Androgen Receptor Expression in Thai Breast Cancer Patients
Abstract
:1. Introduction
2. Methods
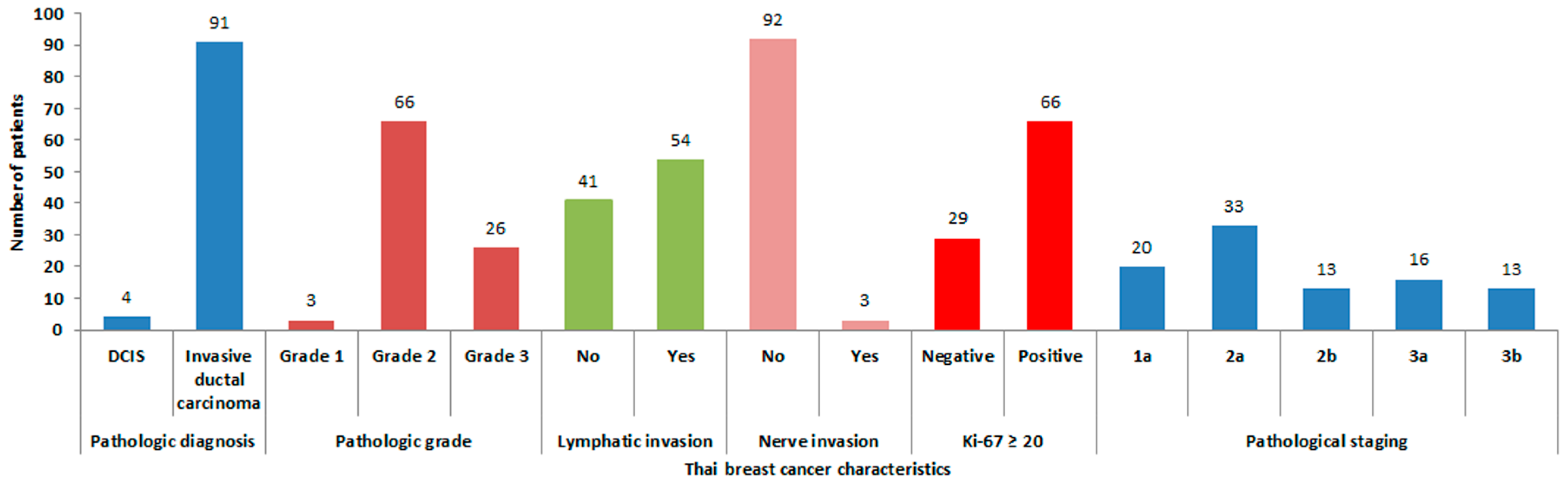
2.1. Thai Breast Cancer Patient and Tumor Characteristics
2.2. Ethical Considerations
