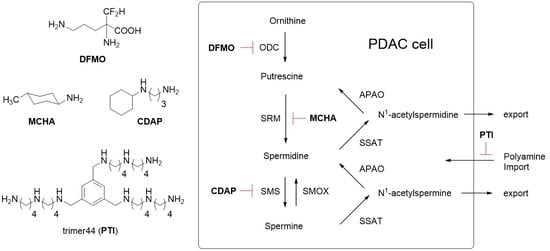
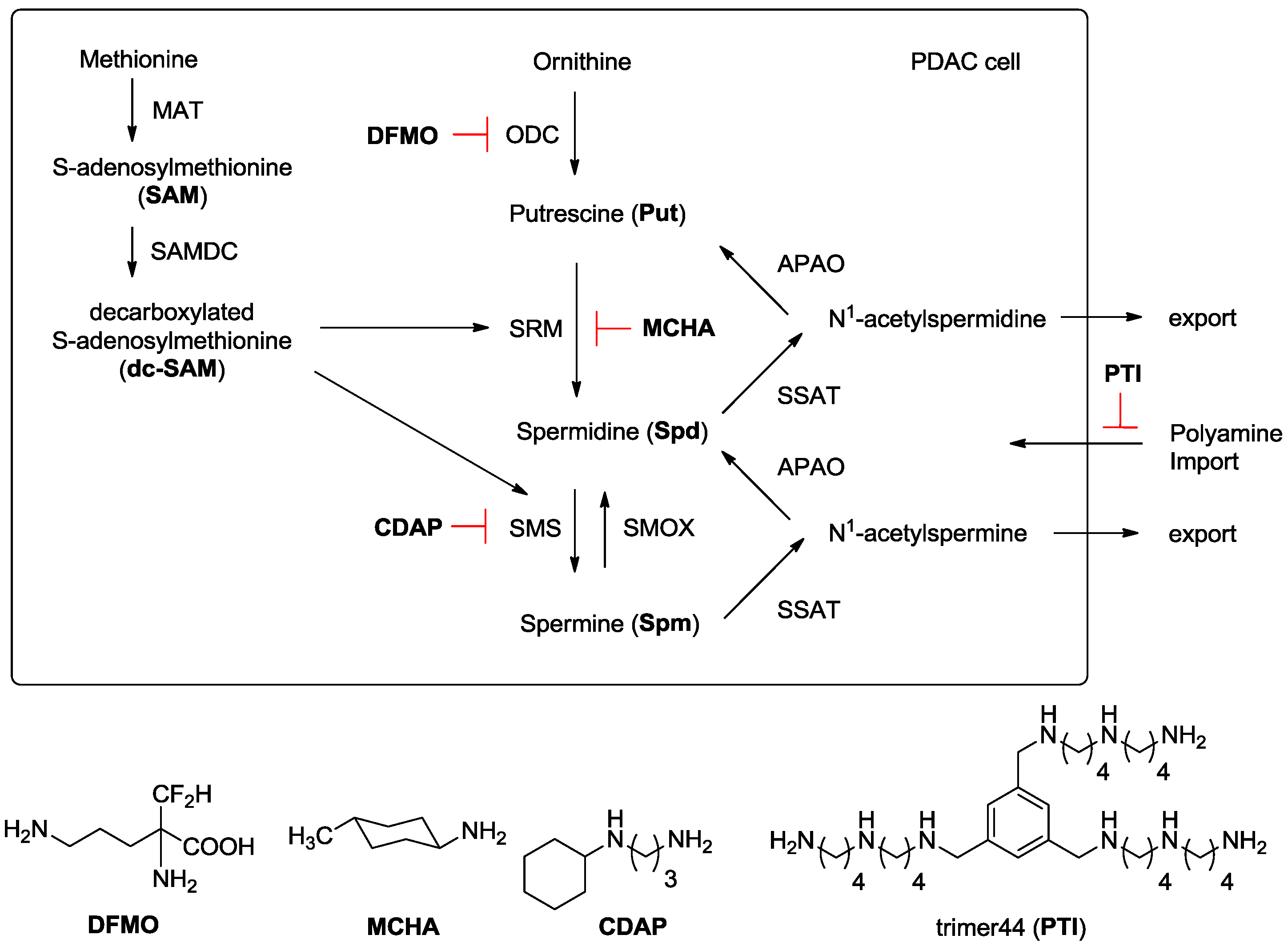
Investigation of Polyamine Metabolism and Homeostasis in Pancreatic Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biological Studies
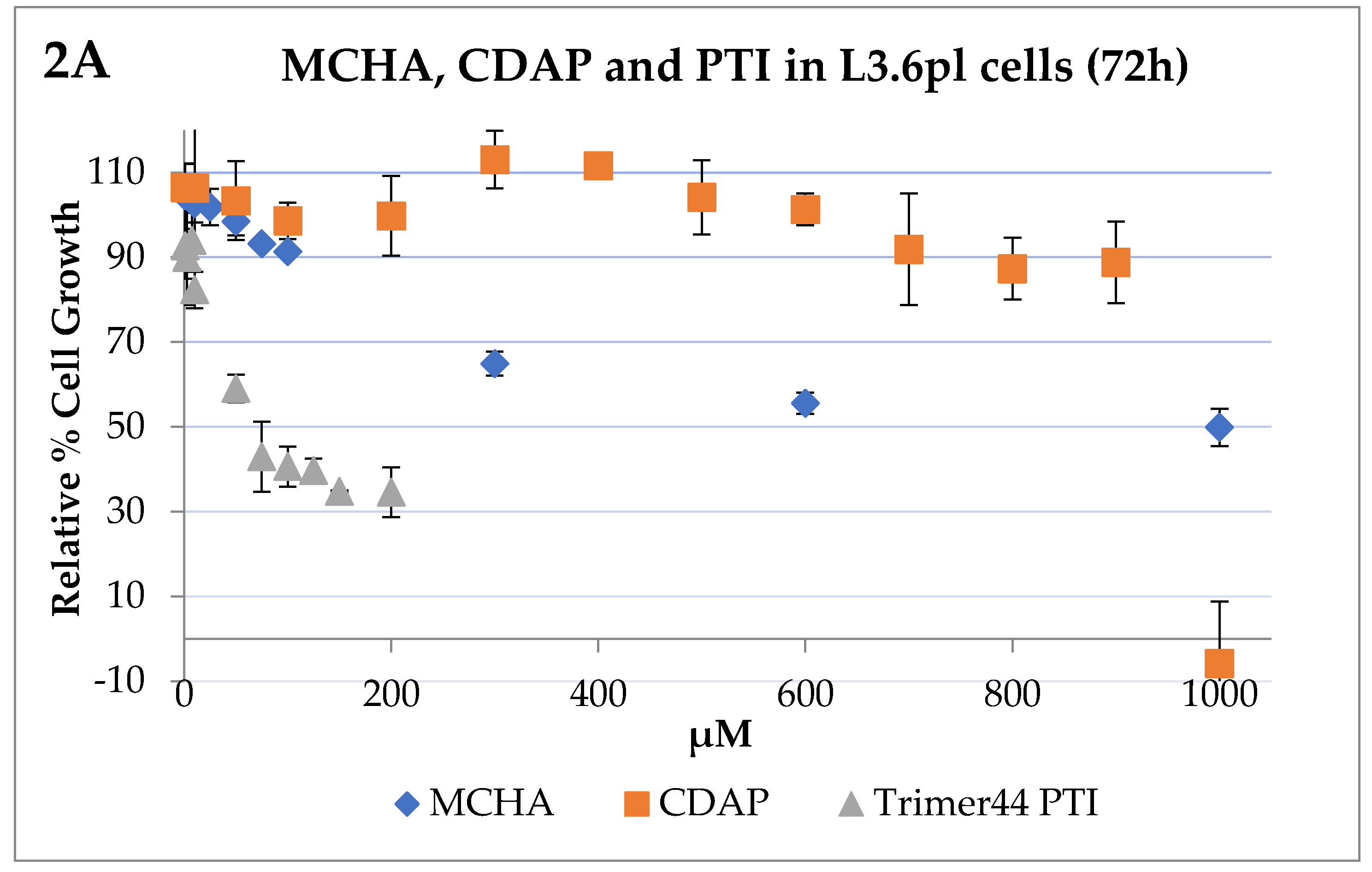
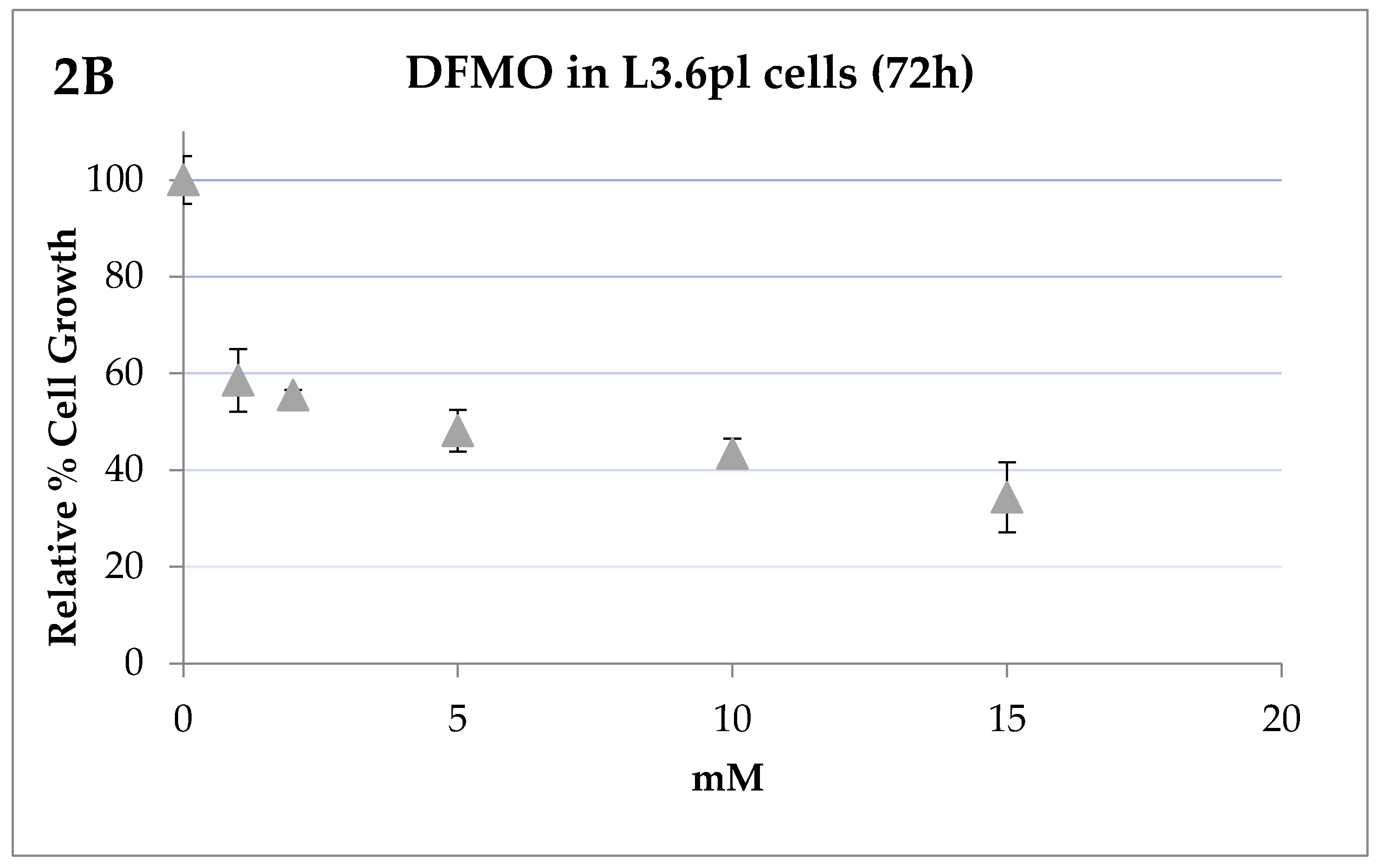
2.3. IC50 Determinations and Cell Growth Studies
2.4. HPLC and Polyamine Level Determination
2.5. Statistical Analysis
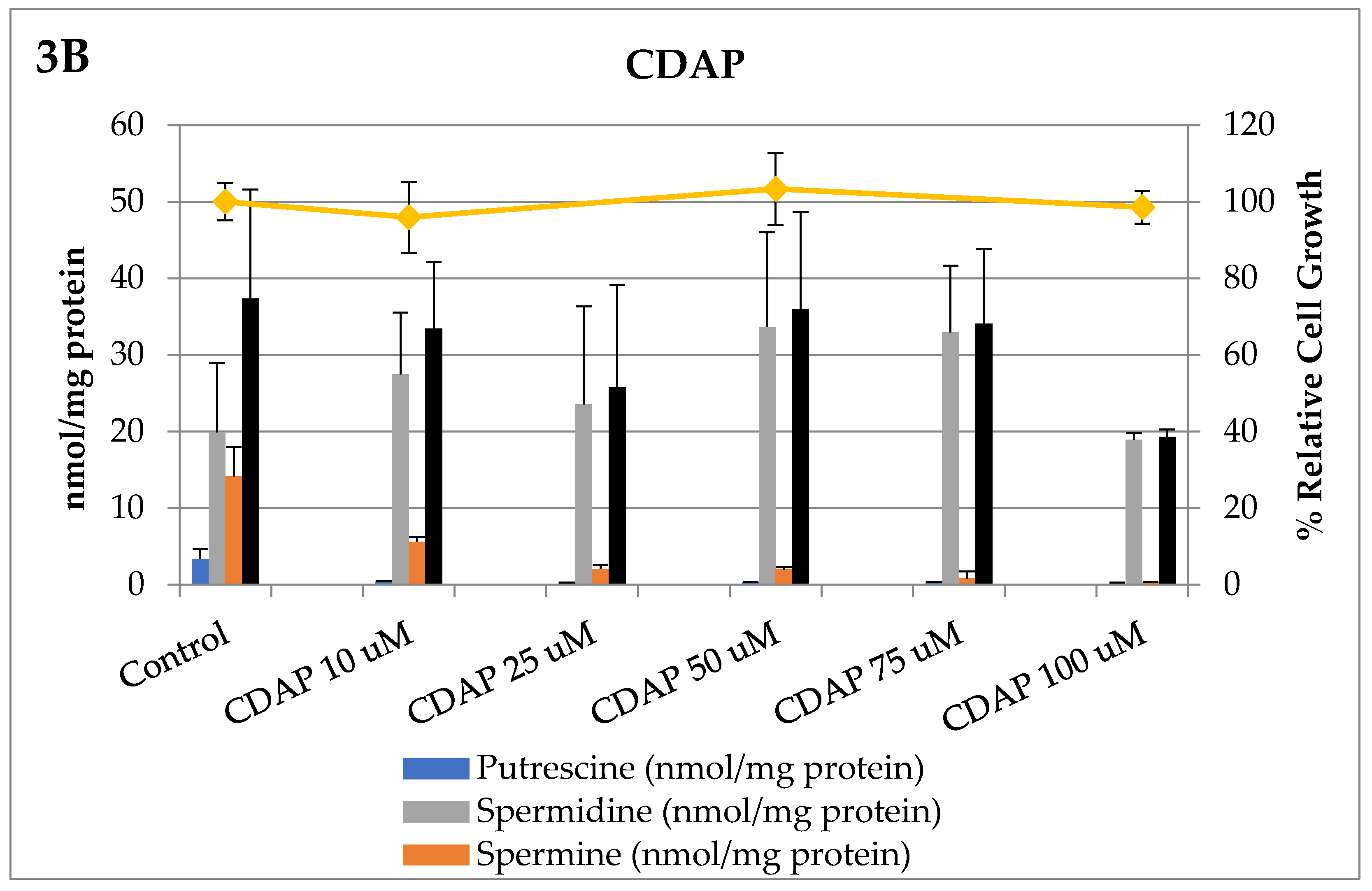
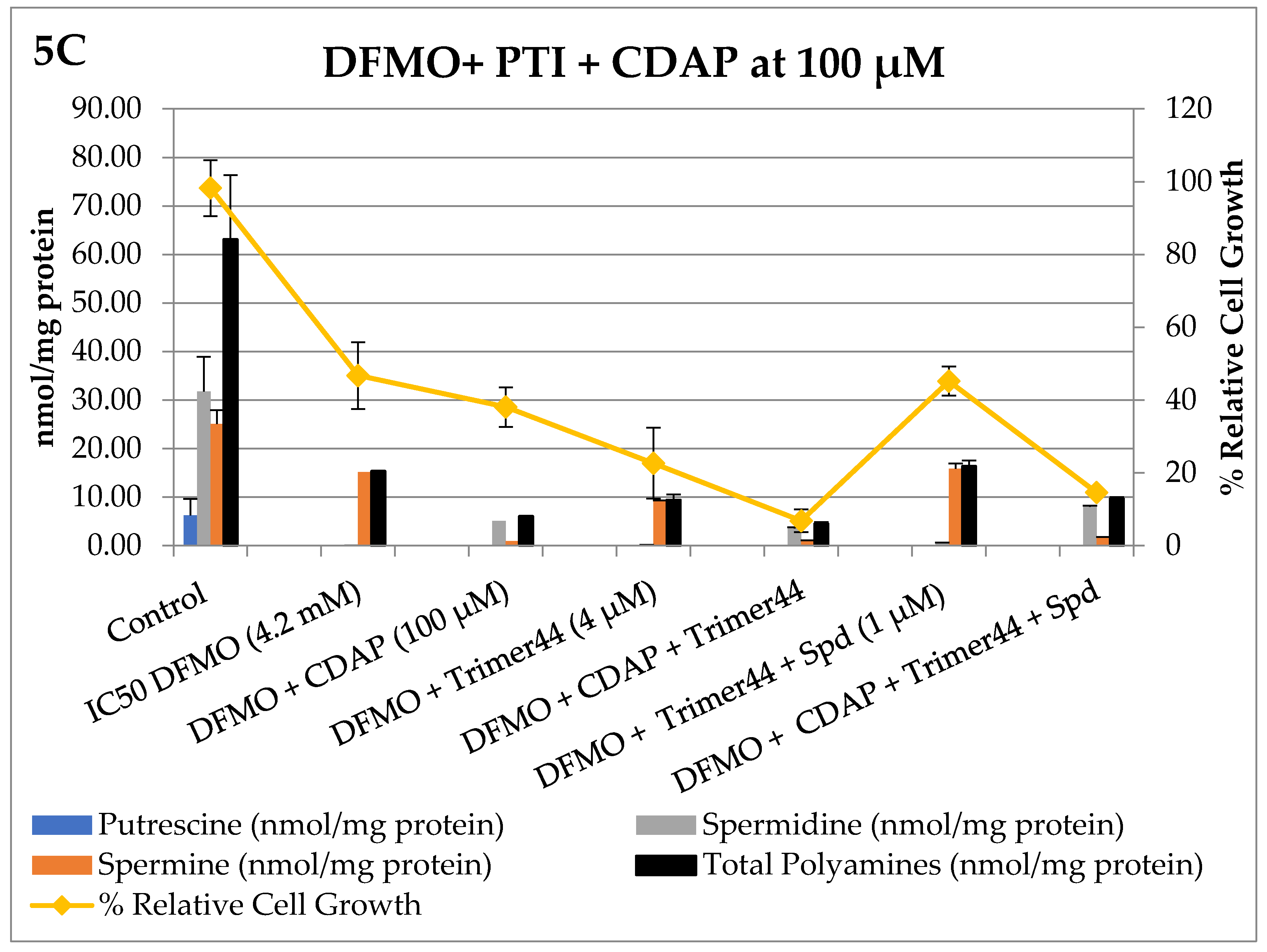
3. Results
Bioevaluation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2016; American Cancer Society: Atlanta, GA, USA, 2016. [Google Scholar]
- Mohammed, A.; Janakiram, N.B.; Madka, V.; Ritchie, R.L.; Brewer, M.; Biddick, L.; Patlolla, J.M.; Sadeghi, M.; Lightfoot, S.; Steele, V.E.; et al. Eflornithine (DFMO) prevents progression of pancreatic cancer by modulating ornithine decarboxylase signaling. Cancer Prev. Res. (Phila) 2014, 7, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Gysin, S.; Rickert, P.; Kastury, K.; McMahon, M. Analysis of genomic DNA alterations and mRNA expression patterns in a panel of human pancreatic cancer cell lines. Genes Chromosom. Cancer 2005, 44, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Loser, C.; Folsch, U.R.; Paprotny, C.; Creutzfeldt, W. Polyamine concentrations in pancreatic tissue, serum, and urine of patients with pancreatic cancer. Pancreas 1990, 5, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hyvonen, M.T.; Merentie, M.; Uimari, A.; Keinanen, T.A.; Janne, J.; Alhonen, L. Mechanisms of polyamine catabolism-induced acute pancreatitis. Biochem. Soc. Trans. 2007, 35, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Loser, C.; Folsch, U.R.; Cleffmann, U.; Nustede, R.; Creutzfeldt, W. Role of ornithine decarboxylase and polyamines in camostate (Foy-305)-induced pancreatic growth in rats. Digestion 1989, 43, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Patel, A.; Skruber, K.; Geerts, D.; Altomare, D.A.; Phanstiel, O., IV. ATP13A3 and Caveolin-1 as Potential Biomarkers for Difluoromethylornithine-based therapies in Pancreatic Cancers. Am. J. Cancer Res. 2016, 6, 1231–1252. [Google Scholar] [PubMed]
- Muth, A.; Madan, M.; Archer, J.J.; Ocampo, N.; Rodriguez, L.; Phanstiel, O. Polyamine transport inhibitors: Design, synthesis, and combination therapies with difluoromethylornithine. J. Med. Chem. 