Distribution of Rare Earth Elements in Sediments of the Marine Lake Mir (Dugi Otok, Croatia)
Abstract
:1. Introduction
Present and Future REY Trends in Water Systems
2. Materials and Methods
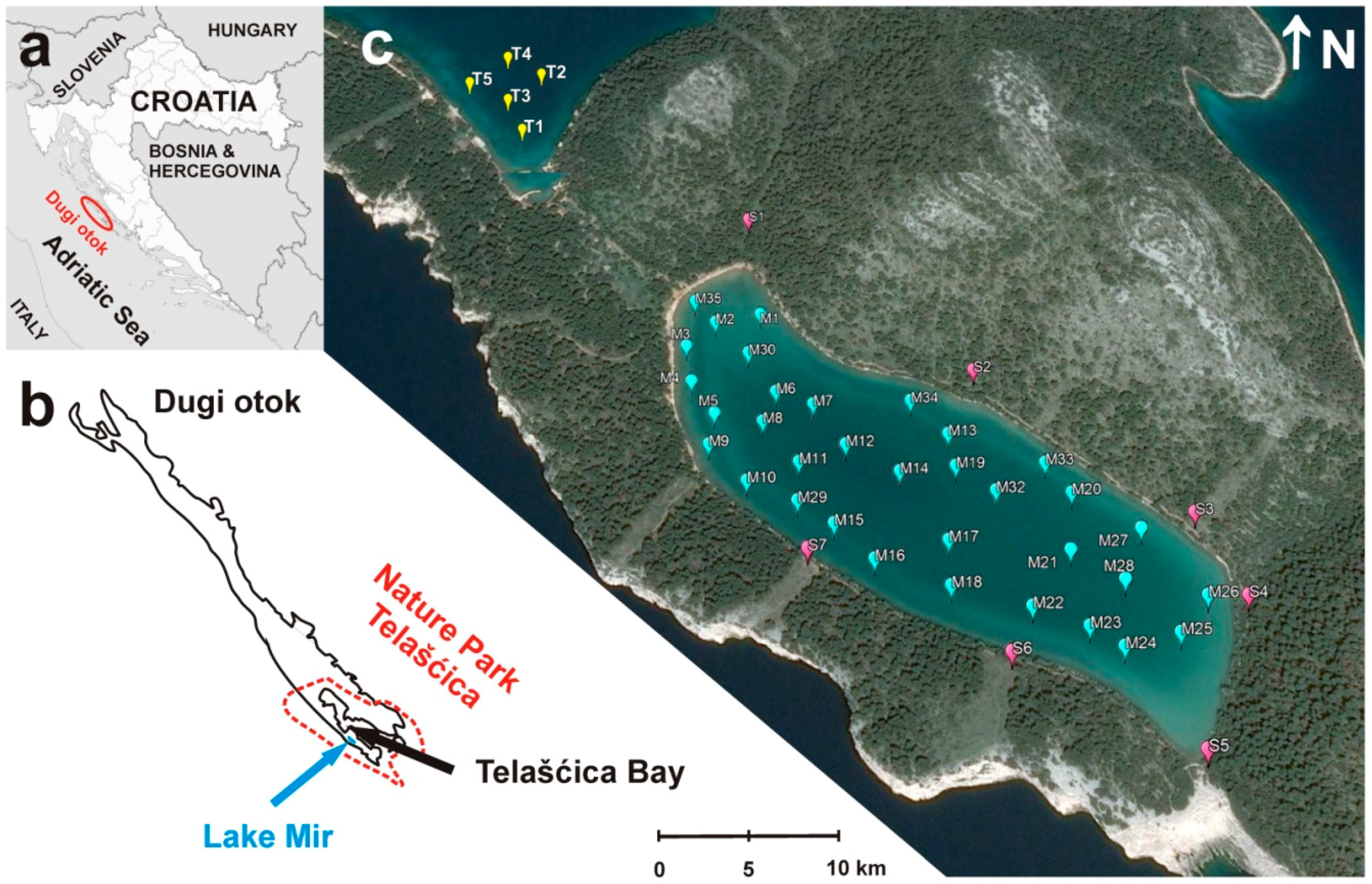
2.1. Study Area
2.2. Sampling and Sample Preparation
2.3. Rare Earth Element Analysis
2.4. REY Ratios and Enrichment
2.5. Statistical Analysis
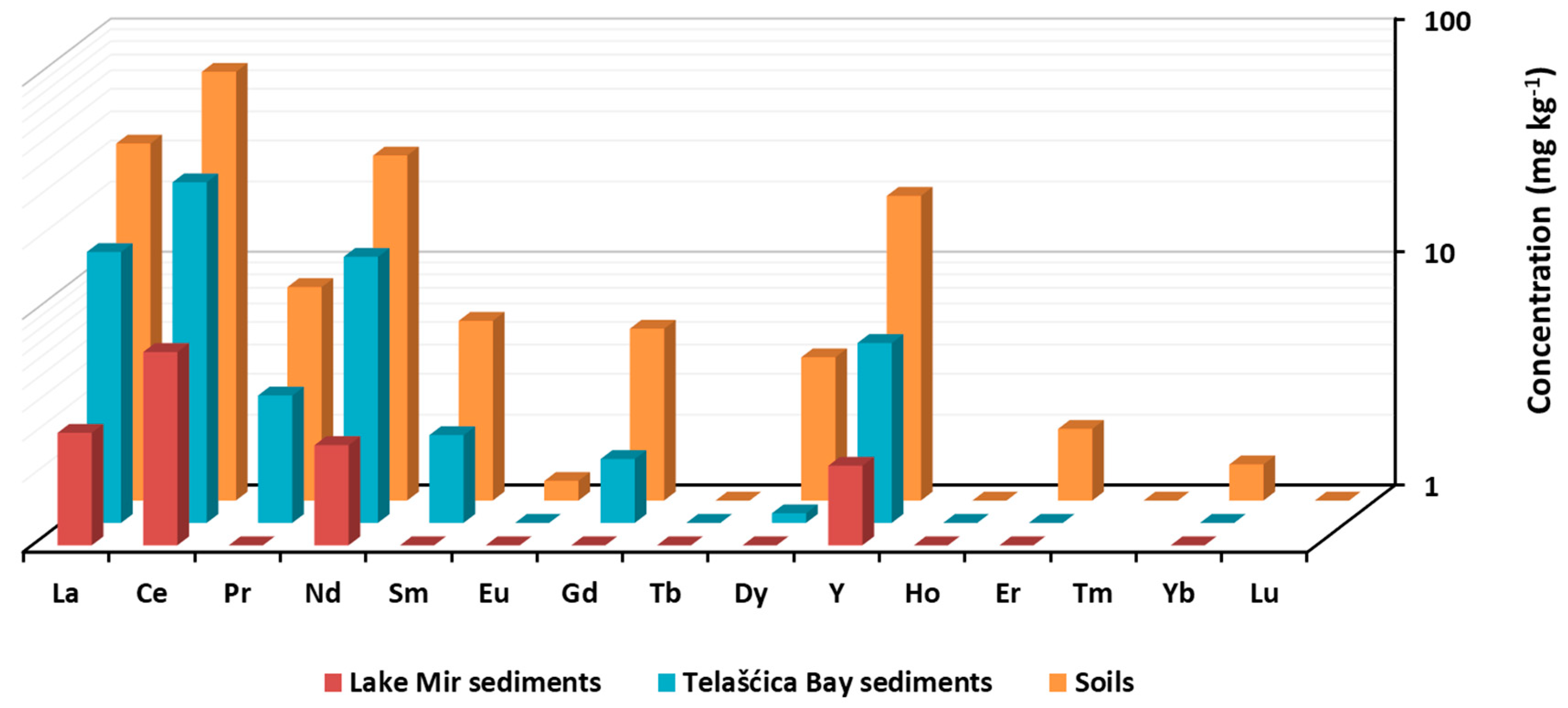
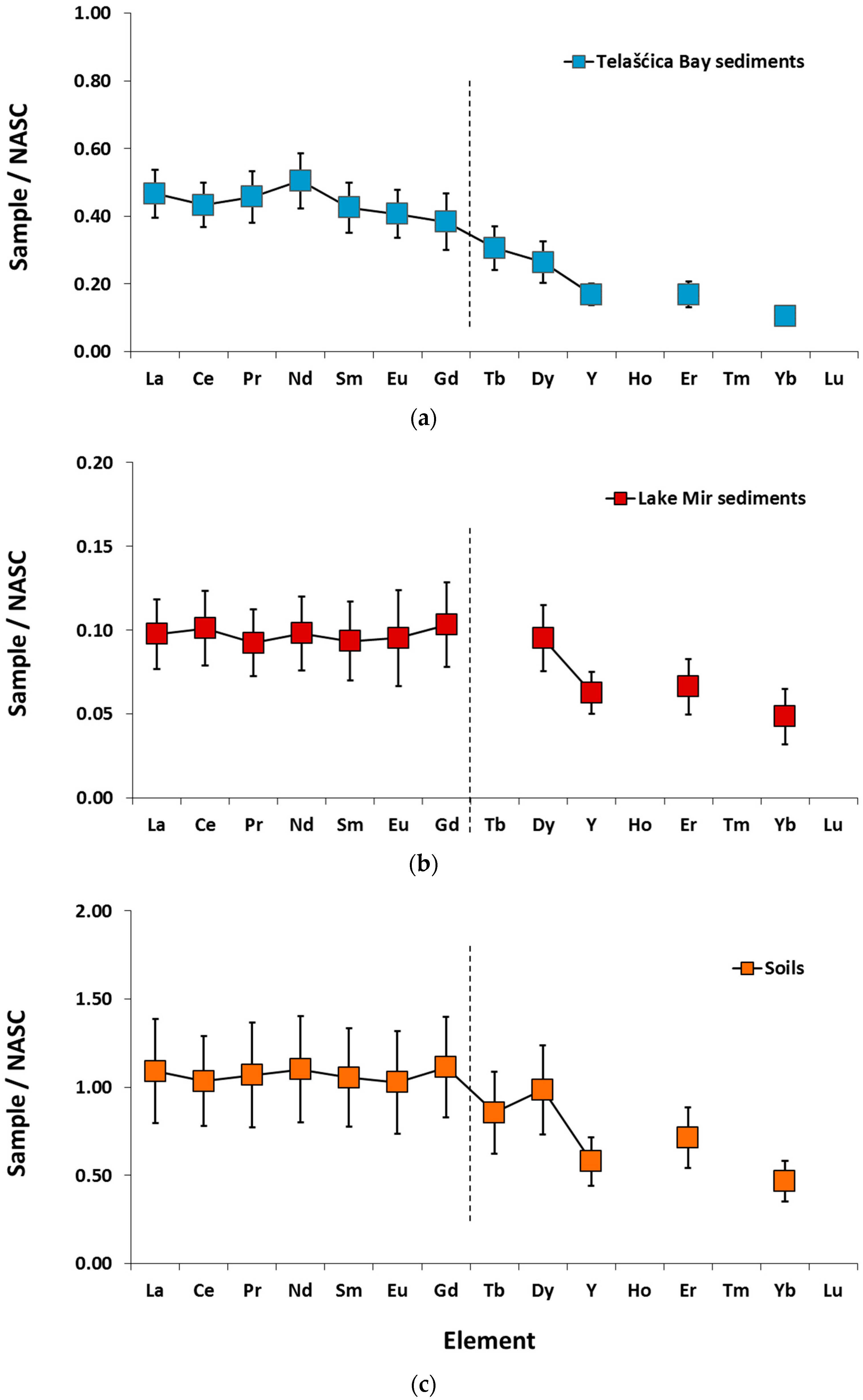
3. Results
4. Discussion
4.1. REY Distribution in Soils and Sediments
4.2. Principal Component Analysis (PCA)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Clay (%) | Silt (%) | Sand (%) | TOC (%) | CaCO3 (%) | |
|---|---|---|---|---|---|
| Lake Mir | 0.3–1.7 | 13–42 | 57–82 | 3.2–8.9 | 41–75 |
| Telašćica Bay | 1.0 | 17 | 81 | 0.4 | 85 |
| Al * | As | Ba | Ca * | Cd | Co | Cu | Fe * | K * | Li | Mg * | Mn | Mo | Ni | Pb | U | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 1.7 | 3.1 | 25.7 | 206 | 0.05 | 0.8 | 2.93 | 0.25 | 1.0 | 3.3 | 5.0 | 361 | 0.96 | 3.6 | 3.9 | 1.7 | 8.6 |
| M2 | 3.2 | 3.8 | 22.3 | 198 | 0.04 | 0.9 | 4.59 | 0.31 | 1.3 | 4.8 | 4.4 | 192 | 2.38 | 4.2 | 2.9 | 1.6 | 8.9 |
| M3 | 2.0 | 2.6 | 23.0 | 226 | 0.05 | 0.9 | 3.12 | 0.23 | 1.1 | 4.0 | 5.1 | 169 | 1.36 | 4.1 | 5.3 | 2.1 | 8.4 |
| M4 | 1.8 | 3.7 | 21.6 | 212 | 0.07 | 1.5 | 7.37 | 0.49 | 1.4 | 4.8 | 4.3 | 151 | 2.42 | 5.8 | 5.5 | 1.9 | 12.5 |
| M5 | 3.8 | 6.2 | 35.2 | 228 | 0.09 | 1.7 | 6.82 | 0.79 | 2.1 | 7.4 | 6.3 | 256 | 2.44 | 7.0 | 8.1 | 2.4 | 14.5 |
| M6 | 2.1 | 4.1 | 22.1 | 150 | 0.01 | 1.0 | 3.63 | 0.44 | 1.5 | 4.2 | 4.0 | 151 | 1.26 | 4.2 | 3.5 | 1.3 | 9.6 |
| M7 | 5.8 | 19.2 | 55.1 | 170 | 0.13 | 2.2 | 6.17 | 0.67 | 3.8 | 10.4 | 10.1 | 426 | 4.01 | 9.0 | 7.8 | 2.2 | 17.3 |
| M8 | 3.9 | 19.6 | 40.0 | 138 | 0.10 | 1.7 | 15.1 | 0.54 | 3.3 | 6.2 | 7.9 | 205 | 4.57 | 7.5 | 6.7 | 1.8 | 20.1 |
| M9 | 2.4 | 5.3 | 24.1 | 152 | 0.08 | 1.4 | 4.33 | 0.49 | 1.7 | 4.0 | 5.3 | 185 | 3.50 | 4.7 | 6.1 | 2.1 | 9.4 |
| M10 | 3.5 | 6.0 | 32.2 | 236 | 0.15 | 1.8 | 6.09 | 0.82 | 2.1 | 6.5 | 5.8 | 215 | 3.10 | 7.1 | 7.9 | 2.5 | 13.4 |
| M11 | 3.7 | 22.3 | 44.4 | 94.4 | 0.06 | 1.5 | 8.94 | 0.4 | 4.0 | 8.8 | 10.7 | 101 | 4.06 | 6.2 | 4.0 | 1.5 | 22.0 |
| M12 | 6.9 | 24.6 | 65.9 | 140 | 0.11 | 2.7 | 25.4 | 1.27 | 4.6 | 11 | 11.4 | 468 | 4.25 | 10.1 | 9.0 | 2.1 | 36.8 |
| M13 | 2.8 | 15.7 | 36.5 | 184 | 0.12 | 2.0 | 23.1 | 0.57 | 2.4 | 6.4 | 5.8 | 189 | 3.85 | 7.0 | 7.6 | 2.1 | 24.5 |
| M14 | 6.5 | 23.2 | 58.2 | 112 | 0.22 | 2.4 | 10.5 | 0.73 | 4.7 | 12.0 | 13.0 | 156 | 9.31 | 11.2 | 9.5 | 2.4 | 25.8 |
| M15 | 6.5 | 31.2 | 61.8 | 96.7 | 0.21 | 2.5 | 10.7 | 0.73 | 6.0 | 14.1 | 15.2 | 121 | 7.11 | 11.1 | 8.6 | 1.6 | 24 |
| M16 | 2.5 | 9.1 | 31.2 | 179 | 0.10 | 1.3 | 6.99 | 0.29 | 2.1 | 5.1 | 6.6 | 362 | 2.37 | 5.1 | 7.1 | 2.3 | 15.5 |
| M17 | 3.7 | 32 | 47.5 | 78.4 | 0.09 | 1.8 | 5.64 | 0.44 | 3.4 | 8.9 | 9.1 | 80 | 10.9 | 7.9 | 6.1 | 1.4 | 17.8 |
| M18 | 6.6 | 48 | 89.8 | 100 | 0.20 | 1.8 | 14.4 | 0.5 | 5.5 | 11.2 | 14.7 | 154 | 4.39 | 8.9 | 8.7 | 1.8 | 30.9 |
| M19 | 4.0 | 28.7 | 60.4 | 161 | 0.09 | 1.7 | 5.22 | 0.4 | 3.8 | 8.7 | 9.5 | 214 | 4.89 | 7.4 | 6 | 2.2 | 13.3 |