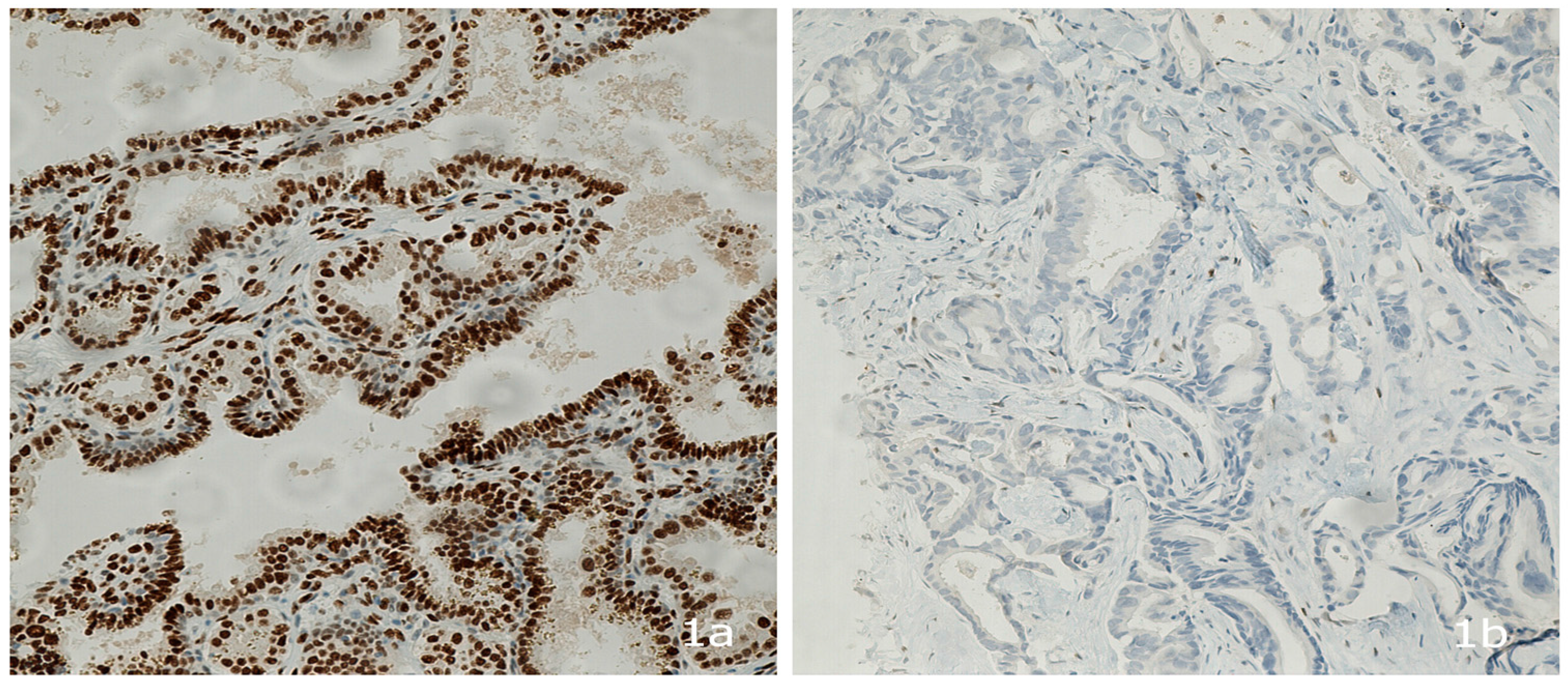
2.3. Androgen Receptor Protein Expression
2.4. Sample Size Calculation for Detecting the Prevalence of AR Expression in Thai Patients
