Releasing Hormone (CRH) Neurocircuitry: Insights into Human Psychiatric Disorders
Abstract
:1. Introduction
1.1. CRH Synthesis
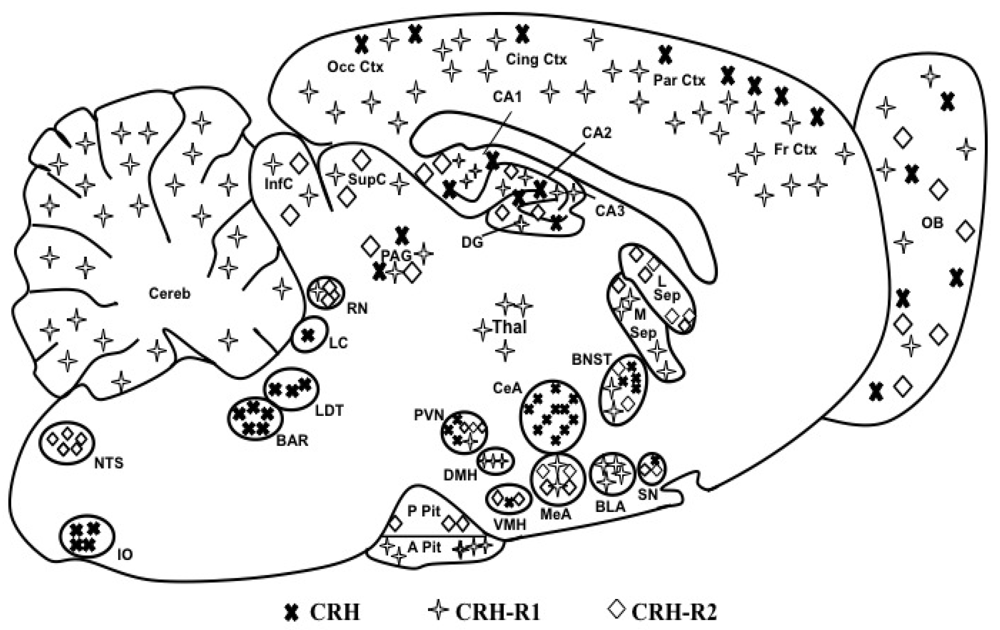
1.2. CRH Receptors
1.3. CRH Binding Protein
1.4. Synaptic CRH Activity
2. CRH Agonists and Antagonists
3. Genetically Altered Rodent Models
3.1. CRH Mutants
3.2. CRH Overexpressing (CRH-OE) Mice
3.2.1. General CRH-OE
3.2.2. Spatially Restricted CRH-OE
3.2.3. Spatially and Temporally Restricted CRH-OE
3.3. CRH-R1 and CRH-R2 Mutants
3.3.1. CRH-R1 Mutants
3.3.2. CRH-R2 Mutants
3.3.3. Combined CRH-R1 and CRH-R2 Mutants
3.3.4. Urocortin Mutants
3.4. CRH-BP Mutants
| CRH Deletion Mutant | |||
|---|---|---|---|
| Line | Manipulation | Main Phenotypes | References |
| CRH- KO | Constitutive deletion of CRH by insertion of a phosphoglycerate kinase neomycin-resistant cassette | Adrenal insufficiency | [57,58,59,60] |
| ↓ Stress CORT | |||
| No behavioral changes | |||
| CRH Overexpression (OE) Mutants | |||
| Line | Manipulation | Main Phenotypes | References |
| CRH-Tg | Mouse metallothionein-1 (MT-1) promoter driven CRH OE in brain, adrenal glands, heart, and testes. | Adrenal Hypertrophy | [61,66,67,70,79,80] |
| Cushingoid phenotype | |||
| Attentional Impairment | |||
| ↑ Basal CORT and ACTH | |||
| ↓ Locomotion | |||
| ↑ Anxiety in OF, EPM, LD, and black/white transition test | |||
| ↑ Active coping in FST | |||
| ↓ Despair in FST | |||
| ↓ Sexual receptivity in females | |||
| ↓ Alcohol preference | |||
| Gene expression changes | |||
| CRH-OE2122 | Thy-1 promoter driven CRH OE in neurons postnatally through adulthood. | Adrenal Hypertrophy | [62,65,69] |
| Cushingoid phenotype at 6 months of age | |||
| ↑ Basal CORT | |||
| Dexamethasone non-suppression | |||
| ↓ Acoustic startle reactivity | |||
| ↓ Habituation to a startle response | |||
| Deficit in pre-pulse inhibition | |||
| ↑ Food and water consumption, and altered heart rate | |||
| CRH-COEDel | Rosa26 (R26) promoter driven CRH OE in the whole body. | Cushingoid phenotype at 3-weeks of age | [64] |
| ↑ Adrenal weight, ↓ thymus weight | |||
| ↑ Basal CORT | |||
| ↑ Anxiety in OF, EPM, LD, and black/white transition test | |||
| ↓ Despair in FST | |||
| CRH Overexpression (OE) Mutants | |||
| Line | Manipulation | Main Phenotypes | References |
| CRH-COEAPit | R26 and POMC promoter driven CRH OE in the anterior and intermediate lobes of the pituitary | Mild Cushingoid phenotype at 5-6 months of age | [64] |
| ↑ Basal CORT | |||
| CRH-COE-Nes | R26 and Nestin promoter driven CRH OE in neurons and glia from embryonic day 10.5 | ↑ Stress-induced CORT and ACTH in male mice | [63,75,76] |
| ↓ Despair in FST and TST tests, reversible with CRH-R1 antagonist treatment | |||
| ↑ REM sleep | |||
| CRH-COE-Cam | R26 and CamK2 promoter driven CRH OE in forebrain glutamatergic neurons from postnatal day 15 | Normal HPA axis activity | |
| ↑ REM sleep | |||
| ↑ Deficit in spatial performance in the MWM and Y-maze tests. | |||
| CRH-COE-Dlx | R26 and Dlx promoter driven CRH OE in GABAergic interneurons from embryonic day 10.5 | Normal HPA axis activity and behavior | |
| FBCRHOElife | CamK2 promoter driven forebrain CRH OE from embryonic day 0 through life | Cushingoid phenotype by 8 weeks of age | [77] |
| ↑ Nadir CORT and ACTH | |||
| Crh-COECamCreERT2 | R26 and Camk2a-CreERT2 promoter driven CRH OE in forebrain glutamatergic neurons (OE induced by tamoxifen at postnatal week 8) | ↑ Anxiety in LD and EPM tests | [22] |
| FBCRHOEdev | CamK2 promoter driven forebrain CRH OE from embryonic day 15 to postnatal day 21 | ↑Basal CORT only during CRH- OE. | [77] |
| ↑ Despair in FST and TST test (↓ despair in FST with antidepressants treatment) | |||
| ↑ Anxiety in OF, EPM, and LD tests | |||
| ↑ CRH-R1 mRNA in the cingulate cortex, dentate gyrus and CA1 region of the hippocampus | |||
| CRF-OE | CamK2 promoter driven forebrain CRH OE from 8 to 11 weeks of age | ↑ Nadir CORT | [78] |
| ↓ Thymus weight in females | |||
| ↓ Locomotion in familiar environment | |||
| ↓ Despair in FST | |||
| Trend towards anxiety in LD test | |||
| CRH Receptor Mutants | |||
| Line | Manipulation | Main Phenotypes | References |
| CRH-R1-/- | Constitutive deletion (exons 5-8) of the CRH-R1 | Adrenal gland atrophy | [79,80] |
| ↑ PVN CRH | |||
| ↓ Basal CORT | |||
| ↓ Stress-induced CORT and ACTH in males | |||
| ↓ Anxiety in LD and EPM tests | |||
| CRH-R1-/- | Constitutive deletion of CRH-R1 (transmembrane regions V, VI, and VII) | ↓ CRH-induced cAMP | [6,81,83,87,88] |
| ↓ Basal ACTH in pituitary cultures | |||
| ↓ Basal CORT in females | |||
| ↑ PVN CRH only in neonates | |||
| ↑ Plasma and PVN vasopressin | |||
| ↓ Stress-induced CORT and ACTH | |||
| ↓ ACTH-induced CORT | |||
| ↓ Anxiety in LD test basally and during alcohol withdrawal | |||
| ↓ Neuronal activity (cFOS) | |||
| Crhr1loxP/loxPCamk2a-cre Or Cam-CRHR1 Or CRHR1Camk2aCre Or CRF1-CKO | Camk2a promoter driven deletion CRH-R1 in forebrain | ↑ Stress-induced CORT and ACTH in adults and neonates | [75,82,84,86] |
| ↑ CRH mRNA in PVN in adults and neonates | |||
| ↓ Anxiety in LD and EPM test in adults | |||
| Cam-CRHR1 | |||
| Hyperactive in OF test in adults | |||
| ↓ Neuronal activity | |||
| ↓ Deficit in spatial memory in Y-maze tests after chronic social defeat stress | |||
| No deficit in spatial learning and memory in MWM or Y-maze tests after early life stress | |||
| No chronic social defeat stress-induced deficits in the novel object recognition test | |||
| No stress-induced atrophy of apical dendrites in CA3 neurons compared to control mice | |||
| No stress-induced reduction of GR mRNA in CA1 and CA3 neurons compared to control mice | |||
| ↑ Weight gain after chronic stress | |||
| No deficit in hippocampal LTP after early life stress | |||
| ↑ High frequency stimulation-induced LTP with early life stress | |||
| Crhr1loxP/loxPNes-cre Nes-CRHR1 | Nes-Cre promoter driven deletion CRH-R1 in all neurons | ↓ Basal CORT in neonates | [85,86] |
| ↑ Stress-induced CORT and ACTH | |||
| ↓ Alcohol consumption after forced swim stress | |||
| CRH Receptor Mutants | |||
| Line | Manipulation | Main Phenotypes | References |
| Crhr1Glu-CKO | Nex-Cre promoter driven CRH-R1 deletion in mature glutamatergic neurons | ↓ Anxiety in LD, EPM, novel object exploration, and modified hole board tests | [22] |
| No effect on despair behavior in FST | |||
| No effect auditory fear conditioning | |||
| ↑ Locomotion in LD test | |||
