Stratum Corneum Hydration and Skin Surface pH Variation Indicate that Organ Blood Flow Is Regulated by Meridian Activity at Certain Hours
Abstract
:1. Introduction
2. Experimental Section
2.1. Test Preparations
2.2. Participants and Experimental Design
2.3. Data Generation and Analysis
2.3.1. Spindle-Shaped Curve
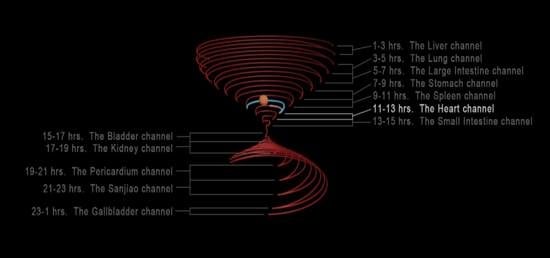
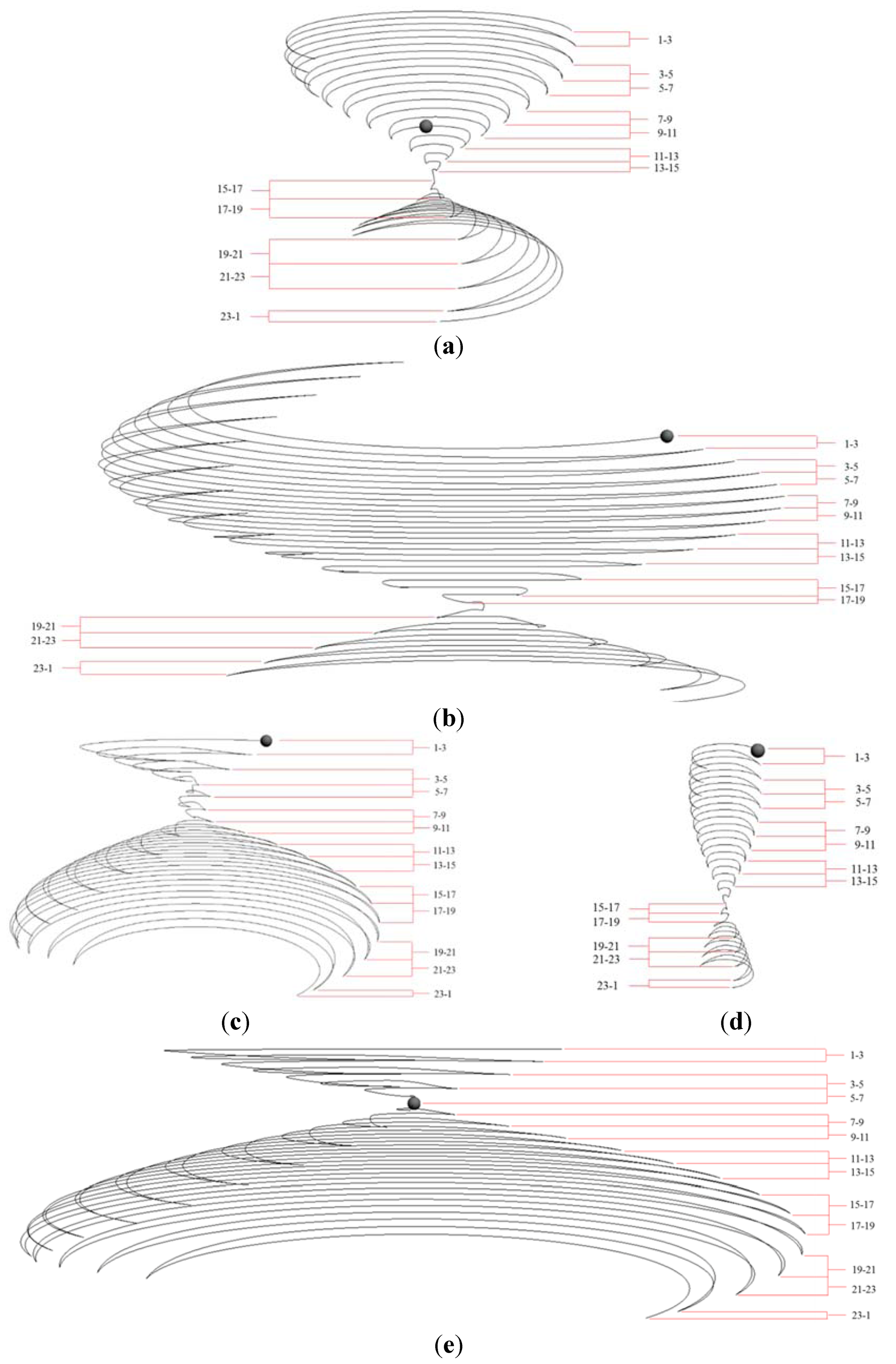
2.3.2. Design Procedure and Concepts of an Hourglass Trajectory Diagram

3. Results and Discussion
3.1. Energy Dependency for Organ Survival
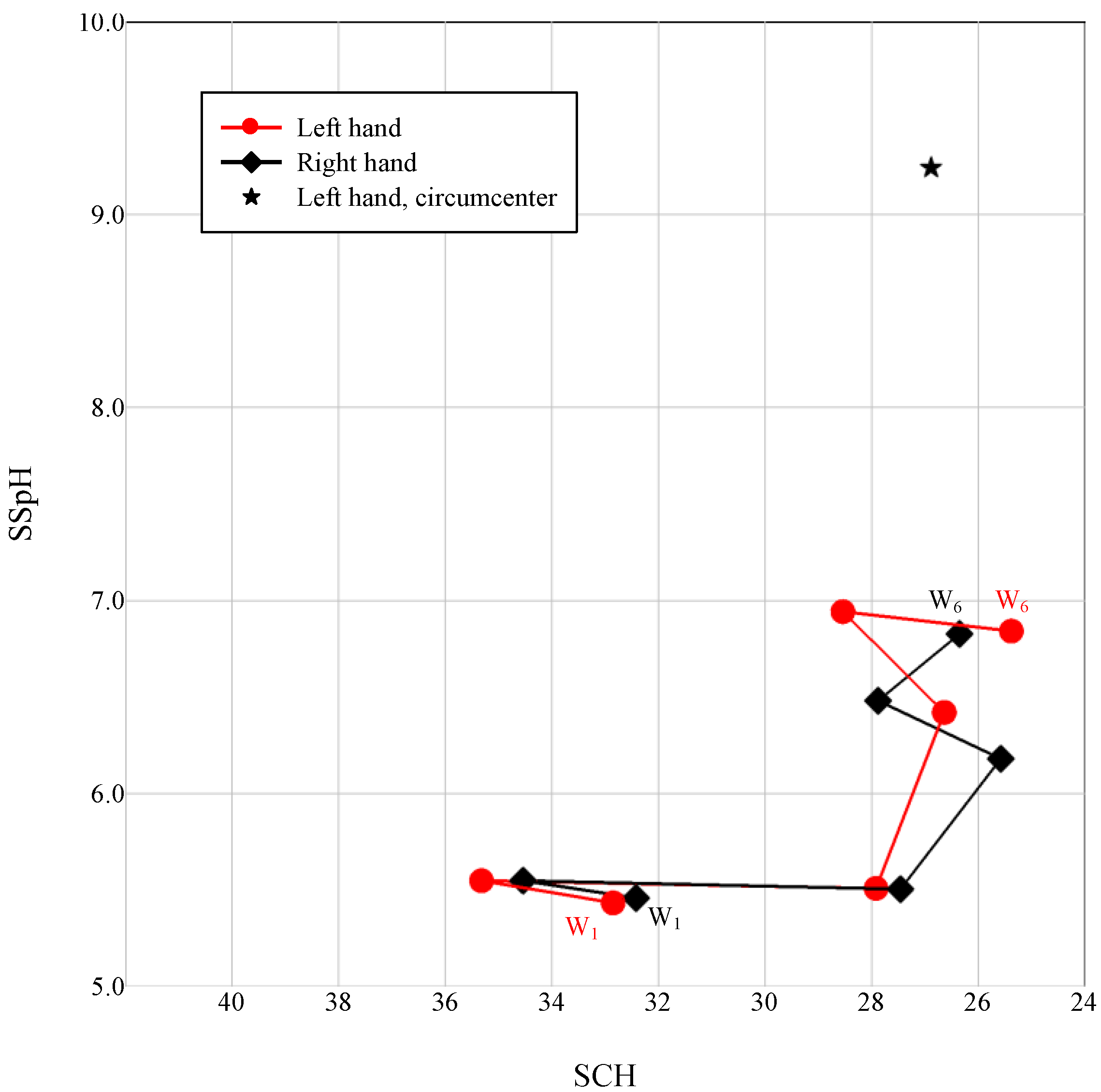
3.2. Case Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Widmaier, E.P.; Raff, H.; Strang, K.T. Regulation of organic metabolism and energy balance. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; Widmaier, E.P., Raff, H., Strang, K.T., Eds.; McGraw-Hill: New York, NY, USA, 2014; pp. 572–601. [Google Scholar]
- Storch, K.F.; Lipan, O.; Leykin, I.; Viswanathan, N.; Davis, F.C.; Wong, W.H.; Weitz, C.J. Extensive and divergent circadian gene expression in liver and heart. Nature 2002, 417, 78–83. [Google Scholar] [PubMed]
- Bray, M.S.; Shaw, C.A.; Moore, M.W.; Garcia, R.A.; Zanquetta, M.M.; Durgan, D.J.; Jeong, W.J.; Tsai, J.Y.; Bugger, H.; Zhang, D.; et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 1036–1047. [Google Scholar]
- Widmaier, E.P.; Raff, H.; Strang, K.T. Cardiovascular and physiology. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; Widmaier, E.P., Raff, H., Strang, K.T., Eds.; McGraw-Hill: New York, NY, USA, 2014; pp. 362–445. [Google Scholar]
- Widmaier, E.P.; Raff, H.; Strang, K.T. The endocrine system. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; Widmaier, E.P., Raff, H., Strang, K.T., Eds.; McGraw-Hill: New York, NY, USA, 2014; pp. 319–361. [Google Scholar]
- Widmaier, E.P.; Raff, H.; Strang, K.T. Neuronal signaling and the structure of the nervous system. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; Widmaier, E.P., Raff, H., Strang, K.T., Eds.; McGraw-Hill: New York, USA, 2014; pp. 138–190. [Google Scholar]