2014, 57, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, A.; Takahashi, N.; Beppu, T.; Hosoda, H.; Samejima, K. Effects of inhibitors of spermidine synthase and spermine synthase on polyamine synthesis in rat tissues. Biochem. Pharmacol. 1993, 45, 1897–1903. [Google Scholar] [CrossRef]
- He, Y.; Shimogori, T.; Kashiwagi, K.; Shirahata, A.; Igarashi, K. Inhibition of cell growth by combination of alpha-difluoromethylornithine and an inhibitor of spermine synthase. J. Biochem. 1995, 117, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.; Kamel, J.; Kaur, N.; Shicora, A.C.; Ayene, I.S.; Gilmour, S.K.; Phanstiel, O. Development of polyamine transport ligands with improved metabolic stability and selectivity against specific human cancers. J. Med. Chem. 2013, 56, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Minocha, S.C.; Minocha, R.; Robie, C.A. High-performance liquid chromatographic method for the determination of dansyl-polyamines. J. Chromatogr. 1990, 511, 177–183. [Google Scholar] [CrossRef]
- Evans, C.H.; Lee, T.S.; Flugelman, A.A. Spermine-directed immunosuppression of cervical carcinoma cell sensitivity to a majority of lymphokine-activated killer lymphocyte cytotoxicity. Nat. Immun. 1995, 14, 157–163. [Google Scholar] [PubMed]