- z = 1.96 at alpha 0.05% or 95% confidence interval
- p = Proportion of androgen receptor positive; 70%, according to Ren et al. [24]
- d = Acceptable error of 10% = 0.01
- n = (1.96 )2 · (0.7) · (1−0.7)/(0.1) · 2 = 81 cases
2.5. Data Analysis
3. Results
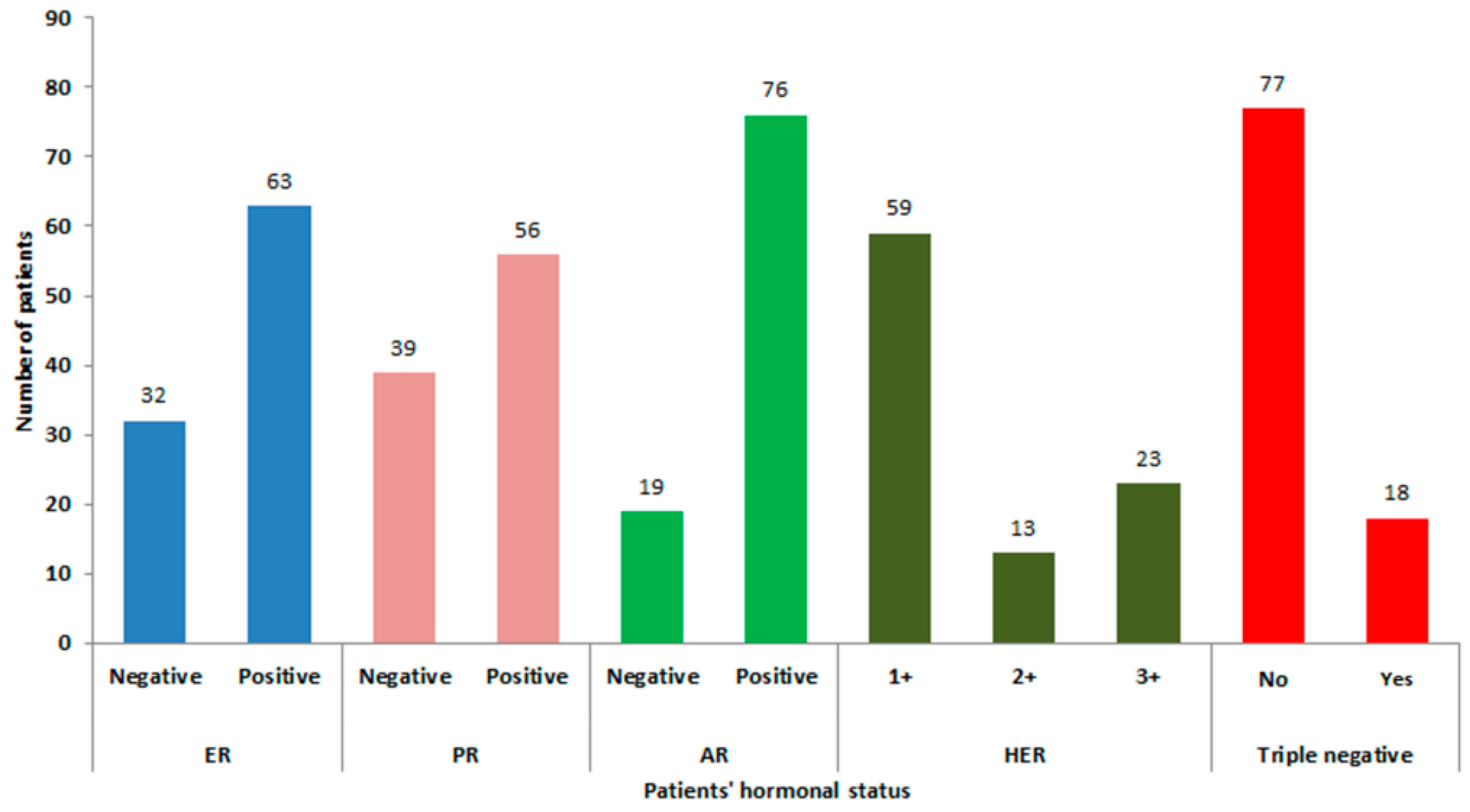
3.1. Patient and Tumor Characteristics and AR Expression
3.2. Association of Tumor Characteristics with AR Expression
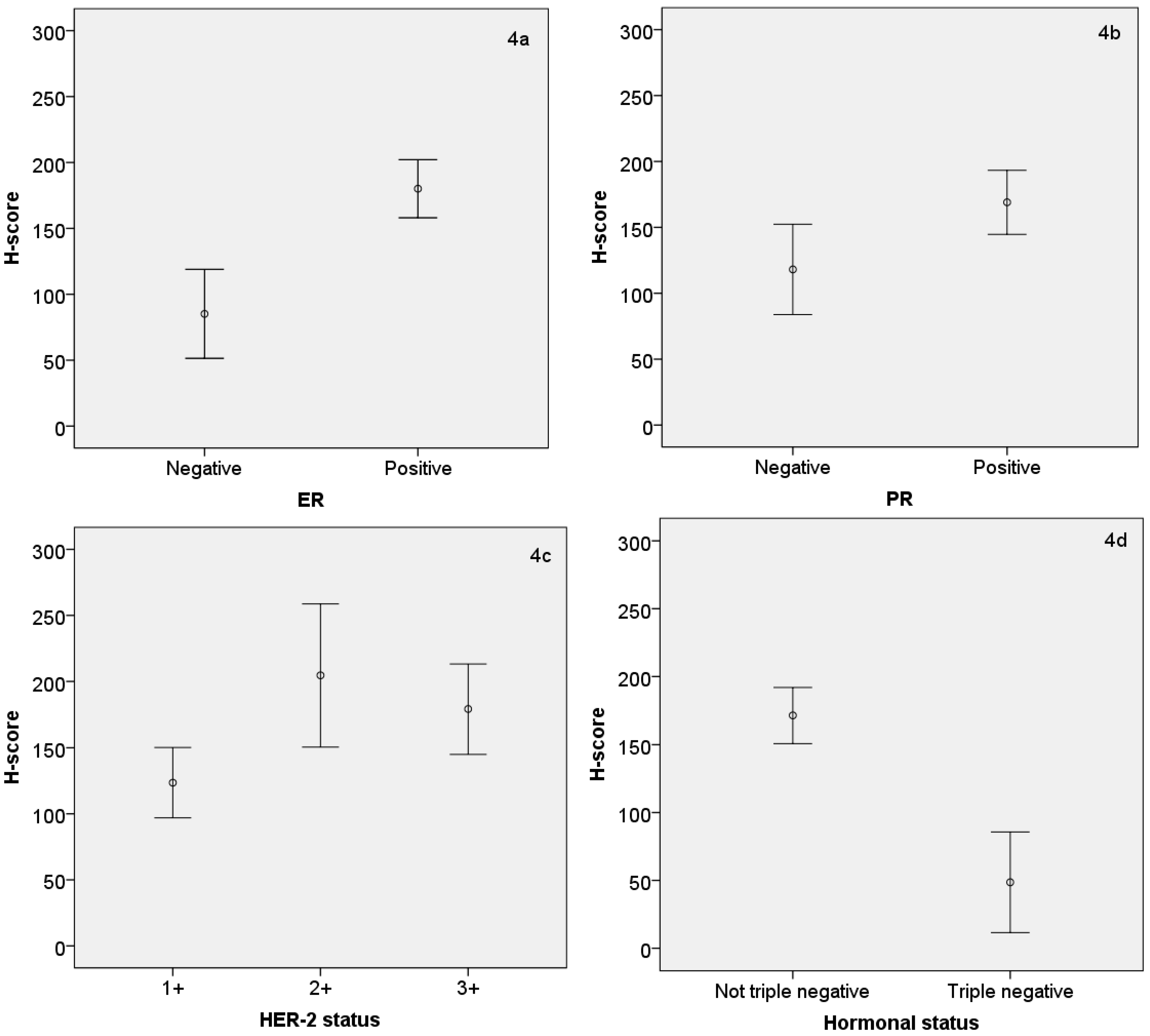