| No effect on basal or stress-induced CORT secretion | |||
| ↓ Excitatory field potentials on glutamatergic neurons in the BLA | |||
| ↓ Facilitation of action potential firing between hippocampal DG-CA3-CA1 network | |||
| Crhr1DA-CKO | Dat-CreERT2 promoter driven CRH-R1 deletion in midbrain dopaminergic neurons | ↑ Anxiety in LD, EPM, novel object exploration, and modified hole board tests | |
| No effect on despair behavior in FST | |||
| No effect auditory fear conditioning | |||
| No effect on basal or stress-induced CORT secretion | |||
| ↓ Response to stress-induced DA release in PFC | |||
| Crhr1GABA-CKO | DLX5/6 promoter driven CRH-R1 deletion in forebrain GABAergic neurons | No effect on anxiety behaviors | [22] |
| No effect on despair behavior in FST | |||
| Crhr15HT-CKO | ePet-Cre promoter driven CRH-R1 deletion in brainstem seratonergic neurons | No effect auditory fear conditioning | |
| No effect on basal or stress-induced CORT secretion | |||
| Crhr1CNS-CKO | Nes-Cre promoter driven CRH-R1 deletion in all neurons | No effect on anxiety behaviors | |
| CRH-R2-/- | Constitutive deletion by replacing 5th-7th transmembrane domains with a neomycin-resistant cassette | ↑ Stress-induced CORT and ACTH | [90,93] |
| ↓ Food intake after stress of food deprivation | |||
| ↑Anxiety in EPM and OF tests | |||
| ↑ Despair in FST | |||
| ↑ CRH mRNA in the CeA | |||
| ↑ Ucn1 mRNA in Edinger-Westphal (EW) nucleus | |||
| CRH-R2-/- | Constitutive deletion by replacing exons of the 3rd intracellular loop with neomycin-resistant cassette | ↑ Anxiety in EPM and LD tests in males | [91,92] |
| ↑ Locomotion in OF test in males | |||
| ↑ Stress-induced anxiety in the OF test in males | |||
| ↓ Neuronal activation measured by levels of phosphorylated CREB | |||
| ↑ Despair behavior in the FST and TST tests that is prevented when MEK/ERK pathway in the hippocampus is inhibited | |||
| CRH-R2-/- | Constitutive deletion by replacing the 3rd and 4th transmembrane domains with a neomycin-resistant cassette | ↓ cAMP activity in cultured cardiomyocytes | [89] |
| ↑ Ucn1 mRNA in Edinger-Westphal (EW) nucleus | |||
| ↑ Stress-induced CORT and ACTH | |||
| ↓ Cardiac function with Ucn administration | |||
| Altered feeding with Ucn administration | |||
| CRH-R1-/-/ | CRH-R1-/- [79] were crossed to CRH-R2-/- [90] to generate these double knockout mice | ↑ PVN CRH | [7] |
| CRH-R2-/- | ↓ Basal CORT and ACTH | ||
| ↓ HPA axis reactivity to stress | |||
| CRH-R1-/-/ | CRH-R1-/-[81] were crossed to CRH-R2-/-[89] to generate these double knockout mice | ↓ Basal CORT and ACTH | [95] |
| CRH-R2-/- | ↓ Anxiety in EPM and OF tests in females | ||
| ↑ PVN CRH and AVP | |||
| Ucn mutants | |||
| Line | Manipulation | Main Phenotypes | References |
| Ucn-/- | Constitutive deletion of Ucn1 by replacement of the coding exon of Ucn with an EGFP-LacZ fusion reporter and a PGKneo selection cassette | Normal HPA axis activity | [97] |
| No change anxiety in the EPM, OF, and LD tests | |||
| Impaired acoustic startle response | |||
| Ucn-/- | Constitutive deletion of Ucn1 by replacement of region encoding the mature peptide with a neomycin-resistant gene cassette | Normal HPA axis activity and feeding behavior | [98,99] |
| ↑ Anxiety in EPM and OF tests | |||
| ↓ CRH-R2 mRNA in the LS | |||
| ↓ Length of hair cell in organ of Corti leading to hearing impairments | |||
| Ucn2 KO | Constitutive deletion of Ucn2 by insertion of a neomycin-resistant gene cassette | ↑ Nocturnal CORT and ACTH in females | [100] |
| ↑ AVP mRNA in the PVN and SON of females - altered drinking habits | |||
| ↓ Despair in the FST and TST in females | |||
| No changes in anxiety in EPM and LD tests or in conditioned fear tests | |||
| ↑ CRH mRNA in the BnST and CeA | |||
| ↓ Ucn3 mRNA in the median preoptic nucleus and perifornical area | |||
| ↑ CRH-R2 mRNA in the BnST, LS and DR | |||
| Ucn1/Ucn2 dKO | Cross breeding Ucn1 [98] and Ucn2 [100] single KOs to generate these double knockout mice. | ↑ Stress-induced plasma CORT in males | [101] |
| ↑ PVN CRH mRNA | |||
| Hypertrophy of the zona fasciculate | |||
| ↓ Anxiety in EPM and OF | |||
| ↓ Behavioral response to acute stress in females | |||
| ↓ CRH-R2 mRNA in the LS | |||
| ↑ Amygdala CRH mRNA | |||
| Ucn3tZ/tZ | Ucn3 gene was disrupted by homologous recombination and the ORF was replaced by a tau-lacZ reporter gene | Normal basal and stress-induced HPA axis responses | [102] |
| No changes in anxiety-related behaviors in EPM, social interaction, and modified hole board tests compared to WT | |||
| No difference in despair behavior in the FST compared to WT | |||
| No genotype effect in the ASR test | |||
| ↑ Cocial discrimination memory | |||
| Ucn tKO | Cross breeding Ucn1, 2, and 3 single KOs from [Vetter, Chen, deussing] | ↓ Basal exploration in OF 24 hours post-stress | [103] |
| ↑ Anxiety in OF, LD and ASR tests 24 hours after an acute stressor | |||
| ↑ Freezing in cued fear conditioning and ASR tests | |||
| ↓ Spatial learning in MWM | |||
| ↑ CRH-R2 mRNA in the LS and DRN | |||
| ↑ CRH-R1 mRNA in amygdala compared to controls 24 hours post- stress | |||
| Lack of stress-induced amygdala gene modification compared to that observed in controls | |||
| CRH-BP mutants | |||
| Line | Manipulation | Main Phenotypes | References |
| CRH-BP (transgenic) | Mouse metallothionein-1 (MT-1) promoter driven CRH-BP OE in the brain and pituitary as well as sites such as the placenta, plasma, and amniotic fluid | Normal CORT and ACTH | [104] |
| ↑ Weight gain (gender specific) | |||
| Blunted ACTH response to LPS injection | |||
| CRH-BP (transgenic) | Pituitary glycoprotein hormone a-subunit (a-GSU) promoter driven CRH-BP OE in the pituitary | Normal CORT and ACTH | [105] |
| ↑ PVN CRH and vasopressin | |||
| ↑ Locomotion | |||
| Trend towards decreased anxiety in the OF | |||
| CRH-BP-/- | Constitutive deletion by replacing exons1-5 with a phosphoglycerate kinase neomycin-resistant cassette | Normal CORT and ACTH | [106] |
| ↑ Anxiety in EPM, OF and defensive withdraw (gender specific) | |||
| ↓ Food intake and weight gain in males | |||
3.5. The Use of Viral Vectors to Modulate CRH Activity
| Line (viral vector) | Manipulation | Main Phenotypes | References |
|---|---|---|---|
| CeA CRF OE | Long-term lentiviral CRH OE in the CeA of adult male mice. | ↓ Basal and stress-induced anxiety in OF and LD tests | [107] |
| (pCSC-SP-PW-rCRF-IRES/GFP) | Behavioral testing began 4 months after lentiviral injections | ||