- Kano, Y.; Soda, K.; Nakamura, T.; Saitoh, M.; Kawakami, M.; Konishi, F. Increased blood spermine levels decrease the cytotoxic activity of lymphokine-activated killer cells: A novel mechanism of cancer evasion. Cancer Immunol. Immunother. 2007, 56, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Nishimoto, N.; Uhlinger, D.J.; Igarashi, K.; Takeshita, M.; Tamura, M. Spermine suppresses the activation of human neutrophil NADPH oxidase in cell-free and semi-recombinant systems. Biochem. J. 1996, 313, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Nakamura, T.; Kasono, K.; Kawakami, M.; Konishi, F. Spermine, a natural polyamine, suppresses LFA-1 expression on human lymphocyte. J. Immunol. 2005, 175, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Borovikova, L.V.; Wang, H.; Metz, C.; Tracey, K.J. Spermine inhibition of monocyte activation and inflammation. Mol. Med. 1999, 5, 595–605. [Google Scholar] [PubMed]
- Zhang, M.; Wang, H.; Tracey, K.J. Regulation of macrophage activation and inflammation by spermine: A new chapter in an old story. Crit. Care Med. 2000, 28, N60–N66. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, A.; Merkow, J.; Edil, B.H.; Zhu, Y. Immunotherapy for pancreatic ductal adenocarcinoma: An overview of clinical trials. Chin. J. Cancer Res. 2015, 27, 376–391. [Google Scholar] [PubMed]
- Kunk, P.R.; Bauer, T.W.; Slingluff, C.L.; Rahma, O.E. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J. Immunother. Cancer 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Coward, J.K.; Talekar, R.R.; Secrist, J.A., 3rd. Effects of certain 5′-substituted adenosines on polyamine synthesis: Selective inhibitors of spermine synthase. Biochemistry 1986, 25, 4091–4097. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Coward, J.K. Effect of N-(n-butyl)-1,3-diaminopropane on polyamine metabolism, cell growth and sensitivity to chloroethylating agents. Biochem. Pharmacol. 1993, 46, 717–724. [Google Scholar] [CrossRef]
- Pegg, A.E.; Wechter, R.; Poulin, R.; Woster, P.M.; Coward, J.K. Effect of S-adenosyl-1,12-diamino-3-thio-9-azadodecane, a multisubstrate adduct inhibitor of spermine synthase, on polyamine metabolism in mammalian cells. Biochemistry 1989, 28, 8446–8453. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; Shicora, A.C.; Keough, M.P.; Snook, A.E.; Burns, M.R.; Gilmour, S.K. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol. Res. 2014, 2, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.S.; Burns, M.R.; Gilmour, S.K. Polyamine blockade promotes antitumor immunity. Oncoimmunology 2014, 3, e27360. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.; Minton, A.; Peters, M.; Phanstiel, O.; Gilmour, S. A Novel Polyamine Blockade Therapy Activates an Anti-Tumor Immune Response. Oncotarget 2017, 8, 84140–84152. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Casero, R.A.; Soulet, D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 2012, 42, 711–723. [Google Scholar] [CrossRef] [PubMed]




| Experiment | DFMO (mM) | Spermidine nmoles/mg Protein | Spermine nmoles/mg Protein | Total Polyamines nmoles/mg Protein | Spd/Spm Ratio |
|---|---|---|---|---|---|
| Control | 0 | 13.0 ± 1.6 | 10.1 ± 0.9 | 23.1 | 1.29 |
| DFMO | 0.1 | 12.0 ± 2.8 | 13.4 ± 4.3 | 25.3 | 0.90 |
| DFMO | 0.5 | 0.7 ± 0.1 | 15.3 ± 2.3 | 16.1 | 0.05 |
| DFMO | 1 | 0.2 ± 0.0 | 14.9 ± 0.8 | 15.2 | 0.01 |
| DFMO | 2 | 0.6 ± 0.7 | 9.2 ± 3.9 | 9.8 | 0.06 |
| DFMO | 3 | 0.1 ± 0.0 | 6.1 ± 0.2 | 6.2 | 0.01 |