| M20 | 5.9 | 13 | 45.0 | 163 | 0.12 | 2.0 | 9.39 | 0.77 | 3.8 | 9.7 | 10.2 | 167 | 5.88 | 8.7 | 8.8 | 2.7 | 18.5 |
| M21 | 6.9 | 48.5 | 84.1 | 113 | 0.16 | 1.9 | 21.2 | 0.58 | 5.8 | 13.1 | 13.3 | 104 | 7.59 | 10.6 | 7.6 | 1.8 | 32.3 |
| M22 | 5.7 | 53 | 89.3 | 126 | 0.14 | 1.5 | 7.48 | 0.51 | 5.5 | 11.9 | 14.0 | 168 | 5.07 | 8.6 | 6.5 | 1.8 | 18.6 |
| M23 | 4.3 | 26.4 | 61.2 | 177 | 0.13 | 1.8 | 6.13 | 0.52 | 4.0 | 9.2 | 9.9 | 187 | 5.23 | 8.2 | 6.0 | 2.4 | 13.7 |
| M24 | 4.1 | 7.3 | 32.6 | 187 | 0.11 | 1.8 | 5.63 | 0.57 | 2.7 | 7.6 | 6.9 | 113 | 5.51 | 8.5 | 8.5 | 2.8 | 12.5 |
| M25 | 5.8 | 6.1 | 40.2 | 189 | 0.16 | 2.2 | 6.22 | 0.67 | 3.4 | 10.7 | 8.2 | 131 | 6.98 | 10.3 | 9.4 | 3.4 | 16.4 |
| M26 | 2.9 | 4.0 | 19.6 | 132 | 0.04 | 0.9 | 3.00 | 0.22 | 1.8 | 5.4 | 5.0 | 71 | 2.05 | 3.9 | 4.6 | 1.6 | 8.1 |
| M27 | 3.4 | 7.7 | 31.0 | 167 | 0.07 | 1.7 | 5.45 | 0.51 | 2.6 | 7.3 | 7.2 | 103 | 3.36 | 7.4 | 7.2 | 2.2 | 11.0 |
| M28 | 6.3 | 52.3 | 78.1 | 107 | 0.17 | 2.0 | 8.83 | 0.63 | 5.0 | 10.6 | 14.3 | 107 | 7.38 | 10.1 | 8.3 | 2.1 | 19.7 |
| M29 | 5.1 | 19.4 | 43.0 | 113 | 0.12 | 5.0 | 5.63 | 0.55 | 4.5 | 10.1 | 14 | 230 | 8.86 | 8.9 | 9.1 | 2.9 | 16.5 |
| M30 | 4.1 | 3.2 | 27.2 | 195 | 0.08 | 1.3 | 3.65 | 0.34 | 1.8 | 7.7 | 5.2 | 136 | 2.58 | 6.4 | 6.3 | 2.8 | 9.6 |
| M31 | 8.2 | 33.8 | 69.3 | 111 | 0.2 | 3.0 | 13.0 | 1.04 | 5.2 | 14.7 | 13.7 | 168 | 8.13 | 13.2 | 10.9 | 1.8 | 27.3 |
| M32 | 7.2 | 36.4 | 72.2 | 133 | 0.19 | 2.7 | 7.95 | 0.74 | 4.6 | 12.9 | 9.5 | 151 | 10.7 | 11.9 | 8.83 | 3.0 | 20.1 |
| M33 | 2.5 | 5.5 | 24.7 | 214 | 0.12 | 1.7 | 4.3 | 0.26 | 1.6 | 5.7 | 5.0 | 73 | 2.88 | 5.1 | 5.5 | 2.8 | 9.7 |
| M34 | 1.7 | 4.3 | 23.1 | 234 | 0.07 | 0.9 | 4.16 | 0.28 | 1.0 | 2.6 | 4.5 | 161 | 1.51 | 4.0 | 4.3 | 2.0 | 9.7 |
| M35 | 1.3 | 1.9 | 19.4 | 222 | 0.01 | 0.8 | 1.59 | 0.2 | 0.9 | 3.0 | 3.9 | 251 | 0.29 | 3.8 | 2.4 | 1.3 | 5.1 |
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | ΣREY | ΣLREE/ΣHREE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 4.2 | 9.87 | 1.0 | 3.98 | 0.8 | 0.2 | 0.8 | 0.1 | 0.6 | 2.99 | <0.1 | 0.3 | <0.1 | 0.2 | <0.1 | 25.0 | 4.98 |
| M2 | 4.3 | 9.95 | 1.0 | 3.94 | 0.7 | 0.2 | 0.7 | <0.1 | 0.6 | 3.34 | 0.1 | 0.3 | <0.1 | 0.2 | <0.1 | 25.3 | 4.58 |
| M3 | 3.3 | 7.43 | 0.8 | 3.01 | 0.6 | 0.1 | 0.6 | <0.1 | 0.5 | 2.44 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 19.2 | 4.74 |
| M4 | 3.7 | 5.70 | 0.6 | 2.32 | 0.4 | <0.1 | 0.4 | <0.1 | 0.3 | 1.97 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 15.7 | 5.11 |
| M5 | 3.5 | 7.94 | 0.9 | 3.11 | 0.6 | 0.1 | 0.6 | <0.1 | 0.4 | 2.66 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 20.2 | 4.84 |
| M6 | 2.0 | 4.66 | 0.5 | 1.70 | 0.3 | <0.1 | 0.3 | <0.1 | 0.3 | 1.57 | <0.1 | 0.1 | <0.1 | <0.1 | <0.1 | 11.4 | 4.80 |
| M7 | 3.5 | 7.78 | 0.8 | 3.18 | 0.6 | 0.1 | 0.6 | <0.1 | 0.4 | 2.56 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 19.9 | 4.93 |
| M8 | 2.6 | 5.80 | 0.6 | 2.17 | 0.4 | <0.1 | 0.4 | <0.1 | 0.3 | 1.72 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 14.3 | 5.16 |
| M9 | 2.1 | 4.81 | 0.5 | 1.87 | 0.4 | <0.1 | 0.4 | <0.1 | 0.3 | 1.58 | <0.1 | 0.1 | <0.1 | 0.1 | <0.1 | 12.2 | 4.85 |
| M10 | 3.6 | 7.75 | 0.8 | 2.96 | 0.6 | 0.1 | 0.6 | <0.1 | 0.5 | 2.74 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 20.1 | 4.51 |