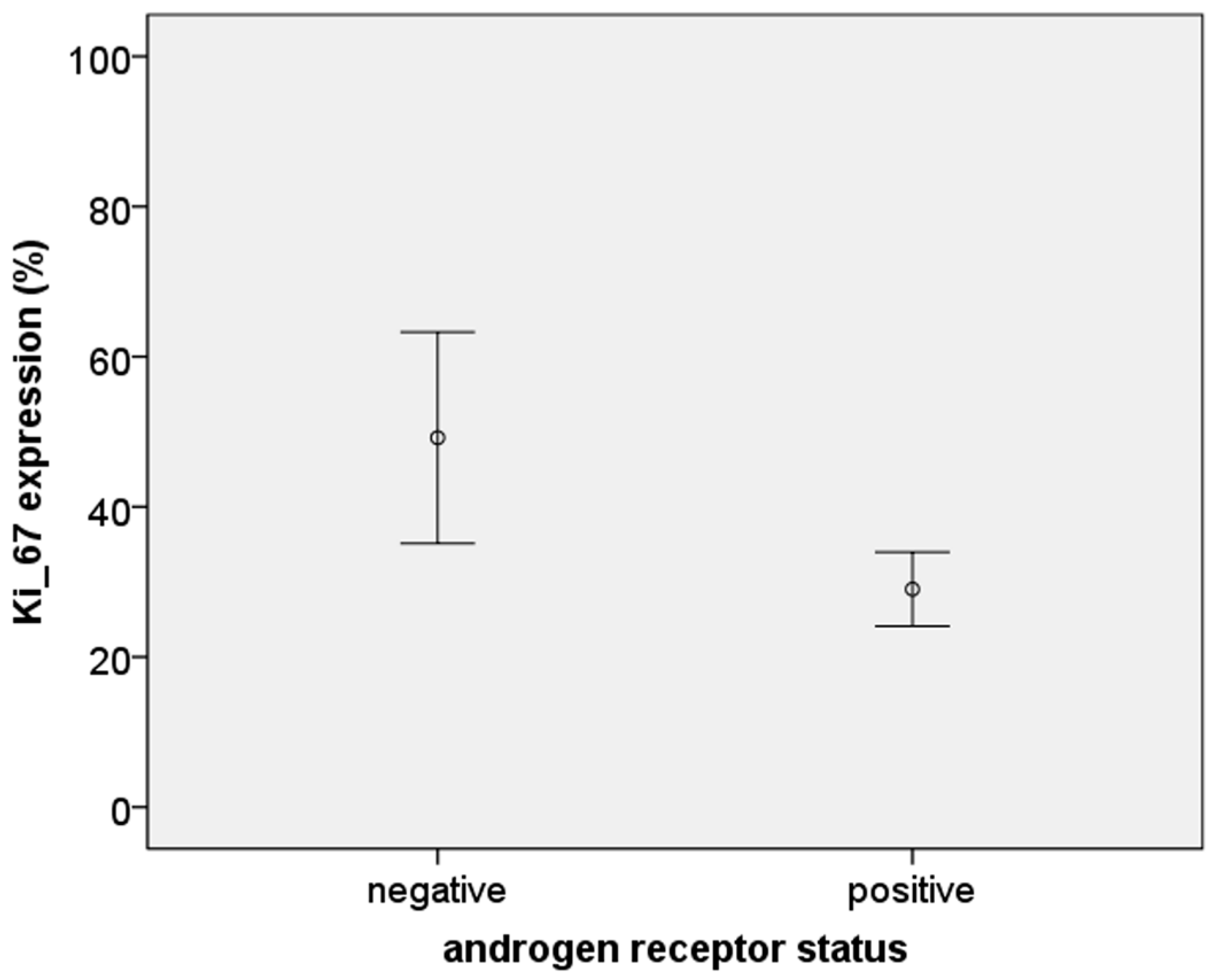
3.3. Relationship between AR Expression (H-Score) and Other Hormonal Receptors and Ki-67
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 4 February 2014).