| ↓ Response to acoustic startle | |||
| Habituation to startle after stress ↑ CRH-R1 mRNA in CeA | |||
| CeA-CRF-OE | Short-term lentiviral CRH OE in the CeA of adult male mice when Dox is administered. | ↑ Stress-induced anxiety in LD test | [108] |
| (rtTA-IRES/GFP + TRE-mCRF-IRES/RFP) | |||
| Behavioral testing began 3 days after Dox administration | No effects on despair in FST or TST | ||
| No effects on fear conditioning | |||
| Lenti-CMV-CRF OE | Lentivirus-induced CRH OE in the CeA of female rats. | Impaired negative feedback of the HPA axis | [110] |
| (LVCRFp3.CRF) | Behavioral testing began 2 weeks after lentiviral injections | ||
| Disrupted reproductive and sexual function. | |||
| ↑ Despair in FST | |||
| ↑ Anxiety in acoustic startle | |||
| CeA CRF OE | Lentivirus-induced CRH OE in the CeA of male rats. | ↑ CRH and vasopressin mRNA in the PVN | [109] |
| LVCRFp3.CRF | Behavioral testing began 4 weeks after lentiviral injections | ||
| ↑Basal ACTH | |||
| Dexamethasone non-suppression | |||
| ↑ Anxiety in EPM and defensive withdrawal tests. | |||
| BnST CRF OE | Long-term lentiviral CRH OE in the BnST of male mice.Behavioral testing began 4 months after lentiviral injections | ↑ Despair in FST | [107] |
| (pCSC-SP-PW-rCRF-IRES/GFP) | ↓ CRH-R1 mRNA in BnST | ||
| BnST CRF OE | CRH OE in the BnST of adult male rats before or after fear conditioning in ASR tests | No changes in anxiety measures in EPM or DW tests | [111] |
| (LVCRFp3.CRF) | |||
| No HPA axis alterations | |||
| Behavioral testing began ~2 weeks after lentiviral injections | ↓ CRH-R1 binding density in the BnST | ||
| ↓ CRH-R2 binding density in the DRN | |||
| CRH OE induced before conditioning to fearful stimulus: | |||
| No differences in baseline ASR | |||
| ↓ Startle sensitization and shock reactivity in ASR | |||
| ↓ FPS, impaired acquisition of associative fear memory | |||
| CRH OE induced after conditioning to fearful stimulus: | |||
| ↑ FPS, enhanced fear memory expression | |||
| BLA CRFR1 KD | CRH-R1 KD in the BLA of adult male mice. | ↓ Anxiety in the LD and OF tests | [112] |
| (Lenti-shCRFR1) | Behavioral testing began ~2 weeks after lentiviral injections | ||
| CeA CRF-KD | CRH KD in the CeA of adult male mice | ↓ Basal anxiety in the EPM test and stress-induced anxiety in LD test | [108] |
| (Lenti-shCRF) | |||
| Behavioral testing began ~2 weeks after lentiviral | |||
| No effects on despair in FST or TST | |||
| No effects on fear conditioning | |||
| ↑ Basal plasma CORT levels | |||
| ↓ Ucn3 mRNA in BnST | |||
| GPe CRFR1 KD | CRH-R1 KD in the GPe of adult male mice. | ↑ Anxiety in the LD, OF and EPM tests | [113] |
| (Lenti-shCRFR1) | Behavioral testing began ~2 weeks after lentiviral injection | ||
| No changes in locomotion | |||
| ↓ Enkephalin protein in GPe, possible mechanism for increased anxiety | |||
| rPFA-Ucn3 OE | Transgenic mice with Ucn3 under the control of a TRE were injected in the rPFA with lentivirus containing rtTA. Ucn3 OE occurs when Dox is administered | ↑ Anxiety in the LD and OF tests | [114] |
| (Lenti-rtTA) | ↑ Metabolic rate, but has no effect on food intake | ||
| ↓ Insulin sensitivity |
4. Human Gene Polymorphisms
4.1. CRH
4.2. CRHR1
4.3. CRHR2
4.4. CRHBP
4.5. The Potential of CRH-Pathways Genetic Studies
| GENE | Polymorphism | Associated Disorder | Effect | References |
|---|---|---|---|---|
| CRH | 173 bp in the dinucleotide repeat marker CRH-PCR1 | Behavioral inhibition | Risk promoting | [115,116] |
| rs6999100 (CC) | ||||
| rs6159 (GG) | ||||
| rs1870393 (CC) | ||||
| CRHR1 | rs7209436 (TT) rs110402 ((AA) rs242924 (TT) | Depression with adverse early life experiences | Protective | [117,118,119,120,121] |
| T-A-T haplotype | ||||
| CRHR1 | T-A-T haplotype rs17689882 | Depression with childhood physical neglect | Risk promoting | [122] |
| rs16940674 | ||||
| rs16940665 | ||||
| CRHR1 | rs110402 (TT) | Depression onset and seasonal episodes | Risk promoting | [125] |
| CRHR1 | rs110402 (GG) | Depression vulnerability | Risk promoting | [16] |
| CRHR1 | G-G-T haplotype | Genetic susceptibility to major depression and response to antidepressant treatment | Risk promoting | [126,127,128] |
| rs1876828 (GG) | ||||
| rs242939 (GG) | ||||
| rs242941 (TT) | ||||
| CRHR1 | rs1876831 (CC) | Alcohol consumption with life stress | Risk promoting | [72,129,130] |
| rs242938 (A) | ||||
| CRHR1 | rs4792887 (TT) | Suicidality after low stress exposure | Risk promoting | [135] |
| CRHR2 | rs2270007 (GG) | Decreased response to antidepressant treatment in depressed patients | Risk promoting | [125] |
| CRHR2 | 5-2-3 haplotype (allele 3 in GT) | Suicidal behavior in bipolar disorder | Risk promoting | [136] |
| CRHBP | rs10473984 (TT) | Remission and decreased depressive symptoms with citalopram treatment | Risk promoting | [35] |
| CRHBP | rs1875999 (TT) | Unipolar depression | Risk promoting | [137] |
| CRHBP | rs10055255 (TT) | Stress-induced alcohol craving and negative mood | Risk promoting | [138] |
| CRHBP CRHR1 | rs3811939 (GG) | Comorbid alcoholism in Schizophrenic patients | Risk promoting | [134] |
| rs110402 (TT) | ||||
| CRHBP CRHR1 | rs1875999 | Suicidal behavior in Schizophrenia | Risk promoting | [139] |
| rs169400665 |
5. Targeting the CRH Pathway for Therapy
6. Conclusions
Acknowledgments
References
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar]
- Lewis, K.; Li, C.; Perrin, M.H.; Blount, A; Kunitake, K.; Donaldson, C.; Vaughan, J.; Reyes, T.M.; Gulyas, J.; Fischer, W.; et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor CRF family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 7570–7575. [Google Scholar]
- Brar, B.; Sanderson, T.; Wang, N.; Lowry, P.J. Post-translational processing of human procorticotrophin-releasing factor in transfected mouse neuroblastoma and Chinese hamster ovary cell lines. J. Endocrinol. 1997, 154, 431–440. [Google Scholar] [CrossRef]
- Grigoriadis, D.E.; Lovenberg, T.W.; Chalmers, D.T.; Liaw, C.; Souza, E.B.D.E. Characterization of Corticotropin- releasing factor receptor subtypes. Ann. NY Acad. Sci. 1996, 780, 60–80. [Google Scholar] [CrossRef]
- Muglia, L.J.; Jacobson, L.; Luedke, C.; Vogt, S.K.; Schaefer, M.L.; Dikkes, P.; Fukuda, S.; Sakai, Y.; Suda, T.; Majzoub, J.A. Corticotropin-releasing hormone links pituitary adrenocorticotropin gene expression and release during adrenal insufficiency. J. Clin. Invest. 2000, 105, 1269–1277. [Google Scholar] [CrossRef]