| M11 | 2.0 | 4.66 | 0.5 | 1.72 | 0.3 | <0.1 | 0.3 | <0.1 | 0.3 | 1.61 | <0.1 | 0.1 | <0.1 | 0.1 | <0.1 | 11.6 | 4.49 |
| M12 | 3.4 | 7.87 | 0.8 | 2.97 | 0.6 | 0.1 | 0.6 | <0.1 | 0.4 | 2.27 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 19.4 | 5.32 |
| M13 | 2.8 | 6.24 | 0.6 | 2.47 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.11 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 16.0 | 4.70 |
| M14 | 3.3 | 7.32 | 0.8 | 2.90 | 0.6 | 0.1 | 0.5 | <0.1 | 0.4 | 2.41 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 18.7 | 4.83 |
| M15 | 3.1 | 6.83 | 0.7 | 2.68 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.18 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 17.3 | 5.00 |
| M16 | 2.8 | 6.16 | 0.6 | 2.56 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.07 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 16.0 | 4.77 |
| M17 | 1.9 | 4.18 | 0.5 | 1.71 | 0.3 | <0.1 | 0.3 | <0.1 | 0.3 | 1.49 | <0.1 | 0.1 | <0.1 | 0.1 | <0.1 | 10.9 | 4.47 |
| M18 | 2.9 | 6.53 | 0.7 | 2.68 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.09 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 16.8 | 4.81 |
| M19 | 3.0 | 6.56 | 0.7 | 2.60 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.03 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 16.7 | 5.11 |
| M20 | 3.3 | 7.19 | 0.8 | 3.06 | 0.6 | 0.1 | 0.5 | <0.1 | 0.4 | 2.20 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 18.6 | 5.18 |
| M21 | 3.3 | 7.25 | 0.8 | 2.85 | 0.6 | 0.1 | 0.5 | <0.1 | 0.4 | 2.27 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 18.5 | 5.02 |
| M22 | 2.7 | 5.95 | 0.6 | 2.33 | 0.4 | <0.1 | 0.4 | <0.1 | 0.3 | 2.02 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 15.0 | 4.73 |
| M23 | 2.7 | 5.89 | 0.6 | 2.30 | 0.4 | <0.1 | 0.4 | <0.1 | 0.4 | 2.02 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 15.0 | 4.52 |
| M24 | 2.8 | 6.11 | 0.7 | 2.43 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.08 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 15.9 | 4.73 |
| M25 | 3.4 | 8.15 | 0.8 | 2.95 | 0.6 | 0.1 | 0.6 | <0.1 | 0.4 | 2.28 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 19.7 | 5.39 |
| M26 | 1.8 | 4.16 | 0.4 | 1.70 | 0.3 | <0.1 | 0.3 | <0.1 | 0.3 | 1.50 | <0.1 | 0.1 | <0.1 | <0.1 | <0.1 | 10.6 | 4.56 |
| M27 | 2.5 | 5.61 | 0.6 | 2.26 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 1.90 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 14.7 | 4.64 |
| M28 | 3.3 | 7.34 | 0.8 | 3.20 | 0.6 | 0.1 | 0.5 | <0.1 | 0.4 | 2.28 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 18.9 | 5.14 |
| M29 | 2.3 | 4.97 | 0.6 | 2.24 | 0.5 | <0.1 | 0.4 | <0.1 | 0.3 | 1.80 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 13.4 | 4.59 |
| M30 | 3.9 | 9.03 | 1.0 | 3.77 | 0.8 | 0.2 | 0.7 | <0.1 | 0.5 | 2.91 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 23.2 | 5.09 |
| M31 | 3.8 | 8.56 | 0.9 | 3.52 | 0.7 | 0.1 | 0.6 | <0.1 | 0.5 | 2.72 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 21.8 | 5.02 |
| M32 | 3.6 | 7.84 | 0.8 | 2.99 | 0.6 | 0.1 | 0.6 | <0.1 | 0.4 | 2.43 | <0.1 | 0.2 | <0.1 | 0.2 | <0.1 | 19.8 | 5.12 |
