1. Introduction
Tumor cell lines represent a unique tool for investigating both tumor biology/profiles and the mechanisms underlying tumor responsiveness to anticancer therapies [
1,
2,
3]. The establishment of tumor cell lines is extremely difficult and the success rate is low and unpredictable [
4]. Although tumors exhibit apparent autonomy from normal regulatory control
in vivo, they often fail to grow when cultured
in vitro [
2].
Several different cell types, other than neoplastic cells, may be obtained from tumor samples, including connective-tissue fibroblasts, infiltrating lymphocytes and elements of normal tissue from which the neoplasia arose. When trying to establish a tumor cell line, the major problem is contamination by fibroblasts or other types of endothelial cells, which grow readily in culture and may also respond to tumor-derived mitogenic factors [
5]. Furthermore, in primary tumor cultures many cells may not be capable of propagation due to genetic or phenotypic aberrations, terminal differentiation or nutritional insufficiency. Nevertheless, some primary cultures can be subcultured, opening up major research possibilities [
6,
7].
Even though established cell lines are commercially available from a number of sources, there is a need for simple procedures to generate solid tumor cell lines that retain the characteristics of the original tumor. This would help to define, for example tumor associated antigens (TAAs) as an aid in developing immunotherapeutic approaches [
8,
9,
10].
We report on the development of an efficient methodology to establish primary cultures from tumor tissues and demonstrate how a few simple modifications in cell culture establishment allow the generation of several cell lines from different types of human tumors.
2. Materials and Methods
2.1. Tumor Samples
Samples were obtained from surgical procedures and neoplastic effusions. In both cases fresh tumor material was collected in accordance with a protocol approved by the local Ethics Committee. The vast majority of tumor samples were derived from metastasis (99 out of 115 samples). Surgical materials not required for histopathologic diagnosis, were placed in sterile tubes containing RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 200 U/mL penicillin and 200 μg/mL streptomycin (all from Life Technologies Inc., Paisley, UK). In case of neoplastic effusion, tumor cells were recovered by centrifugation. When possible, a portion of the tumor sample was cryopreserved in freezing medium containing 90% FBS and 10% dimethyl sulphoxide (DMSO) (Wak-Chemie Medical GMBH, Steinbach/TS, Germany), and stored in liquid nitrogen for further studies.
2.2. Establishment of Primary Cell Cultures from Surgical Samples
Two different approaches to obtain viable tumor cells from tumor specimens were utilized, the first included mechanical disaggregation with a cell scraper, while the second, representing an improvement of this methodology, included the use of the Gentle MACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). In the first case, after removal of debris (i.e., fat and necrotic material), surgical samples were cut into 2–4 mm square sections and washed with sterile phosphate buffered saline (PBS, Life Technologies Inc.). Tumor cells were released from the samples by enzymatic treatment with 0.1% collagenase (Sigma Aldrich, Irvine, UK) and 50 U/mL dispase (GIBCO/Invitrogen Life Technologies, Carlsbad, CA, USA), in a 37 °C water bath for at least two hours, agitating every10–20 min. At the end of incubation, the same amount of ice-cold RPMI supplemented with 20% FBS (GIBCO/Invitrogen Life Technologies) was added, the tumor cell suspension was passed through a 85-μm nylon mesh cell strainer (Becton Dickinson, San Jose, CA, USA) and the undigested tissues squeezed with a cell scraper (mechanical disaggregation). In the case of soft tumor samples (i.e., liver metastases) we used only mechanical disaggregation until the tumor tissue was completely dispersed.
Tumor specimens processed with the Gentle MACS Dissociator were treated when necessary, with 0.1% collagenase for an half hour, and then disaggregated using different protocols depending on the tissue characteristics.
After both procedures, tumor cells were passed in filters (Miltenyi Biotec) to remove clusters and then collected by centrifugation at 1000 rpm for 10 min, checked for viability with trypan blue dye exclusion, resuspended at a concentration of 0.5–1 × 106 cells/mL of CellGro SCGM (Cell Genix, Freiburg, Germany),supplemented with 20% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (complete medium) (all from Life Technologies Inc.) and cultured in 25 cm2 tissue flasks (Corning, Stone Staffordshire, UK) at 37 °C and 5% CO2. Viable tumor cells attached to the flask within 12–24 h. At the first medium change, rather than discarding medium containing unattached cells that may grow and provide a backup culture, we put this into a fresh flask. Cultures at 75% to 100% confluence were selected for subculture by trypsinization with 0.25% trypsin and 0.02% EDTA (Life Technologies Inc.) in a calcium/magnesium-free balanced solution. The culture medium was changed twice a week and cellular homogeneity evaluated microscopically every 24–48 h. When possible, early passage and late passage primary cultures were frozen in 90% FBS and 10% DMSO and stored in liquid nitrogen for further experiments.