- Muller, M.B. Selective activation of the hypothalamic vasopressinergic system in mice deficient for the Corticotropin-Releasing Hormone Receptor 1 is dependent on glucocorticoids. Endocrinology 2000, 141, 4262–4269. [Google Scholar] [CrossRef]
- Preil, J.; Müller, M.B.; Gesing, A.; Reul, J.M.; Sillaber, I.; van Gaalen, M.M.; Landgrebe, J.; Holsboer, F.; Stenzel-Poore, M.; Wurst, W. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology 2001, 142, 4946–4955. [Google Scholar] [CrossRef]
- Surget, A.; Belzung, C. Involvement of vasopressin in affective disorders. Eur. J. Pharmacol. 2008, 583, 340–349. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. Corticotropin-releasing hormone and arginine vasopressin in depression focus on the human postmortem hypothalamus. Vitam. Horm. 2010, 82, 339–365. [Google Scholar] [CrossRef]
- Chalmers, D.T.; Lovenberg, T.W.; De Souza, E.B. Localization of novel corticotropin-releasing factor receptor CRF2 mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF1 receptor mRNA expression. J. Neurosci. 1995, 15, 6340–6350. [Google Scholar]
- Reul, J. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002, 2, 23–33. [Google Scholar] [CrossRef]
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin II: A member of the corticotropin-releasing factor CRF neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848. [Google Scholar]
- Vaughan, J.; Donaldson, C.; Bittencourt, J.; Perrin, M.H.; Lewis, K.; Sutton, S.; Chan, R.; Turnbull, A.V.; Lovejoy, D.; Rivier, C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 1995, 378, 287–292. [Google Scholar]
- Kostich, W.A.; Grzanna, R.; Lu, N.Z.; Largent, B.L. Immunohistochemical visualization of corticotropin-releasing factor type 1 (CRF1) receptors in monkey brain. J. Comp. Neurol. 2004, 478, 111–125. [Google Scholar] [CrossRef]
- Sánchez, M.M.; Young, L.J.; Plotsky, P.M.; Insel, T.R. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J. Comp. Neurol. 1999, 408, 365–377. [Google Scholar] [CrossRef]
- Hsu, D.T.; Mickey, B.J.; Langenecker, S.A.; Heitzeg, M.M.; Love, T.M.; Wang, H.; Kennedy, S.E.; Peciña, M.; Shafir, T.; Hodgkinson, C.A.; et al. Variation in the Corticotropin-Releasing Hormone Receptor 1 (CRHR1) Gene Influences fMRI Signal Responses during Emotional Stimulus Processing. J. Neurosci. 2012, 32, 3253–3260. [Google Scholar]
- Hiroi, N.; Wong, M.L.; Licinio, J.; Park, C.; Young, M.; Gold, P.W.; Chrousos, G.P.; Bornstein, S.R. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol. Psychiatr. 2001, 6, 540–546. [Google Scholar] [CrossRef]
- Chen, R.; Lewis, K.A.; Perrin, M.H.; Vale, W.W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 8967–8971. [Google Scholar] [CrossRef]
- Pisarchik, A.; Slominski, A.T. Alternative splicing of CRH-R1 receptors in human and mouse skin: Identification of new variants and their differential expression. FASEB J. 2001, 15, 2754–2756. [Google Scholar]
- Perrin, M.; Donaldson, C.; Chen, R.; Blount, A.; Berggren, T.; Bilezikjian, L.; Sawchenko, P.; Vale, W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. USA 1995, 92, 2969–2973. [Google Scholar]
- Lovenberg, T.W.; Liaw, C.W.; Grigoriadis, D.E.; Clevenger, W.; Chalmers, D.T.; De Souza, E.B.; Oltersdorf, T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA 1995, 92, 836–840. [Google Scholar]
- Refojo, D.; Schweizer, M.; Kuehne, C.; Ehrenberg, S.; Thoeringer, C.; Vogl, A.M.; Dedic, N.; Schumacher, M.; von Wolff, G.; Avrabos, C.; et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR. Science 2011, 333, 1903–1907. [Google Scholar]
- McLean, M.; Bisits, A.; Davies, J.; Woods, R.; Lowry, P.; Smith, R. A placental clock controlling the length of human pregnancy. Nature Med. 1995, 1, 460–463. [Google Scholar]
- Behan, D.P.; De Souza, E.B.; Lowry, P.J.; Potter, E.; Sawchenko, P.; Vale, W.W. Corticotropin releasing factor CRF binding protein: A novel regulator of CRF and related peptides. Front Neuroendocrin. 1995, 16, 362–382. [Google Scholar] [CrossRef]
- Orth, D.N.; Mount, C.D. Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma. Biochem. Biophys. Res. Commun. 1987, 143, 411–417. [Google Scholar] [CrossRef]
- Potter, E.; Behan, D.P.; Fischer, W.H.; Linton, E.A.; Lowry, P.J.; Vale, W.W. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature 1991, 349, 423–426. [Google Scholar] [CrossRef]
- Seasholtz, A.F.; Burrows, H.L.; Karolyi, I.J.; Camper, S.A. Mouse models of altered CRH-binding protein expression. Peptides 2001, 22, 743–751. [Google Scholar] [CrossRef]
- Potter, E.; Behan, D.P.; Linton, E.A.; Lowry, P.J.; Sawchenko, P.E.; Vale, W.W. The central distribution of a corticotropin-releasing factor CRF-binding protein predicts multiple sites and modes of interaction with CRF. Proc. Natl. Acad. Sci. USA 1992, 89, 4192–4196. [Google Scholar]
- Behan, D.P.; Khongsaly, O.; Ling, N.; De Souza, E.B. Urocortin interaction with corticotropin-releasing factor CRF binding protein CRF-BP: A novel mechanism for elevating “free” CRF levels in human brain. Brain Res. 1996, 725, 263–267. [Google Scholar]
- Gallagher, J.P.; Orozco-Cabal, L.F.; Liu, J.; Shinnick-Gallagher, P. Synaptic physiology of central CRH system. Eur. J. Pharmacol. 2008, 583, 215–225. [Google Scholar] [CrossRef]
- Liu, J.; Yu, B.; Neugebauer, V.; Grigoriadis, D.E.; Rivier, J.; Vale, W.W.; Shinnick-Gallagher, P.; Gallagher, J.P. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J. Neurosci. 2004, 24, 4020–4029. [Google Scholar]
- Blank, T.; Nijholt, I.; Eckart, K.; Spiess, J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: Implications for hippocampus-dependent learning. J. Neurosci. 2002, 22, 3788–3794. [Google Scholar]
- Nemeroff, C.B.; Widerlov, E.; Bissette, G.; Walleus, H.; Karlsson, I.; Eklund, K.; Kilts, C.D.; Loosen, P.T.; Vale, W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984, 226, 1342–1344. [Google Scholar]
- Bremner, J.D.; Licinio, J.; Darnell, A.; Krystal, J.H.; Owens, M.J.; Southwick, S.M.; Nemeroff, C.B.; Charney, D.S. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatr. 1997, 154, 624–629. [Google Scholar]
- Binder, E.B.; Owens, M.J.; Liu, W.; Deveau, T.C.; Rush, a J.; Trivedi, M.H.; Fava, M.; Bradley, B.; Ressler, K.J.; Nemeroff, C.B. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch. Gen. Psychiatr. 2010, 67, 369–379. [Google Scholar] [CrossRef]
- Mitchell, A. The role of corticotropin releasing factor in depressive illness: A critical review. Neurosci. Biobehav. Rev. 1998, 22, 635–651. [Google Scholar] [CrossRef]