| M33 | 2.8 | 6.02 | 0.7 | 2.59 | 0.5 | 0.1 | 0.5 | <0.1 | 0.4 | 2.17 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 16.1 | 4.60 |
| M34 | 3.5 | 7.90 | 0.8 | 3.0 | 0.6 | 0.1 | 0.7 | <0.1 | 0.5 | 2.56 | <0.1 | 0.2 | <0.1 | 0.1 | <0.1 | 20.0 | 4.94 |
| M35 | 2.6 | 6.06 | 0.6 | 2.39 | 0.4 | <0.1 | 0.4 | <0.1 | 0.3 | 1.82 | <0.1 | 0.1 | <0.1 | 0.1 | <0.1 | 14.8 | 5.37 |
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | ΣREY | ΣLREE/ΣHREE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 16.2 | 31.8 | 3.8 | 15 | 2.6 | 0.5 | 2.2 | 0.3 | 1.3 | 6.4 | 0.2 | 0.5 | <0.1 | 0.4 | <0.1 | 81.2 | 7.92 |
| T2 | 16.6 | 33.5 | 4.2 | 16.4 | 2.8 | 0.6 | 2.1 | 0.3 | 1.2 | 6.48 | 0.2 | 0.5 | <0.1 | 0.3 | <0.1 | 85.2 | 8.49 |
| T3 | 11.3 | 22.7 | 2.7 | 10.8 | 1.8 | 0.4 | 1.3 | 0.2 | 0.7 | 3.89 | 0.1 | 0.3 | <0.1 | 0.2 | <0.1 | 56.4 | 9.46 |
| T4 | 15.2 | 30.2 | 3.7 | 14.6 | 2.6 | 0.5 | 2.2 | 0.3 | 1.3 | 6.5 | 0.2 | 0.6 | <0.1 | 0.4 | <0.1 | 78.3 | 7.42 |
| T5 | 13.3 | 26.3 | 3.2 | 12.4 | 2.1 | 0.4 | 1.6 | 0.2 | 1.0 | 6.28 | 0.2 | 0.5 | <0.1 | 0.3 | <0.1 | 67.8 | 6.99 |
| S1 | 33.1 | 68.6 | 8.0 | 29.3 | 5.8 | 1.2 | 5.4 | 0.7 | 4.0 | 19.8 | 0.7 | 2.0 | 0.3 | 1.4 | 0.2 | 181 | 5.20 |
| S2 | 36.9 | 76.6 | 8.9 | 32.7 | 6.3 | 1.3 | 5.8 | 0.8 | 4.5 | 22.3 | 0.8 | 2.2 | 0.3 | 1.6 | 0.2 | 201 | 5.15 |
| S3 | 38.6 | 81 | 9.4 | 34.4 | 6.7 | 1.4 | 6.2 | 0.8 | 4.7 | 22.8 | 0.8 | 2.3 | 0.3 | 1.6 | 0.3 | 211 | 5.29 |
| S4 | 35.6 | 76 | 8.7 | 31.7 | 6.3 | 1.3 | 5.9 | 0.8 | 4.4 | 21.2 | 0.8 | 2.2 | 0.3 | 1.5 | 0.2 | 197 | 5.27 |
| S5 | 14.7 | 32.1 | 3.4 | 12.9 | 2.6 | 0.5 | 2.5 | 0.3 | 1.9 | 10.1 | 0.4 | 1.0 | 0.1 | 0.7 | 0.1 | 83.3 | 4.71 |
| S6 | 34.7 | 68.3 | 8.5 | 30.9 | 6.1 | 1.2 | 5.5 | 0.8 | 4.1 | 20.3 | 0.8 | 2.0 | 0.3 | 1.4 | 0.2 | 185 | 5.19 |
| S7 | 44.0 | 79.9 | 10.7 | 39.3 | 7.5 | 1.6 | 6.9 | 0.9 | 5.2 | 25.3 | 0.9 | 2.5 | 0.3 | 1.8 | 0.3 | 227 | 5.10 |
References
- Hamner, W.M.; Gilmer, R.W.; Hamner, P.P. The physical, chemical, biological characteristics of a stratified, saline, sulfide lake in Palau. Limnol. Oceanogr. 1982, 27, 896–909. [Google Scholar] [CrossRef]
- Burnett, W.C.; Landing, W.M.; Lyons, W.B.; Orem, W. Jellyfish Lake, Palau: A model anoxic environment for geochemical studies. Eos Trans. Am. Geophys. Union 1989, 70, 777–783. [Google Scholar] [CrossRef]
- Landing, W.M.; Burnett, W.C.; Lyons, W.B.; Orem, W.H. Nutrient cycling, the biogeochemistry of manganese, iron, zinc in Jellyfish Lake, Palau. Limnol. Oceanogr. 1991, 36, 515–525. [Google Scholar] [CrossRef]
- Orem, W.H.; Burnett, W.C.; Landing, W.M.; Lyons, W.B.; Showers, W. Jellyfish Lake, Palau: Early diagenesis of organic matter in sediments of an anoxic marine lake. Limnol. Oceanogr. 1991, 36, 526–543. [Google Scholar] [CrossRef] [Green Version]
- Bates, A.L.; Spiker, E.C.; Orem, W.H.; Burnett, W.C. Speciation, isotopic composition of sulfur in sediments from Jellyfish Lake, Palau. Chem. Geol. 1993, 106, 63–76. [Google Scholar] [CrossRef]
- Lyons, W.B.; Lent, R.M.; Burnett, W.C.; Chin, P.; Landing, W.M.; Orem, W.H.; McArthur, J.M. Jellyfish Lake, Palau: Regeneration of C, N, Si, P in anoxic marine sediments. Limnol. Oceanogr. 1996, 41, 1394–1403. [Google Scholar] [CrossRef]