2.3. Establishment of Primary Cell Cultures from Pleural or Peritoneal Effusions
Under sterile conditions, neoplastic effusions were transferred to 50 mL sterile tubes (Corning, Shiphol Rijk, The Netherlands) and centrifuged at 1200 rpm for 10 min. The supernatant was discarded and cells were washed and resuspended in complete medium. After evaluation of viability, the cells were seeded at a density of 3 × 106 cells/mL in 25 cm2 tissue culture flasks (Corning) at 37 °C and 5% CO2. Culture medium was changed twice a week and cellular homogeneity was evaluated microscopically every 24–48 h. When cultures reached sub-confluence, cells were collected as previously described and subcultured at a dilution of 1:3 until they began to grow rapidly, and then serially subcultured at a dilution of 1:5 every three days.
2.4. Ascite-Derived Ovarian Cancer Cells
Approximately 200 mL of ascitic fluid was obtained from ten patients. Ascitic fluid was transferred to 50 mL tubes, centrifuged and resuspended in an appropriate volume with PBS (Gibco, Grand Island, NY, USA). After pathology evaluation for the presence of tumor cells, a fraction of the cells was cryopreserved for later use and seeded in 25 cm2 flasks at a concentration of 0.5 × 106/mL.
Alternatively, mononuclear cells were isolated by Ficoll gradient separation and then incubated with the CD326 (EpCAM) Tumor Cell Enrichment and Detection Kit (Miltenyi Biotec) according to the manufacturer’s instructions. Cells isolated from ascitic fluid were phenotypically and histochemically characterized to confirm their neoplastic origin and then cryopreserved or alternatively cultured as described above, and further expanded. In selected cases, when a large amount of cells was available, cell culture purity was improved by negative selection with anti-fibroblast microbeads (MiniMacs, Miltenyi Biotec), performed according to the manufacturer’s instructions. At every fifth passage a portion of the cells was frozen in 90% FBS and 10% DMSO and stored in liquid nitrogen for further experiments.
2.5. Phenotypical Analysis
Two different approaches were employed to evaluate the neoplastic component of cultured cells derived from tumor samples. For cells obtained from the first 75 samples, the morphological and immunocytochemical characteristics of tumor cell lines were studied at the 4–5th passage, and every 20 passages thereafter, by immuno-enzymatic staining with tumor-specific monoclonal antibodies. Cultures were fixed in 10% neutral-buffered formalin, embedded in paraffin and then stained with haematoxylin-eosin and with periodic acid-Schiff stain (PAS) to define the presence of malignant cells on the basis of cytomorphology. For the identification of specific tumor cells, we tested monoclonal antibodies against cytokeratin, vimentin/Clone V9, CD99 and HER2/neu (Dako, Glostruo, Denmark), EGFR (Neomarkers, Fremont, CA, USA), CD326 (Miltenyi Biotec) using indirect immuno-enzymatic staining according to the manufacturers’ instructions. Cultured tumor cells from ovarian cancer were also evaluated by cytofluorimetric analysis for the expression of CD326 antigen. Evaluation of CD326 expression was performed by direct immunofluorescence, using phycoerythrin (PE)-anti CD326 specific monoclonal antibody (Miltenyi Biotec), according to previously reported methods (8). Examination of growing cultures was performed using a direct phase-contrast microscope. Alternatively, at least three cytospins were performed using 105 cultured cells/cytospin obtained after 3–5 passages, for morphological and immunocytochemical analysis. Cells were fixed in 95% alcohol and one slide was stained with haematoxylin-eosin to identify malignant cells on the basis of cytomorphology. To distinguish tumor cells from hyperplastic mesothelial cells, the other slides were tested with monoclonal antibodies against cytokeratin CAM 5.2 (Dako) and calretinin (Invitrogen), using indirect immuno-enzymatic staining according to the manufacturers’ instructions. Tumor cells were assessed by a semiquantative method.