- Bonne, O.; Gill, J.M.; Luckenbaugh, D.A.; Collins, C.; Owens, M.J.; Alesci, S.; Neumeister, A.; Yuan, P.; Kinkead, B.; Manji, H.K.; et al. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance P in the cerebrospinal fluid of civilians with posttraumatic stress disorder before and after treatment with paroxetine. J. Clin. Psychiatr. 2011, 72, 1124–1128. [Google Scholar]
- Imaki, T.; Shibasaki, T.; Hotta, M.; Demura, H. Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: Comparison with pattern of c-fos mRNA induction after stress. Brain Res. 1993, 616, 114–125. [Google Scholar] [CrossRef]
- Campbell, B.M.; Morrison, J.L.; Walker, E.L.; Merchant, K.M. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol. Biochem. Behav. 2004, 77, 447–455. [Google Scholar] [CrossRef]
- Sutton, R.E.; Koob, G.F.; Le Moal, M.; Rivier, J.; Vale, W. Corticotropin releasing factor produces behavioural activation in rats. Nature 1982, 297, 331–333. [Google Scholar]
- Dunn, A. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses. Brain Res. Rev. 1990, 15, 71–100. [Google Scholar] [CrossRef]
- Rivest, S.; Deshaies, Y.; Richard, D. Effects of corticotropin-releasing factor on energy balance in rats are sex dependent. Am. J. Phys. 1989, 257, R1417–R1422. [Google Scholar]
- Glowa, J.R.; Barrett, J.E.; Russell, J.; Gold, P.W. Effects of corticotropin releasing hormone on appetitive behaviors. Peptides 1992, 13, 609–621. [Google Scholar] [CrossRef]
- Sherman, J.E.; Kalin, N.H. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol. Biochem. Behav. 1988, 30, 801–807. [Google Scholar] [CrossRef]
- Britton, K.T.; Lee, G.; Vale, W.; Rivier, J.; Koob, G.F. Corticotropin releasing factor CRF receptor antagonist blocks activating and “anxiogenic” actions of CRF in the rat. Brain Res. 1986, 369, 303–306. [Google Scholar] [CrossRef]
- Heinrichs, S.C.; Pich, E.M.; Miczek, K.A.; Britton, K.T.; Koob, G.F. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992, 581, 190–197. [Google Scholar] [CrossRef]
- Kalin, N.H.; Sherman, J.E.; Takahashi, L.K. Antagonism of endogenous CRH systems attenuates stress-induced freezing behavior in rats. Brain Res. 1988, 457, 130–135. [Google Scholar] [CrossRef]
- Heinrichs, S.C.; Lapsansky, J.; Lovenberg, T.W.; De Souza, E.B.; Chalmers, D.T. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul. Pept. 1997, 71, 15–21. [Google Scholar] [CrossRef]
- Liebsch, G.; Landgraf, R.; Engelmann, M.; Lörscher, P.; Holsboer, F. Differential behavioural effects of chronic infusion of CRH 1 and CRH 2 receptor antisense oligonucleotides into the rat brain. J. Psychiatr. Res. 1999, 33, 153–163. [Google Scholar] [CrossRef]
- Bagosi, Z.; Jászberényi, M.; Szabó, G.; Telegdy, G. The effects of CRF and the urocortins on [3H]GABA release from the rat amygdala—An in vitro superfusion study. Brain Res. Bull. 2008, 75, 15–17. [Google Scholar] [CrossRef]
- Bagosi, Z.; Csabafi, K.; Jászberényi, M.; Telegdy, G. The effects of corticotropin-releasing factor and the urocortins on hypothalamic gamma-amino butyric acid release—The impacts on the hypothalamic-pituitary-adrenal axis. Neurochem. Int. 2012, 60, 350–354. [Google Scholar] [CrossRef]
- Rainnie, D.G.; Bergeron, R.; Sajdyk, T.J.; Patil, M.; Gehlert, D.R.; Shekhar, A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J. Neurosci. 2004, 24, 3471–3479. [Google Scholar] [CrossRef]
- Valdez, G.R.; Inoue, K.; Koob, G.F.; Rivier, J.; Vale, W.; Zorrilla, E.P. Human urocortin II: Mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002, 943, 142–150. [Google Scholar] [CrossRef]
- Valdez, G.R.; Zorrilla, E.P.; Rivier, J.; Vale, W.W.; Koob, G.F. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003, 980, 206–212. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Joppa, M.; Ling, N.; Foster, A.C. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides 2004, 25, 659–666. [Google Scholar] [CrossRef]
- Chen, P.; Vaughan, J.; Donaldson, C.; Vale, W.; Li, C. Injection of Urocortin 3 into the ventromedial hypothalamus modulates feeding, blood glucose levels, and hypothalamic POMC gene expression but not the HPA axis. Am. J. Physiol-Endoc. Metab. 2010, 298, E337–E345. [Google Scholar] [CrossRef]
- Muglia, L.; Jacobson, L.; Majzoub, J.A. Production of corticotropin-releasing hormone-deficient mice by targeted mutation in embryonic stem cells. Ann. NY Acad. Sci. 1996, 780, 49–59. [Google Scholar] [CrossRef]
- Muglia, L.; Jacobson, L.; Dikkes, P.; Majzoub, J.A. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 1995, 373, 427–432. [Google Scholar]
- Weninger, S.C. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc. Natl. Acad. Sci. USA 1999, 96, 8283–8288. [Google Scholar] [CrossRef]
- Dunn, A.J.; Swiergiel, A.H. Behavioral responses to stress are intact in CRF-deficient mice. Brain Res. 1999, 845, 14–20. [Google Scholar] [CrossRef]
- Stenzel-Poore, M.P.; Cameron, V.A.; Vaughan, J.; Sawchenko, P.E.; Vale, W. Development of Cushing’s syndrome in corticotropin-releasing factor transgenic mice. Endocrinology 1992, 130, 3378–3386. [Google Scholar] [CrossRef]
- Dirks, A.; Groenink, L.; Schipholt, M.I.; van der Gugten, J.; Hijzen, T.H.; Geyer, M.A.; Olivier, B. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol. Psychiatr. 2002, 51, 583–590. [Google Scholar] [CrossRef]
- Lu, S.; Steiner, M.A.; Whittle, N.; Vogl, A.M.; Walser, S.M.; Ableitner, M.; Refojo, D.; Ekker, M.; Rubenstein, J.L.; Stalla, G.K.; et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol. Psychiatr. 2008, 13, 1028–1042. [Google Scholar] [CrossRef]
- Dedic, N.; Touma, C.; Romanowski, C.P.; Schieven, M.; Kühne, C.; Ableitner, M.; Lu, A.; Holsboer, F.; Wurst, W.; Kimura, M.; Deussing, J.M. Assessing behavioural effects of chronic HPA axis activation using conditional CRH-overexpressing mice. Cell Mol. Neurobiol. 2011. [Epub ahead of print].. [Google Scholar]
- Groenink, L.; Dirks, A.; Verdouw, P.M.; Schipholt, M.L.; Veening, J.G.; van der Gugten, J.; Olivier, B. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol. Psychiatr. 2002, 51, 875–881. [Google Scholar] [CrossRef]
- Stenzel-Poore, M.P.; Heinrichs, S.C.; Rivest, S.; Koob, G.F.; Vale, W.W. Overproduction of corticotropin-releasing factor in transgenic mice: A genetic model of anxiogenic behavior. J. Neurosci. 1994, 14, 2579–2584. [Google Scholar]
- van Gaalen, M.M.; Stenzel-Poore, M.P.; Holsboer, F.; Steckler, T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur. J. Neurosci. 2002, 15, 2007–2015. [Google Scholar] [CrossRef]