- Hamner, W.M.; Hamner, P.P. Stratified marine lakes of Palau (Western Caroline Islands). Phys. Geogr. 1998, 19, 175–220. [Google Scholar]
- Cerrano, C.; Azzini, F.; Bavestrello, G.; Calcinai, B.; Pansini, M.; Sarti, M.; Thung, D.C. Marine lakes of karst islands in Ha Long Bay (Vietnam). Chem. Ecol. 2006, 22, 489–500. [Google Scholar] [CrossRef]
- Colin, P.L. Marine Environments of Palau; Indo-Pacific Press: San Diego, CA, USA, 2009; p. 414. [Google Scholar]
- Mlakar, M.; Fiket, Ž.; Geček, S.; Cukrov, N.; Cuculić, V. Marine lake as in situ laboratory for studies of organic matter influence on speciation and distribution of trace metals. Cont. Shelf Res. 2015, 103, 1–11. [Google Scholar] [CrossRef]
- Davranche, M.; Grybos, M.; Gruau, G.; Pedrot, M.; Dia, A.; Marsac, R. Rare earth element patterns: A tool for identifying trace metal sources during wetland soil reduction. Chem. Geol. 2011, 284, 127–137. [Google Scholar] [CrossRef]
- McLennan, S.M.; Taylor, S.R. Geology, Geochemistry, and Natural Abundances of the Rare Earth Elements. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Hannigan, R.; Dorval, E.; Jones, C. The rare earth element chemistry of estuarine surface sediments in Chesapeake Bay. Chem. Geol. 2010, 272, 20–30. [Google Scholar] [CrossRef]
- López-González, N.; Borrego, J.; Carro, B.; Grande, J.A.; De la Torre, M.L.; Valente, T. Rare-earth-element fractionation patterns in estuarine sediments as a consequence of acid mine drainage: A case study in SW Spain. Bol. Geol. Min. 2012, 123, 55–64. [Google Scholar]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Influence of the geological setting on the REE geochemistry of estuarine sediments: A case study of the Zrmanja River estuary (eastern Adriatic coast). J. Geochem. Explor. 2017, 182, 70–79. [Google Scholar] [CrossRef]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare Earth Elements: Critical Resources for High Technology. U.S. Geological Survey Fact Sheet 087-02; 2002. Available online: http://pubs.usgs.gov/fs/2002/ fs087-02/ (accessed on 9 August 2018).
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States—A Summary of Domestic Deposits and A Global Perspective; U.S. Geological Survey: Reston, VA, USA, 2010; p. 104.
- Livergood, R. Rare Earth Elements: A Wrench in the Supply Chain. Center for Strategic and International Studies. 2010. Available online: http://csis.org/files/publication/101005_DIIG_Current_Issues_no22_Rare_earth_elements.pdf (accessed on 9 August 2018).
- Wang, Y.; Sun, J.; Chen, H.; Guo, F. Determination of the contents and distribution characteristics of REE in natural plants by NAA. J. Radioanal. Nucl. Chem. 1997, 219, 99–103. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, X.H.; Zhou, Q. Effects of rare earth elements on the distribution of mineral elements and heavy metals in horseradish. Chemosphere 2008, 73, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Zhang, M.; Zhang, N.; Li, M. Effects of rare earth elements on growth and metabolism of medicinal plants. Acta Pharm. Sin. B 2013, 3, 20–24. [Google Scholar] [CrossRef]
- Pamić, J.; Gušić, I.; Jelaska, V. Geodynamic evolution of the Central Dinarides. Tectonophysics 1998, 297, 251–268. [Google Scholar] [CrossRef]