- Widmaier, E.P.; Raff, H.; Strang, K.T. Consciousness, the Brain, and Behavior. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; Widmaier, E.P., Raff, H., Strang, K.T., Eds.; McGraw-Hill: New York, NY, USA, 2014; pp. 234–256. [Google Scholar]
- Cicek, D.; Kandi, B.; Berilgen, M.S.; Bulut, S.; Tekatas, A.; Dertlioglu, S.B.; Ozel, S.; Saral, Y. Does autonomic dysfunction play a role in atopic dermatitis? Br. J. Dermatol. 2008, 159, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Pérgola, P.E.; Kellogg, D.L.; Johnson, J.M.; Kosiba, W.A.; Solomon, D.E. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am. J. Physiol. 1993, 265, 785–792. [Google Scholar]
- Dung, H.C. Acupuncture points of the brachial plexus. Am. J. Chin. Med. 1985, 13, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Stücker, M.; Struk, A.; Altmeyer, P.; Herde, M.; Baumgärtl, H.; Lübbers, D.W. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J. Physiol. 2002, 538, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Deadman, P.; Al-Khafaji, M.; Kevin, B. A Manual of Acupuncture. Available online: https://amanualofacupuncture.com/about-manual-of-acupuncture (accessed on 26 November 2014).
- Whitehead, K.; Hedges, J. Photodegradation and photosensitization of mycosporine-like amino acids. J. Photochem. Photobiol. B 2005, 80, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.F.; Chou, H.N.; Sung, P.J. Porphyra-334 isolated from the marine algae Bangia atropurpurea: Conformational performance for energy conversion. Mar. Drugs 2014, 12, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, M.; Gorbushina, A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006, 255, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Schürch, C.; Zülli, F. UVA-screening compounds from red algae protect against photoageing. Personal Care 2004, 1, 29–31. [Google Scholar]