- Heinrichs, S.C.; Min, H.; Tamraz, S.; Carmouché, M.; Boehme, S.A.; Vale, W.W. Anti-sexual and anxiogenic behavioral consequences of corticotropin-releasing factor overexpression are centrally mediated. Psychoneuroendocrinology 1997, 22, 215–224. [Google Scholar] [CrossRef]
- Dirks, A.; Groenink, L.; Bouwknecht, J.A.; Hijzen, T.H.; van der Gugten, J.; Ronken, E.; Verbeek, J.S.; Veening, J.G.; Dederen, P.J.W.C.; Korosi, A.; et al. Overexpression of corticotropin-releasing hormone in transgenic mice and chronic stress-like autonomic and physiological alterations. Eur. J. Neurosci. 2002, 16, 1751–1760. [Google Scholar] [CrossRef]
- Palmer, A.A.; Sharpe, A.L.; Burkhart-Kasch, S.; McKinnon, C.S.; Coste, S.C.; Stenzel-Poore, M.P.; Phillips, T.J. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology 2004, 176, 386–397. [Google Scholar] [CrossRef]
- Hansson, A.C.; Cippitelli, A.; Sommer, W.H.; Fedeli, A.; Björk, K.; Soverchia, L.; Terasmaa, A.; Massi, M.; Heilig, M.; Ciccocioppo, R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. USA 2006, 103, 15236–15241. [Google Scholar]
- Treutlein, J.; Kissling, C.; Frank, J.; Wiemann, S.; Dong, L.; Depner, M.; Saam, C.; Lascorz, J.; Soyka, M.; Preuss, U.W.; et al. Genetic association of the human corticotropin releasing hormone receptor 1 CRHR1 with binge drinking and alcohol intake patterns in two independent samples. Mol. Psychiatr. 2006, 11, 594–602. [Google Scholar] [CrossRef]
- Heilig, M.; Koob, G.F. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007, 30, 399–406. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Hendriksen, H.; van Oorschot, R.; Cook, J.M.; Rallipalli, S.; Huang, S.; Millan, M.J.; Olivier, B.; Groenink, L. Lifelong CRF overproduction is associated with altered gene expression and sensitivity of discrete GABA(A) and mGlu receptor subtypes. Psychopharmacology 2012, 219, 897–908. [Google Scholar] [CrossRef]
- Wang, X.D.; Rammes, G.; Kraev, I.; Wolf, M.; Liebl, C.; Scharf, S.H.; Rice, C.J.; Wurst, W.; Holsboer, F.; Deussing, J.M.; et al. Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011, 31, 13625–13634. [Google Scholar]
- Kimura, M.; Müller-Preuss, P.; Lu, A.; Wiesner, E.; Flachskamm, C.; Wurst, W.; Holsboer, F.; Deussing, J.M. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol. Psychiatr. 2010, 15, 154–165. [Google Scholar] [CrossRef]
- Kolber, B.J.; Boyle, M.P.; Wieczorek, L.; Kelley, C.L.; Onwuzurike, C.C.; Nettles, S.A.; Vogt, S.K.; Muglia, L.J. Transient early-life forebrain corticotropin-releasing hormone elevation causes long-lasting anxiogenic and despair-like changes in mice. J. Neurosci. 2010, 30, 2571–2581. [Google Scholar]
- Vicentini, E.; Arban, R.; Angelici, O.; Maraia, G.; Perico, M.; Mugnaini, M.; Ugolini, A.; Large, C.; Domenici, E.; Gerrard, P.; et al. Transient forebrain over-expression of CRF induces plasma corticosterone and mild behavioural changes in adult conditional CRF transgenic mice. Pharmacol. Biochem. Behav. 2009, 93, 17–24. [Google Scholar] [CrossRef]
- Smith, G.W.; Aubry, J.M.; Dellu, F.; Contarino, A.; Bilezikjian, L.M.; Gold, L.H.; Chen, R.; Marchuk, Y.; Hauser, C.; Bentley, C.A.; et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998, 20, 1093–1102. [Google Scholar] [CrossRef]
- Contarino, A.; Dellu, F.; Koob, G.F.; Smith, G.W.; Lee, K.F.; Vale, W.; Gold, L.H. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999, 835, 1–9. [Google Scholar] [CrossRef]
- Timpl, P.; Spanagel, R.; Sillaber, I.; Kresse, A.; Reul, J.M.; Stalla, G.K.; Blanquet, V.; Steckler, T.; Holsboer, F.; Wurst, W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Gen. 1998, 19, 162–166. [Google Scholar] [CrossRef]
- Müller, M.B.; Zimmermann, S.; Sillaber, I.; Hagemeyer, T.P.; Deussing, J.M.; Timpl, P.; Kormann, M.S.D.; Droste, S.K.; Kühn, R.; Reul, J.M.H.M.; et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003, 6, 1100–1107. [Google Scholar]
- Nguyen, N.K.; Keck, M.E.; Hetzenauer, A.; Thoeringer, C.K.; Wurst, W.; Deussing, J.M.; Holsboer, F.; Müller, M.B.; Singewald, N. Conditional CRF receptor 1 knockout mice show altered neuronal activation pattern to mild anxiogenic challenge. Psychopharmacology 2006, 188, 374–385. [Google Scholar] [CrossRef]
- Wang, X.D.; Chen, Y.; Wolf, M.; Wagner, K.V.; Liebl, C.; Scharf, S.H.; Harbich, D.; Mayer, B.; Wurst, W.; Holsboer, F.; et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol. Dis. 2011, 42, 300–310. [Google Scholar] [CrossRef]
- Molander, A.; Vengeliene, V.; Heilig, M.; Wurst, W.; Deussing, J.M.; Spanagel, R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology 2012, 37, 1047–1056. [Google Scholar] [CrossRef]
- Schmidt, M.V.; Deussing, J.M.; Oitzl, M.S.; Ohl, F.; Levine, S.; Wurst, W.; Holsboer, F.; Müller, M.B.; de Kloet, E.R. Differential disinhibition of the neonatal hypothalamic- pituitary-adrenal axis in brain-specific CRH receptor 1-knockout mice. Eur. J. Neurosci. 2006, 24, 2291–2298. [Google Scholar]
- Schmidt, M. Regulation of the developing hypothalamic-pituitary-adrenal axis in corticotropin releasing hormone receptor 1-deficient mice. Neuroscience 2003, 119, 589–595. [Google Scholar] [CrossRef]
- Müller, M.B.; Preil, J.; Renner, U.; Zimmermann, S.; Kresse, A.E.; Stalla, G.K.; Keck, M.E.; Holsboer, F.; Wurst, W. Expression of CRHR1 and CRHR2 in mouse pituitary and adrenal gland: Implications for HPA system regulation. Endocrinology 2001, 142, 4150–4153. [Google Scholar] [CrossRef]
- Coste, S.C.; Kesterson, R.A.; Heldwein, K.A.; Stevens, S.L.; Heard, A.D.; Hollis, J.H.; Murray, S.E.; Hill, J.K.; Pantely, G.A.; Hohimer, A.R.; et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Gen. 2000, 24, 403–409. [Google Scholar] [CrossRef]
- Bale, T.L.; Contarino, A.; Smith, G.W.; Chan, R.; Gold, L.H.; Sawchenko, P.E.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Gen. 2000, 24, 410–414. [Google Scholar] [CrossRef]
- Kishimoto, T.; Radulovic, J.; Radulovic, M.; Lin, C.R.; Schrick, C.; Hooshmand, F.; Hermanson, O.; Rosenfeld, M.G.; Spiess, J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Gen. 2000, 24, 415–419. [Google Scholar] [CrossRef]
- Todorovic, C.; Sherrin, T.; Pitts, M.; Hippel, C.; Rayner, M.; Spiess, J. Suppression of the MEK/ERK signaling pathway reverses depression-like behaviors of CRF2-deficient mice. Neuropsychopharmacology 2009, 34, 1416–1426. [Google Scholar] [CrossRef]
- Bale, T.L.; Vale, W.W. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: Sexually dichotomous responses. J. Neurosci. 2003, 23, 5295–5301. [Google Scholar]