- Surić, M. Submerged Karst—Dead or Alive? Examples from the Eastern Adriatic Coast (Croatia). Geoadria 2005, 10, 5–12. [Google Scholar] [CrossRef]
- Šegota, T. The sea level and vertical movements of the Adriatic Sea bottom since Ris-Würm glaciations till today. Geol. Vjesnik Zagreb 1982, 35, 93–109. (In Croatian) [Google Scholar]
- Pirazzoli, P.A. A review of possible eustatic, isostatic and tectonic contributions in eight late—Holocene relative sea-level histories from the Mediterranean area. Quat. Sci. Rev. 2005, 24, 1989–2001. [Google Scholar] [CrossRef]
- Bates, R.L.; Jackson, J.A. Glossary of Geology; American Geological Institute: Falls Church, VA, USA, 1987. [Google Scholar]
- Miko, S.; Durn, G.; Prohić, E. Evaluation of terra rossa geochemical baselines from Croatian karst regions. J. Geochem. Explor. 1999, 66, 173–182. [Google Scholar] [CrossRef]
- Gromet, P.L.; Dymek, P.F.; Haskin, L.A.; Korotev, R.L. The North American Shale Composite: Its Composition, Major and Minor Element Characteristics. Geochim. Cosmochim. Acta 1984, 48, 2469–2482. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A. The Characteristics, Occurrence, and Geochemical Behavior of Rare Earth Elements in the Environment: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 429–471. [Google Scholar] [CrossRef]
- Feng, J.L. Behaviour of rare earth elements and yttrium in ferromanganese concretions, gibbsite spots, and the surrounding terra rossa over dolomite during chemical weathering. Chem. Geol. 2010, 271, 112–132. [Google Scholar] [CrossRef]
- Yalcin, M.G.; Ilhan, S. Major and Trace Element geochemistry of Terra Rossa Soil in the Kucukkoras Region, Karaman, Turkey. Geochem. Int. 2008, 46, 1038–1054. [Google Scholar] [CrossRef]
- Carvahlo, L.; Figueira, P.; Monteiro, R.; Reis, A.T.; Almeida, J.; Catry, T.; Lourenço, P.M.; Catry, P.; Barbosa, C.; Catry, I.; et al. Major, minor, trace and rare earth elements in sediments of the Bijagós archipelago, Guinea-Bissau. Mar. Pollut. Bull. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, N.; Frančišković-Bilinski, S.; Hlača, B.; Barišić, D. A recent history of metal accumulation in the sediments of Rijeka harbor, Adriatic Sea, Croatia. Mar. Pollut. Bull. 2011, 62, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, G.; Anschutz, P.; Lavaux, G.; Blanc, G. Rare earth elements in the modern sediments of the Bay of Biscay (France). Mar. Chem. 2006, 100, 39–52. [Google Scholar] [CrossRef]
- Deepulal, P.M. Behaviour of ERZs in a tropical estuary and adjacent continental shelf of southwest coast of India: Evidence from anomalies. J. Earth Syst. Sci. 2012, 121, 1215–1227. [Google Scholar] [CrossRef]
- Vlasov, K.A. Geochemistry and Mineralogy of Rare Elements and Genetic Types of their Deposits—Vol. 1 Geochemistry of Rare Earth Elements; IPST No. 2123; Israel Program for Scientific Translations: Jerusalem, Israel, 1996; p. 945. [Google Scholar]
- Sholkovitz, E.R. The geochemistry of rare earth elements in the Amazon River estuary. Geochim. Cosmochim. Acta 1993, 57, 2181–2190. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Elderfield, H.; Szymczak, R.; Casey, K. Island weathering: River sources of rare earth elements to the Western Pacific Ocean. Mar. Chem. 1999, 68, 39–57. [Google Scholar] [CrossRef]