- The American Cancer Society. Cancer Facts & Figures 2013; The American Cancer Society: Atlanta, GA, USA, 2015; p. 52. [Google Scholar]
- Attasara, P.B.R. Hospital-Based Cancer Registry; National Cancer Institute: Bangkok, Thailand, 2007. [Google Scholar]
- Tung, N. What is the optimal endocrine therapy for postmenopausal women with hormone receptor-positive early breast cancer? J. Clin. Oncol. 2013, 31, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Newman, L.A. Management of patients with locally advanced breast cancer. Surg. Clin. N. Am. 2007, 87, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.D.; Chen, C.M.; Chen, X.S.; Liu, G.Y.; Di, G.H.; Wu, J.; Lu, J.S.; Yang, W.T.; Chen, J.Y.; Shao, Z.M.; et al. Neoadjuvant chemotherapy with vinorelbine-containing regimens in elderly patients with locally advanced breast cancer. Anticancer Res. 2008, 28, 3093–3097. [Google Scholar] [PubMed]
- McGuire, W.L. Estrogen receptors in human breast cancer. J. Clin. Investig. 1973, 52, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.M.; Neven, P.; Giobbie-Hurder, A.; Goldhirsch, A.; Ejlertsen, B.; Mauriac, L.; Forbes, J.F.; Smith, I.; Lang, I.; Wardley, A.; et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: The BIG 1–98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011, 12, 1101–1108. [Google Scholar] [CrossRef]
- Ortmann, O.; Pagani, O.; Jones, A.; Maass, N.; Noss, D.; Rugo, H.; van de Velde, C.; Aapro, M.; Coleman, R. Which factors should be taken into account in perimenopausal women with early breast cancer who may become eligible for an aromatase inhibitor? Recommendations of an expert panel. Cancer Treat. Rev. 2010, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Wang, S.S.; Gao, Y.; Su, Z.Y.; Luo, W.B.; Guan, Z.Z. Clinical characteristics and prognosis of triple-negative breast cancer: A report of 305 cases. Ai Zheng 2008, 27, 561–565. [Google Scholar] [PubMed]
- Kaplan, H.G.; Malmgren, J.A. Impact of triple negative phenotype on breast cancer prognosis. Breast J. 2008, 14, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lara-Medina, F.; Perez-Sanchez, V.; Saavedra-Perez, D.; Blake-Cerda, M.; Arce, C.; Motola-Kuba, D.; Villarreal-Garza, C.; Gonzalez-Angulo, A.M.; Bargallo, E.; Aguilar, J.L.; et al. Triple-negative breast cancer in Hispanic patients: High prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer 2011, 117, 3658–3669. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, R.; Joseph, T.; Dhadda, A.; Chaturvedi, A.; Upadhyay, S. Prognosis of patients with triple-negative breast cancer and brain metastasis. Clin. Oncol. (R. Coll. Radiol.) 2009, 21, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Nahleh, Z. Androgen receptor as a target for the treatment of hormone receptor-negative breast cancer: An unchartered territory. Future Oncol. 2008, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Montiel, R.; Li, B.; Huang, E.; Zeng, L.; Huang, Y. Association between androgen receptor gene CAG repeat polymorphism and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2010, 124, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Chottanapund, S.; Van Duursen, M.B.; Navasumrit, P.; Hunsonti, P.; Timtavorn, S.; Ruchirawat, M.; Van den Berg, M. Effect of androgens on different breast cancer cells co-cultured with or without breast adipose fibroblasts. J. Steroid Biochem. Mol. Biol. 2013, 138, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.O.; Corte, M.D.; Junquera, S.; Bongera, M.; Rodriguez, J.C.; Vizoso, F.J. Expression of androgen receptor and two androgen-induced proteins (apolipoprotein D and pepsinogen C) in ductal carcinoma in situ of the breast. Histopathology 2007, 50, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, P.; Fassan, M.; Cascione, L.; Guler, G.; Balci, S.; Irkkan, C.; Paisie, C.; Lovat, F.; Morrison, C.; Zhang, J.; et al. Androgen receptor status is a prognostic marker in non-basal triple negative breast cancers and determines novel therapeutic options. PLoS ONE 2014, 9, e88525. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, L.A.; Dabbs, D.J.; Beriwal, S.; Striebel, J.M.; Bhargava, R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 2009, 23, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.A.; Dabbs, D.J.; Cooper, K.L.; Amin, M.; Jones, T.E.; Jones, M.W.; Chivukula, M.; Trucco, G.A.; Bhargava, R. Interobserver agreement among pathologists for semiquantitative hormone receptor scoring in breast carcinoma. Am. J. Clin. Pathol. 2012, 138, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.R.; Yagle, K.J.; Swanson, P.E.; Krohn, K.A.; Rajendran, J.G. A robust automated measure of average antibody staining in immunohistochemistry images. J. Histochem. Cytochem. 2009, 58, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhang, L.; Ruoff, R.; Ha, S.; Wang, J.; Jain, S.; Reuter, V.; Gerald, W.; Giri, D.D.; Melamed, J.; et al. Expression of androgen receptor and its phosphorylated forms in breast cancer progression. Cancer 2013, 119, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society. Cancer Facts & Figures; The American Cancer Society: Atlanta, GA, USA, 2013. [Google Scholar]
- Li, C.I.; Daling, J.R.; Malone, K.E. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J. Clin. Oncol. 2003, 21, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; McGregor, S.R. Prevalence and management of HER2/neu-positive early breast cancer in a single institution following availability of adjuvant trastuzumab. Intern. Med. J. 2011, 42, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Khan, R.; Bui, M.M. A review of triple-negative breast cancer. Cancer Control 2010, 17, 173–176. [Google Scholar] [PubMed]
- Swede, H.; Gregorio, D.I.; Tannenbaum, S.H.; Brockmeyer, J.A.; Ambrosone, C.; Wilson, L.L.; Pensa, M.A.; Gonsalves, L.; Stevens, R.G.; Runowicz, C.D. Prevalence and prognostic role of triple-negative breast cancer by race: A surveillance study. Clin. Breast Cancer 2011, 11, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, X.; Liu, Y.; Liu, G.; Dong, G. Ki67 as a predictor of poor prognosis in patients with triple-negative breast cancer. Oncol. Lett. 2014, 9, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Vandorpe, T.; Smeets, A.; Forceville, K.; Brouwers, B.; Neven, P.; Janssens, H.; Deraedt, K.; Moerman, P.; Van Calster, B.; et al. Triple negative breast cancer: Clinical characteristics in the different histological subtypes. Breast 2013, 22, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Traina, T.A. Triple-negative breast cancer: Role of the androgen receptor. Cancer J. 2010, 16, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Ahn, S.; Cheney, M.D.; Yepuru, M.; Miller, D.D.; Steiner, M.S.; Dalton, J.T. Selective androgen receptor modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial: Mesenchymal stem cell signaling. PLoS ONE 2014, 9, e103202. [Google Scholar] [CrossRef] [PubMed]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; Marotti, J.D.; Hankinson, S.E.; Colditz, G.A.; Tamimi, R.M. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin. Cancer Res. 2011, 17, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Shi, Y.X.; Li, Z.M.; Jiang, W.Q. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin. J. Cancer 2010, 29, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Localio, R.; Rebbeck, T.R. Evaluating bias due to population stratification in epidemiologic studies of gene-gene or gene-environment interactions. Cancer Epidemiol. Biomark. Prev. 2006, 15, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Millikan, R.C. Re: Population stratification in epidemiologic studies of common genetic variants and cancer: Quantification of bias. J. Natl. Cancer Inst. 2001, 93, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.; Dainese, E.; Caprara, G.; Rocca, P.C.; Massarelli, G.; Tot, T.; Capella, C.; Eusebi, V. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch. 2005, 447, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Hai, E.; Matsumoto, K.; Ikeda, K.; Tokunaga, S.; Nagahara, H.; Sakurai, K.; Inoue, T.; Nishiguchi, Y. Androgen receptor expression in breast cancer: Relationship with clinicopathological factors and biomarkers. Int. J. Clin. Oncol. 2008, 13, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.O.; Corte, M.D.; Vazquez, J.; Junquera, S.; Sanchez, R.; Alvarez, A.C.; Rodriguez, J.C.; Lamelas, M.L.; Vizoso, F.J. Androgen receptor expression in breast cancer: Relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer 2008, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Soreide, J.A.; Lea, O.A.; Varhaug, J.E.; Skarstein, A.; Kvinnsland, S. Androgen receptors in operable breast cancer: Relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatment. Eur. J. Surg. Oncol. 1992, 18, 112–118. [Google Scholar] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Inwald, E.C.; Klinkhammer-Schalke, M.; Hofstadter, F.; Zeman, F.; Koller, M.; Gerstenhauer, M.; Ortmann, O. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, A.; Perrone, V.; Marone, A. Clinical considerations on the use of a new synthetic androgen, Calusterone, in the treatment of advanced breast cancer. Miner. Med. 1977, 68, 2499–2504. [Google Scholar]
- Scholl, A. Therapeutic results of androgen treatment and ovariectomy in breast cancer. Norm Pathol. Anat. (Stuttg.) 1975, 30, 1–46. [Google Scholar] [PubMed]
- Battelli, T.; Bonsignori, M.; Manocchi, P.; Perilli, A. Skin metastases of male breast cancer improved after androgen treatment. Miner. Med. 1977, 68, 1663–1666. [Google Scholar]
- Kennedy, B.J. Fluoxymesterone therapy in advanced breast cancer. N. Engl. J. Med. 1958, 259, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr. Relat. Cancer 2014, 21, R235–R246. [Google Scholar] [CrossRef] [PubMed]
- Garay, J.P.; Park, B.H. Androgen receptor as a targeted therapy for breast cancer. Am. J. Cancer Res. 2012, 2, 434–435. [Google Scholar] [PubMed]
- Dubsky, P.C.; Jakesz, R.; Mlineritsch, B.; Postlberger, S.; Samonigg, H.; Kwasny, W.; Tausch, C.; Stoger, H.; Haider, K.; Fitzal, F.; et al. Tamoxifen and anastrozole as a sequencing strategy: A randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian Breast and Colorectal Cancer Study Group. J. Clin. Oncol. 2012, 30, 722–728. [Google Scholar] [CrossRef] [PubMed]
| Factors | Androgen Status | p Value | ||
|---|---|---|---|---|
| Negative (n = 19) | Positive (n = 76) | |||
| Menstrual status | Pre-menopause | 13 | 30 | 0.092 |
| Peri-menopause | 1 | 10 | ||
| Menopause | 5 | 36 | ||
| ER | Negative | 15 | 17 | <0.001 |
| Positive | 4 | 59 | ||
| PR | Negative | 14 | 25 | <0.001 |
| Positive | 5 | 51 | ||
| HER2 | 1+ | 17 | 42 | 0.022 |
| 2+ | 1 | 12 | ||
| 3+ | 1 | 22 | ||
| Ki-67 ≥20 | Negative | 2 | 27 | 0.034 |
| Positive | 17 | 49 | ||
| Hormonal status | Not triple negative | 7 | 70 | <0.001 |
| Triple negative | 12 | 6 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chottanapund, S.; Van Duursen, M.B.M.; Ratchaworapong, K.; Navasumrit, P.; Ruchirawat, M.; Van den Berg, M. Androgen Receptor Expression in Thai Breast Cancer Patients. Med. Sci. 2016, 4, 15. https://doi.org/10.3390/medsci4030015
Chottanapund S, Van Duursen MBM, Ratchaworapong K, Navasumrit P, Ruchirawat M, Van den Berg M. Androgen Receptor Expression in Thai Breast Cancer Patients. Medical Sciences. 2016; 4(3):15. https://doi.org/10.3390/medsci4030015
Chicago/Turabian StyleChottanapund, Suthat, M. B. M. Van Duursen, Kumpol Ratchaworapong, Panida Navasumrit, Mathuros Ruchirawat, and Martin Van den Berg. 2016. "Androgen Receptor Expression in Thai Breast Cancer Patients" Medical Sciences 4, no. 3: 15. https://doi.org/10.3390/medsci4030015