- Gresack, J.; Powell, S.; Geyer, M.; Poore, M.S.; Coste, S.; Risbrough, V. CRF2 null mutation increases sensitivity to isolation rearing effects on locomotor activity in mice. Neuropeptides 2010, 44, 349–353. [Google Scholar] [CrossRef]
- Bale, T.L.; Picetti, R.; Contarino, A.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J. Neurosci. 2002, 22, 193–199. [Google Scholar]
- Gysling, K.; Forray, M.I.; Haeger, P.; Daza, C.; Rojas, R. Corticotropin-releasing hormone and urocortin: Redundant or distinctive functions? Brain Res. Rev. 2004, 47, 116–125. [Google Scholar] [CrossRef]
- Wang, X.; Su, H.; Copenhagen, L.D.; Vaishnav, S.; Pieri, F.; Shope, C.D.; Brownell, W.E.; De Biasi, M.; Paylor, R.; Bradley, A. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol. Cell. Biol. 2002, 22, 6605–6610. [Google Scholar]
- Vetter, D.E.; Li, C.; Zhao, L.; Contarino, A.; Liberman, M.C.; Smith, G.W.; Marchuk, Y.; Koob, G.F.; Heinemann, S.F.; Vale, W.; Lee, K.F. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat. Gen. 2002, 31, 363–369. [Google Scholar]
- Giardino, W.J.; Cocking, D.L.; Kaur, S.; Cunningham, C.L.; Ryabinin, A.E. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PLoS One 2011, 6, e26997. [Google Scholar]
- Chen, A.; Zorrilla, E.; Smith, S.; Rousso, D.; Levy, C.; Vaughan, J.; Donaldson, C.; Roberts, A.; Lee, K.F.; Vale, W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J. Neurosci. 2006, 26, 5500–5510. [Google Scholar] [CrossRef]
- Neufeld-Cohen, A.; Evans, A.K.; Getselter, D.; Spyroglou, A.; Hill, A.; Gil, S.; Tsoory, M.; Beuschlein, F.; Lowry, C.A.; Vale, W.; Chen, A. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol. Psychiatr. 2010, 15, 426–441. [Google Scholar] [CrossRef]
- Deussing, J.M.; Breu, J.; Kühne, C.; Kallnik, M.; Bunck, M.; Glasl, L.; Yen, Y.C.; Schmidt, M.V.; Zurmühlen, R.; Vogl, A.M.; et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. 2010, 30, 9103–9116. [Google Scholar]
- Neufeld-cohen, A.; Tsoory, M.M.; Evans, A.K.; Getselter, D.; Gil, S. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc. Natl. Acad. Sci. USA 2010, 107, 19020–19025. [Google Scholar]
- Lovejoy, D.A.; Aubry, J.M.; Turnbull, A.; Sutton, S.; Potter, E.; Yehling, J.; Rivier, C.; Vale, W.W. Ectopic expression of the CRF-binding protein: Minor impact on HPA axis regulation but induction of sexually dimorphic weight gain. J. Neuroendocrinol. 1998, 10, 483–491. [Google Scholar]
- Burrows, H.L.; Nakajima, M.; Lesh, J.S.; Goosens, K.A.; Samuelson, L.C.; Inui, A.; Camper, S.A.; Seasholtz, A.F. Excess corticotropin releasing hormone-binding protein in the hypothalamic-pituitary-adrenal axis in transgenic mice. J. Clin. Invest. 1998, 101, 1439–1447. [Google Scholar] [CrossRef]
- Karolyi, I.J.; Burrows, H.L.; Ramesh, T.M.; Nakajima, M.; Lesh, J.S.; Seong, E.; Camper, S.A.; Seasholtz, A.F. Altered anxiety and weight gain in corticotropin-releasing hormone-binding protein-deficient mice. Proc. Natl. Acad. Sci. USA 1999, 96, 11595–11600. [Google Scholar]
- Regev, L.; Neufeld-Cohen, A.; Tsoory, M.; Kuperman, Y.; Getselter, D.; Gil, S.; Chen, A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol. Psychiatr. 2010, 16, 714–728. [Google Scholar]
- Regev, L.; Tsoory, M.; Gil, S.; Chen, A. Site-Specific Genetic Manipulation of Amygdala Corticotropin-Releasing Factor Reveals Its Imperative Role in Mediating Behavioral Response to Challenge. Biol. Psychiatr. 2011, 71, 317–326. [Google Scholar]
- Flandreau, E.I.; Ressler, K.J.; Owens, M.J.; Nemeroff, C.B. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 2012, 37, 27–38. [Google Scholar] [CrossRef]
- Keen-Rhinehart, E.; Michopoulos, V.; Toufexis, D.J.; Martin, E.I.; Nair, H.; Ressler, K.J.; Davis, M.; Owens, M.J.; Nemeroff, C.B.; Wilson, M.E. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol. Psychiatr. 2009, 14, 37–50. [Google Scholar] [CrossRef]
- Sink, K.S.; Walker, D.L.; Freeman, S.M.; Flandreau, E.I.; Ressler, K.J.; Davis, M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol. Psychiatr. 2012. [Google Scholar]
- Sztainberg, Y.; Kuperman, Y.; Tsoory, M.; Lebow, M.; Chen, A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol. Psychiatr. 2010, 15, 905–917. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Kuperman, Y.; Justice, N.; Chen, A. An anxiolytic role for CRF receptor type 1 in the globus pallidus. J. Neurosci. 2011, 31, 17416–17424. [Google Scholar] [CrossRef]
- Kuperman, Y.; Issler, O.; Regev, L.; Musseri, I.; Navon, I.; Neufeld-Cohen, A.; Gil, S.; Chen, A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 8393–8398. [Google Scholar]
- Smoller, J.W.; Rosenbaum, J.F.; Biederman, J.; Kennedy, J.; Dai, D.; Racette, S.R.; Laird, N.M.; Kagan, J.; Snidman, N.; Hirshfeld-Becker, D. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol. Psychiatr. 2003, 54, 1376–1381. [Google Scholar] [CrossRef]
- Smoller, J.W.; Yamaki, L.H.; Fagerness, J.A.; Biederman, J.; Racette, S.; Laird, N.M.; Kagan, J.; Snidman, N.; Faraone, S.V.; Hirshfeld-Becker, D.; et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol. Psychiatr. 2005, 57, 1485–1492. [Google Scholar] [CrossRef]
- Bradley, R.G.; Binder, E.B.; Epstein, M.P.; Tang, Y.; Nair, H.P.; Liu, W.; Gillespie, C.F.; Berg, T.; Evces, M.; Newport, D.J.; et al. Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatr. 2008, 65, 190–200. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Feinn, R.; Nelson, E.C.; Covault, J.; Anton, R.F.; Farrer, L.; Gelernter, J.A. CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am. J. Med. Genet. B 2011, 156B, 960–968. [Google Scholar]
- Tyrka, A.R.; Price, L.H.; Gelernter, J.; Schepker, C.; Anderson, G.M.; Carpenter, L.L. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects on hypothalamic-pituitary-adrenal axis reactivity. Biol. Psychiatr. 2009, 66, 681–685. [Google Scholar] [CrossRef]
- Heim, C.; Bradley, B.; Mletzko, T.C.; Deveau, T.C.; Musselman, D.L.; Nemeroff, C.B.; Ressler, K.J.; Binder, E.B. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Front. Behav. Neurosci. 2009, 3, 41. [Google Scholar]
- Polanczyk, G.; Caspi, A.; Williams, B.; Price, T.S.; Danese, A.; Sugden, K.; Uher, R.; Poulton, R.; Moffitt, T.E. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: Replication and extension. Arch. Gen. Psychiatr. 2009, 66, 978–985. [Google Scholar] [CrossRef]