- Windom, H.L.; Schropp, S.J.; Calder, F.D.; Ryan, J.D.; Smith, R.G.; Burney, L.C.; Lewis, F.G.; Rawlinson, C.H. Natural trace metal concentrations in estuarine and coastal marine sediments of the southeastern United States. Environ. Sci. Technol. 1989, 23, 314–320. [Google Scholar] [CrossRef]
- Durn, G. Terra Rossa in the Mediterranean Region: Parent Materials, Composition and Origin. Geol. Croat. 2003, 56, 83–100. [Google Scholar]
- Macleod, D.A. The origin of the red Mediterranean soils in Epirus, Greece. J. Soil Sci. 1980, 31, 125–136. [Google Scholar] [CrossRef]
- Garcia-Gonzales, M.T.; Recio, P. Geochemistry and mineralogy of the clay fraction from some Spanish terra rossa. Agrochimica 1988, 32, 161–170. [Google Scholar]
- Boero, V.; Premoli, A.; Melis, P.; Barberis, E.; Arduino, E. Influence of climate on the iron oxide mineralogy of terra rossa. Clays Clay Miner. 1992, 40, 8–13. [Google Scholar] [CrossRef]
- Bronger, A.; Bruhn-Lobin, N. Paleopedology of Terrae rossae—Rhodoxeralfs from Quaternary calcarenites in NW Morocco. Catena 1997, 28, 279–295. [Google Scholar] [CrossRef]
- Moresi, M.; Mongelli, G. The relation between the terra rossa and the carbonate-free residue of the underlying limestones and dolostones in Apulia, Italy. Clay Miner. 1988, 23, 439–446. [Google Scholar] [CrossRef]
- Altay, I. Red Mediterranean soils in some karstic regions of Taurus mountains, Turkey. Catena 1997, 247–260. [Google Scholar]
- Chappaz, A.; Gobeil, C.; Tessier, A. Controls on uranium distribution in lake sediments. Geochim. Cosmochim. Acta 2010, 74, 203–214. [Google Scholar] [CrossRef]


| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | ΣREY | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soils | ||||||||||||||||
| min | 14.7 | 32.1 | 3.4 | 12.9 | 2.6 | 0.5 | 2.5 | 0.3 | 1.9 | 10.1 | 0.4 | 1.0 | 0.1 | 0.7 | 0.1 | 83.3 |
| max | 44.0 | 81.0 | 10.7 | 39.3 | 7.5 | 1.6 | 6.9 | 0.9 | 5.2 | 25.3 | 0.9 | 2.5 | 0.3 | 1.8 | 0.3 | 227 |
| avg | 33.9 | 68.9 | 8.2 | 30.2 | 5.9 | 1.2 | 5.5 | 0.7 | 4.1 | 20.3 | 0.7 | 2.0 | 0.3 | 1.4 | 0.2 | 184 |
| SD | 9.2 | 17.0 | 2.3 | 8.3 | 1.6 | 0.3 | 1.4 | 0.2 | 1.1 | 4.8 | 0.2 | 0.5 | 0.1 | 0.3 | 0.1 | 46.9 |
| Telašćica Bay Sediments | ||||||||||||||||
| min | 11.3 | 22.7 | 2.7 | 10.8 | 1.8 | 0.4 | 1.3 | 0.2 | 0.7 | 3.9 | 0.1 | 0.3 | <0.1 | 0.2 | <0.1 | 56.4 |
| max | 16.6 | 33.5 | 4.2 | 16.4 | 2.8 | 0.6 | 2.2 | 0.3 | 1.3 | 6.5 | 0.2 | 0.6 | <0.1 | 0.4 | <0.1 | 85.2 |
| avg | 14.5 | 28.9 | 3.5 | 13.8 | 2.4 | 0.5 | 1.9 | 0.3 | 1.1 | 5.9 | 0.2 | 0.5 | - | 0.3 | - | 73.8 |
| SD | 2.2 | 4.4 | 0.6 | 2.2 | 0.4 | 0.1 | 0.4 | 0.1 | 0.3 | 1.1 | 0.0 | 0.1 | - | 0.1 | - | 11.7 |
| Lake Mir Sediments | ||||||||||||||||
| min | 1.8 | 4.2 | 0.4 | 1.7 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 1.5 | 0.1 | 0.1 | <0.1 | 0.1 | <0.1 | 10.6 |
| max | 4.3 | 10.0 | 1.0 | 4.0 | 0.8 | 0.2 | 0.8 | 0.1 | 0.6 | 3.3 | 0.1 | 0.3 | <0.1 | 0.2 | <0.1 | 25.3 |
| avg | 3.0 | 6.7 | 0.7 | 2.7 | 0.5 | 0.1 | 0.5 | 0.1 | 0.4 | 2.2 | 0.1 | 0.2 | - | 0.1 | - | 17.2 |
| SD | 0.7 | 1.5 | 0.2 | 0.6 | 0.1 | 0.0 | 0.1 | 0.1 | 0.4 | 0.0 | - | 0.1 | - | 3.8 | ||