4. Discussion
Techniques to grow human cells and tissues on a plastic surface or in suspension have significantly contributed to the success of many biomedical investigations. Successful isolation and growth of tumor cells from freshly excised tumors, depends on several factors including receipt of tissue that contains a significant number of malignant cells for culture, the type of tumor and the
in vitro manipulation, which may or may not result in a stable cell line. In general, the explant culture system is technically simpler, requiring almost no special experience or reagents, but fibroblasts will eventually overgrow tumor cells in conventional culture medium [
5,
11]. In addition, new growth in the culture is limited to cells that have migrated out from the initial tissue fragment. Recent data demonstrated that a large number of melanoma cell lines can be successfully obtained and characterized [
12]. However, no data are available on the possibility of efficiently expanding tumor cells derived from different types of solid tumors. Here we describe a simple and rapid method for the establishment of primary cultures from solid tumors and neoplastic effusions. In the first group of experiments when tumor specimens were disaggregated with a cell scraper, in less than 50% of the clinical samples we were able to generate primary cultures that could be maintained for a significant number of passages
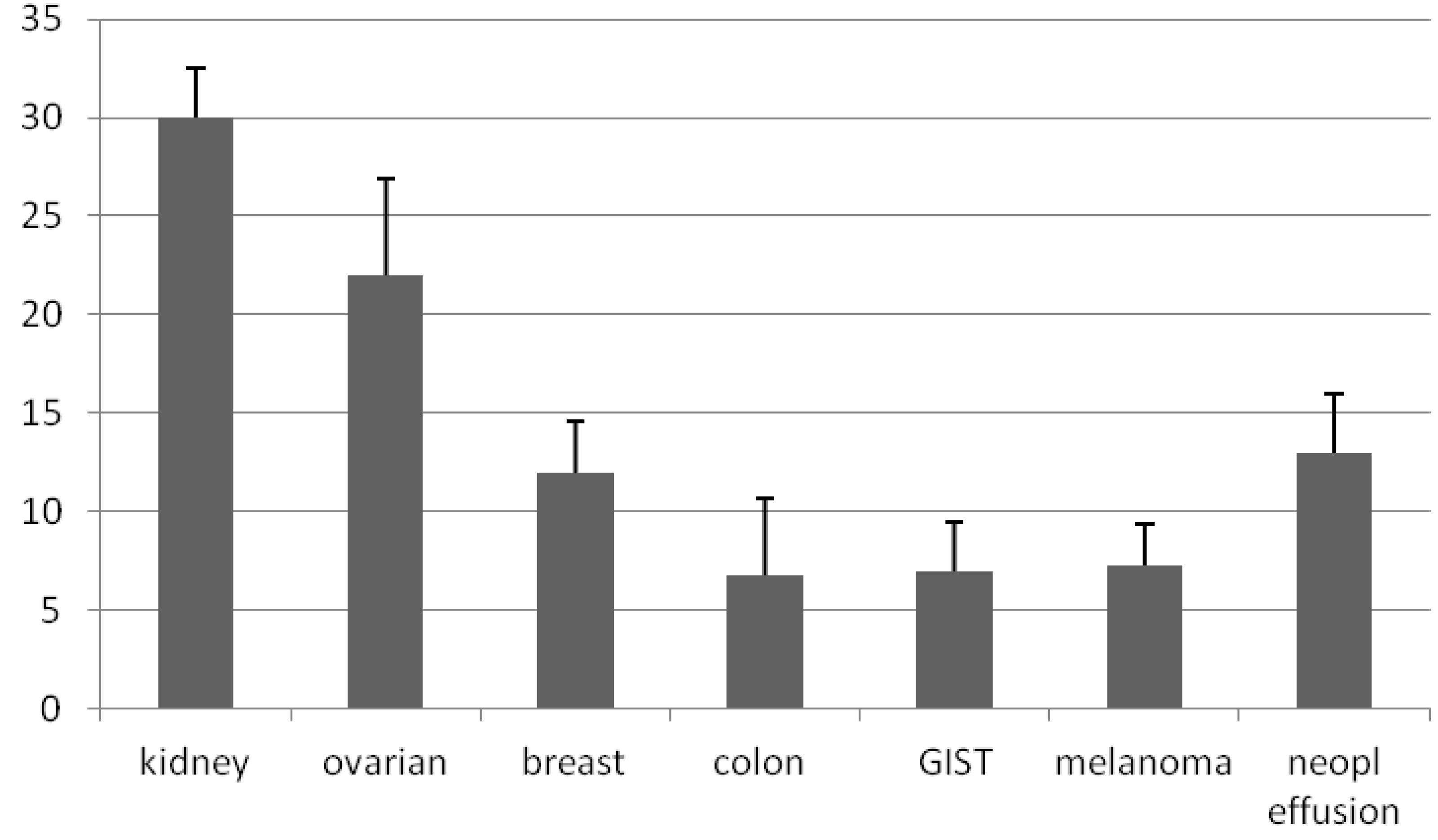
in vitro. Even though the method we described is efficient in establishing primary culture from almost every type of solid tumor, the best results were obtained with kidney and ovarian tumors. Primary cultures were characterized by a number of evaluation criteria including morphological and immunological parameters to confirm that tumor-derived primary cultures retain the characteristics and behavior of malignant cells. These results support the concept that in short-term culture systems based on enzymatic-mechanical disaggregation of tumor specimens, it is possible to grow malignant cells from a wide variety of solid tumors and, in some cases, to obtain primary tumor cell lines.