- Perez, J.C. Codon populations in single-stranded whole human genome DNA are fractal and fine-tuned by the Golden Ratio 1.618. Interdiscip. Sci. 2010, 2, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H. Epidermal turnover time. J. Dermatol. Sci. 1994, 8, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zeng, Q.Q.; Peng, Z.Y. Explore the relationship between record of ZangFu and Jingmai in Huangdi Neijing and systematic anatomy entity. J. Liaoning Univ. TCM 2010, 12, 85–88. (In Chinese) [Google Scholar]
- Somers, V.K.; Dyken, M.E.; Mark, A.L.; Abboud, F.M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrin. Met. 2010, 21, 277–286. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Y.; Huang, W.Z.; Chen, S.X.; Zeng, Q.Q.; Chen, Q.S.; Li, W.; Sun, Z.F. Cardiovascular system and the nervous system has the identity in Huangdi Neijing–Comparing the difference between traditional Chinese medicine and modern medicine in the organizational structure of organs and explore the Luo Entity. J. Liaoning Univ. TCM 2011, 13, 115–118. (In Chinese) [Google Scholar]
- Widmaier, E.P.; Raff, H.; Strang, K.T. The kidneys and regulation of water and inorganic ions. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; Widmaier, E.P., Raff, H., Strang, K.T., Eds.; McGraw-Hill: New York, NY, USA, 2014; pp. 490–532. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, L.-F.; Chou, H.-N.; Hsu, C.-K.; Chou, H.-S.; Sung, P.-J.; Chen, F.-G. Stratum Corneum Hydration and Skin Surface pH Variation Indicate that Organ Blood Flow Is Regulated by Meridian Activity at Certain Hours. Med. Sci. 2014, 2, 161-172. https://doi.org/10.3390/medsci2040161
Chuang L-F, Chou H-N, Hsu C-K, Chou H-S, Sung P-J, Chen F-G. Stratum Corneum Hydration and Skin Surface pH Variation Indicate that Organ Blood Flow Is Regulated by Meridian Activity at Certain Hours. Medical Sciences. 2014; 2(4):161-172. https://doi.org/10.3390/medsci2040161
Chicago/Turabian StyleChuang, Li-Fan, Hong-Nong Chou, Chin-Kong Hsu, Hung-Shih Chou, Ping-Jyun Sung, and Fu-Gin Chen. 2014. "Stratum Corneum Hydration and Skin Surface pH Variation Indicate that Organ Blood Flow Is Regulated by Meridian Activity at Certain Hours" Medical Sciences 2, no. 4: 161-172. https://doi.org/10.3390/medsci2040161