| DFMO | 4.2 | 0.2 ± 0.0 | 7.7 ± 1.0 | 7.9 | 0.02 |
| Experiment | trimer44 (µM) | Spermidine nmoles/mg Protein | Spermine nmoles/mg Protein | Total Polyamines nmoles/mg Protein | Spd/Spm Ratio |
|---|---|---|---|---|---|
| Control | 0 | 14.0 ± 3.6 | 8.5 ± 1.8 | 22.5 | 1.65 |
| trimer 44 | 1 | 17.0 ± 6.3 | 12.9 ± 3.4 | 29.9 | 1.31 |
| trimer 44 | 2 | 12.2 ± 2.8 | 8.9 ± 1.2 | 21.1 | 1.36 |
| trimer 44 | 4 | 12.0 ± 5.3 | 10.4 ± 2.1 | 22.4 | 1.15 |
| trimer 44 | 5 | 11.4 ± 2.5 | 13.5 ± 0.8 | 24.9 | 0.84 |
| trimer 44 | 10 | 5.5 ± 1.5 | 11.2 ± 2.1 | 16.7 | 0.49 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, C.; Thomas, J.; Phanstiel, O., IV. Investigation of Polyamine Metabolism and Homeostasis in Pancreatic Cancers. Med. Sci. 2017, 5, 32. https://doi.org/10.3390/medsci5040032
Massaro C, Thomas J, Phanstiel O IV. Investigation of Polyamine Metabolism and Homeostasis in Pancreatic Cancers. Medical Sciences. 2017; 5(4):32. https://doi.org/10.3390/medsci5040032
Chicago/Turabian StyleMassaro, Chelsea, Jenna Thomas, and Otto Phanstiel, IV. 2017. "Investigation of Polyamine Metabolism and Homeostasis in Pancreatic Cancers" Medical Sciences 5, no. 4: 32. https://doi.org/10.3390/medsci5040032