In the early phase, cell cultures may contain a variable number of contaminating fibroblast-like cells; these usually disappear after a number of passages, but magnetic fibroblast depletion is required in a minority of cases. It is worth noting that cultures from almost all tumor types were initially adversely affected by the presence of trypsin in the enzymatic digestion mixture (data not shown), but this effect did not persist, even though the rate of growth was in general impaired.
Moreover, the efficiency in establishing and maintaining tumor cells in culture depends on the characteristics of the original sample, which in most cases could be rich in connective tissue. In addition with this methodology, in most cases small fragments were obtained and it was not possible to estimate how many cells were plated.
To overcome some of these problems, in a further set of experiments, we evaluated whether it was possible to obtain tumor cell lines using the GentleMACS instrument, which allows automated dissociation of tumor tissues into single-cell suspensions in a closed and sterile system. In preliminary experiments we demonstrated that in most cases tumor samples could be treated without previous digestion, while for samples requiring digestion a short time incubation before dissociation with collagenase was sufficient to obtain a suspension of cells. The use of enzymatic digestion could be a limitation, at least for some types of solid tumors, in that it has been described that culture of isolated cells from protease-digested solid tumors includes the risk of an overgrowth by fibroblasts or stromal cells [
13]. In addition, according to the pathologist’s indications we treated the portion of the tumor sample richest in tumor cells, resulting in highly efficiency obtainment of tumor cell lines without fibroblast contamination. Failure was mainly due to bacterial or fungal contamination of the original tumor sample or to an intrinsic inability of the tumor cells to expand
in vitro. We are still working with surgeons and pathologists to reduce sample contamination. This methodology is very simple and reproducible, since after dissociation it is possible to filter the material and obtain a suspension of cells, cluster free, that can be counted and plated at an opportune concentration. For the clinical translational of this approach it is important to assess the molecular features of tumor cell lines. However, considering that several types of tumor were treated, and there was some difficulty in defining specific tumor-associated antigens, it was not possible to conduct a full molecular characterization of the tumor cell lines obtained in comparison with the primary tumor. So, in order to define the neoplastic origin of the expanding cells they were stained with haematoxylin-eosin to identify malignant cells on the basis of cytomorphology by pathologists.
Another matter of concern is the possibility of obtaining sizeable numbers of ovarian tumor cells from ascitic fluid even when they are initially present at a low percentage. For this reason, an efficient method for enriching and purifying ovarian tumor cells would facilitate tumor cell line cultures. It has been demonstrated that EpCAM represents an optimal antigenic target for separation of malignant cells of epithelial origin, as it is expressed on the great majority of epithelial tumor cells and does not react with fibroblasts, mesothelial cells or other cells types that are usually present in ascitic fluid of ovarian cancer patients [
14]. Our results clearly demonstrate that the growth of contaminating cells present in ascitic fluid can overgrow malignant cells present in the specimens, even if initially present at high percentages. By using specific microbeads directed against CD326 (EpCam) we were able to positively select malignant cells and to clear the majority of contaminants. The cells recovered were highly enriched CD326 cells, even when they were present in a very low percentage in the original sample. Of note in most cases positive cells recovered after enrichment were sufficient for cryopreservation for later use, otherwise, when the collected amount was low, cell culture allowed obtainment of ovarian tumor cell lines that maintained the characteristics of the original tumor and reached a consistent number of malignant cells.
5. Conclusions
Overall the methodology described here presents several advantages including the simplicity of culture maintenance, purity of the cell populations, possibility to obtain sufficient number of tumor cells after only a few culture passages, as well as preservation of the morphological and phenotypical characteristics of the original tumor.
Cell lines generated by this method may prove useful, as
in vitro models for anticancer drug testing/development, and for facilitating the clinical development of CTL-based immunotherapeutic strategies based on the use of vital tumor cells as source of tumor antigens [
8,
14,
15,
16,
17]. As previously demonstrated by our group, treatment with specific autologous antitumor CTLs
in vitro generated induced immunological responses and long-lasting clinical benefit in a patient with RCC [
18]. Using, as stimulator cells, tumor cells obtained with the approach here described, is safe and in an ongoing study at our institution we will define, in a larger series of patients, whether this form of immunotherapy has a role in advanced pre-treated cancer patients failing conventional therapies.