- Grabe, H.J.; Schwahn, C.; Appel, K.; Mahler, J.; Schulz, A.; Spitzer, C.; Fenske, K.; Barnow, S.; Lucht, M.; Freyberger, H.J.; et al. Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am. J. Med. Genet. B 2010, 153B, 1483–1493. [Google Scholar] [CrossRef]
- Pitts, M.W.; Todorovic, C.; Blank, T.; Takahashi, L.K. The central nucleus of the amygdala and corticotropin-releasing factor: Insights into contextual fear memory. J. Neurosci. 2009, 29, 7379–7388. [Google Scholar] [CrossRef]
- Roozendaal, B.; Schelling, G.; McGaugh, J.L. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: Dependence on glucocorticoid receptor activation. J. Neurosci. 2008, 28, 6642–6651. [Google Scholar]
- Papiol, S.; Arias, B.; Gastó, C.; Gutiérrez, B.; Catalán, R.; Fañanás, L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J. Affect. Disord. 2007, 104, 83–90. [Google Scholar] [CrossRef]
- Licinio, J.; O’Kirwan, F.; Irizarry, K.; Merriman, B.; Thakur, S.; Jepson, R.; Lake, S.; Tantisira, K.G.; Weiss, S.T.; Wong, M.L. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol. Psychiatr. 2004, 9, 1075–1082. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, F.; Wang, G.; Xiao, Z.; Tang, J.; Liu, W.; Wang, H.; Liu, H.; Wang, X.; Wu, Y.; et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci.Lett. 2007, 414, 155–158. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Gao, K.; Wan, Q.; Yang, C.; Wang, H.; Wang, X.; Wang, G.; Liu, Z. Interaction between CRHR1 and BDNF genes increases the risk of recurrent major depressive disorder in Chinese population. PLoS One 2011, 6, e28733. [Google Scholar]
- Blomeyer, D.; Treutlein, J.; Esser, G.; Schmidt, M.H.; Schumann, G.; Laucht, M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol. Psychiatr. 2008, 63, 146–151. [Google Scholar] [CrossRef]
- Schmid, B.; Blomeyer, D.; Treutlein, J.; Zimmermann, U.S.; Buchmann, A.F.; Schmidt, M.H.; Esser, G.; Rietschel, M.; Banaschewski, T.; Schumann, G.; Laucht, M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int. J. Neuropsychopharmacol. 2010, 13, 703–714. [Google Scholar] [CrossRef]
- Barr, C.S.; Dvoskin, R.L.; Yuan, Q.; Lipsky, R.H.; Gupte, M.; Hu, X.; Zhou, Z.; Schwandt, M.L.; Lindell, S.G.; McKee, M.; et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch. Gen. Psychiatr. 2008, 65, 934–944. [Google Scholar] [CrossRef]
- Dahl, J.P.; Doyle, G.A.; Oslin, D.W.; Buono, R.J.; Ferraro, T.N.; Lohoff, F.W.; Berrettini, W.H. Lack of association between single nucleotide polymorphisms in the corticotropin releasing hormone receptor 1 (CRHR1) gene and alcohol dependence. J. Psychiatr. Res. 2005, 39, 475–479. [Google Scholar] [CrossRef]
- Soyka, M.; Preuss, U.W.; Koller, G.; Zill, P.; Hesselbrock, V.; Bondy, B. No association of CRH1 receptor polymorphism haplotypes, harm avoidance and other personality dimensions in alcohol dependence: Results from the Munich gene bank project for alcoholism. Addict. Biol. 2004, 9, 73–79. [Google Scholar] [CrossRef]
- Ribbe, K.; Ackermann, V.; Schwitulla, J.; Begemann, M.; Papiol, S.; Grube, S.; Sperling, S.; Friedrichs, H.; Jahn, O.; Sillaber, I.; et al. Prediction of the risk of comorbid alcoholism in schizophrenia by interaction of common genetic variants in the corticotropin-releasing factor system. Arch. Gen. Psychiatr. 2011, 68, 1247–1256. [Google Scholar] [CrossRef]
- Wasserman, D.; Sokolowski, M.; Rozanov, V.; Wasserman, J. The CRHR1 gene: A marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008, 7, 14–19. [Google Scholar]
- De Luca, V.; Tharmalingam, S.; Kennedy, J.L. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. Eur. Psychiatr. 2007, 22, 282–287. [Google Scholar] [CrossRef]
- Claes, S.; Villafuerte, S.; Forsgren, T.; Sluijs, S.; Del-Favero, J.; Adolfsson, R.; van Broeckhoven, C. The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol. Psychiatr. 2003, 54, 867–872. [Google Scholar] [CrossRef]
- Ray, L.A. Stress-induced and cue-induced craving for alcohol in heavy drinkers: Preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol Clin. Exp. Res. 2011, 35, 166–174. [Google Scholar] [CrossRef]
- De Luca, V.; Tharmalingam, S.; Zai, C.; Potapova, N.; Strauss, J.; Vincent, J.; Kennedy, J.L. Association of HPA axis genes with suicidal behaviour in schizophrenia. J. Psychopharmacol. 2010, 24, 677–682. [Google Scholar] [CrossRef]
- Claes, S.J. Corticotropin-releasing hormone (CRH) in psychiatry: From stress to psychopathology. Ann. Med. 2004, 36, 50–61. [Google Scholar] [CrossRef]
- Austin, M.C.; Janosky, J.E.; Murphy, H.A. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol. Psychiatr. 2003, 8, 324–332. [Google Scholar] [CrossRef]
- Zobel, A. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: The first 20 patients treated. J. Psychiatr. Res. 2000, 34, 171–181. [Google Scholar] [CrossRef]
- Held, K.; Künzel, H.; Ising, M.; Schmid, D.A.; Zobel, A.; Murck, H.; Holsboer, F.; Steiger, A. Treatment with the CRH1-receptor-antagonist R121919 improves sleep-EEG in patients with depression. J. Psychiatr. Res. 2004, 38, 129–136. [Google Scholar] [CrossRef]
- Künzel, H.E.; Zobel, A.W.; Nickel, T.; Ackl, N.; Uhr, M.; Sonntag, A.; Ising, M.; Holsboer, F. Treatment of depression with the CRH-1-receptor antagonist R121919: Endocrine changes and side effects. J. Psychiatr. Res. 2003, 37, 525–533. [Google Scholar] [CrossRef]
- Coric, V.; Feldman, H.H.; Oren, D.A.; Shekhar, A.; Pultz, J.; Dockens, R.C.; Wu, X.; Gentile, K.A.; Huang, S.P.; Emison, E.; et al. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress. Anxiety 2010, 27, 417–425. [Google Scholar] [CrossRef]
- Binneman, B.; Feltner, D.; Kolluri, S.; Shi, Y.; Qiu, R.; Stiger, T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am. J. Psychiatr. 2008, 165, 617–620. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Laryea, G.; Arnett, M.G.; Muglia, L.J. Behavioral Studies and Genetic Alterations in Corticotropin-Releasing Hormone (CRH) Neurocircuitry: Insights into Human Psychiatric Disorders. Behav. Sci. 2012, 2, 135-171. https://doi.org/10.3390/bs2020135
Laryea G, Arnett MG, Muglia LJ. Behavioral Studies and Genetic Alterations in Corticotropin-Releasing Hormone (CRH) Neurocircuitry: Insights into Human Psychiatric Disorders. Behavioral Sciences. 2012; 2(2):135-171. https://doi.org/10.3390/bs2020135
Chicago/Turabian StyleLaryea, Gloria, Melinda G. Arnett, and Louis J. Muglia. 2012. "Behavioral Studies and Genetic Alterations in Corticotropin-Releasing Hormone (CRH) Neurocircuitry: Insights into Human Psychiatric Disorders" Behavioral Sciences 2, no. 2: 135-171. https://doi.org/10.3390/bs2020135