| Sample | EuN/EuN* | CeN/CeN* | (La/Yb)N | (Nd/Yb)N | Sample | EuN/EuN* | CeN/CeN* | (La/Yb)N | (Nd/Yb)N |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 1.11 | 1.12 | 2.07 | 2.00 | M25 | 0.74 | 1.15 | 1.67 | 1.65 |
| M2 | 1.27 | 1.11 | 2.12 | 2.43 | M26 | n/a | 1.14 | n/a | n/a |
| M3 | 0.74 | 1.06 | 1.62 | 2.29 | M27 | 0.89 | 1.06 | 2.46 | 2.52 |
| M4 | n/a | 0.89 | 3.64 | 2.25 | M28 | 0.81 | 1.05 | 1.62 | 1.79 |
| M5 | 0.74 | 1.04 | 1.72 | 2.52 | M29 | n/a | 0.98 | 2.26 | 2.50 |
| M6 | n/a | 1.08 | n/a | 2.47 | M30 | 1.19 | 1.06 | 1.92 | 2.11 |
| M7 | 0.74 | 1.08 | 1.72 | 2.51 | M31 | 0.68 | 1.07 | 1.87 | 1.97 |
| M8 | n/a | 1.08 | 2.56 | 2.14 | M32 | 0.74 | 1.07 | 1.77 | 1.67 |
| M9 | n/a | 1.09 | 2.07 | 2.30 | M33 | 0.89 | 1.00 | 2.75 | 2.89 |
| M10 | 0.74 | 1.06 | 1.77 | 2.27 | M34 | 0.68 | 1.10 | 3.44 | 3.35 |
| M11 | n/a | 1.08 | 1.97 | 2.03 | M35 | n/a | 1.13 | 2.56 | 2.67 |
| M12 | 0.74 | 1.11 | 1.67 | 2.54 | T1 | 0.93 | 0.94 | 3.98 | 4.19 |
| M13 | 0.89 | 1.12 | 2.75 | 2.42 | T2 | 1.10 | 0.93 | 5.44 | 6.11 |
| M14 | 0.81 | 1.05 | 1.62 | 2.39 | T3 | 1.16 | 0.95 | 5.56 | 6.03 |
| M15 | 0.89 | 1.08 | 3.05 | 2.55 | T4 | 0.93 | 0.93 | 3.74 | 4.08 |
| M16 | 0.89 | 1.10 | 2.75 | 2.44 | T5 | 0.97 | 0.94 | 4.36 | 4.62 |
| M17 | n/a | 0.99 | 1.87 | 2.01 | |||||
| M18 | 0.89 | 1.06 | 1.43 | 2.25 | S1 | 0.95 | 0.98 | 2.33 | 2.34 |
| M19 | 0.89 | 1.05 | 2.95 | 2.56 | S2 | 0.95 | 0.98 | 2.27 | 2.28 |
| M20 | 0.81 | 1.03 | 1.62 | 2.46 | S3 | 0.96 | 0.99 | 2.37 | 2.40 |
| M21 | 0.81 | 1.04 | 1.62 | 2.42 | S4 | 0.95 | 1.00 | 2.34 | 2.36 |
| M22 | n/a | 1.08 | 2.66 | 2.11 | S5 | 0.87 | 1.05 | 2.07 | 2.06 |
| M23 | n/a | 1.07 | 2.66 | 1.91 | S6 | 0.92 | 0.92 | 2.44 | 2.46 |
| M24 | 0.89 | 1.01 | 2.75 | 2.47 | S7 | 0.99 | 0.85 | 2.41 | 2.44 |
| Sediments | Terra rossa Soil | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lake Mir * | Telašćica Bay * | Zrmanja Estuary, Croatia [15] | Bijagós Archipelago [32] | Cochin, India [35] | Odiel Spain [14] | Chesapeake Bay, USA [13] | Biscay, France [34] | Rijeka, Croatia [33] | Dugi Otok * | Zrmanja Estuary, Croatia [15] | Turkey [31] | China [30] | ||
| La | 3.04 | 14.5 | 23.5 | 4.4–22 | 14.0–51.5 | 30.1 | 15.0–120 | 7.03–27.7 | 10.7 | 33.9 | 13.1 | 86.0 | 69.7–385 | |
| Ce | 6.74 | 28.9 | 45.8 | 9.1–47 | 27.7–103 | 49.3 | 29.7–240 | 12.8–56.5 | 23.1 | 68.9 | 27.2 | 184 | 170–510 | |
| Pr | 0.71 | 3.52 | 5.10 | 0.9–5.0 | 2.77–10.0 | 8.29 | 3.01–26.4 | 1.74–6.63 | 2.91 | 8.2 | 2.92 | 18.9 | 15.0–160 | |
| Nd | 2.69 | 13.8 | 22.0 | 3.1–18 | 11.1–38.8 | 33.3 | 11.0–101 | 6.53–25.2 | 11.4 | 30.2 | 12.8 | 80.1 | 51.6–673 | |
| Sm | 0.52 | 2.38 | 3.85 | 0.52–3.3 | 1.93–6.91 | 7.70 | 1.87–17.4 | 1.19–4.93 | 2.49 | 5.9 | 2.33 | 14.0 | 9.24–225 | |
| Eu | 0.11 | 0.48 | 0.795 | 0.21–0.87 | 0.54–1.65 | 1.56 | 0.32–3.08 | 0.25–1.15 | 0.60 | 1.2 | 0.508 | 2.97 | 1.77–42.1 | |
| Gd | 0.51 | 1.88 | 3.02 | 0.49–3.0 | 1.37–5.67 | 7.71 | 1.61–14.3 | 1.06–4.36 | 2.59 | 5.5 | 1.99 | 10.4 | 6.46–109 | |
| Tb | <0.1 | 0.26 | 0.513 | - | 0.19–0.89 | 1.12 | 0.23–2.01 | 0.13–0.58 | 0.38 | 0.7 | 0.322 | 1.89 | 0.98–17.9 | |
| Dy | 0.40 | 1.1 | 2.61 | 0.35–2.1 | 1.01–4.92 | 6.28 | 1.05–10.5 | 0.64–3.04 | 2.00 | 4.1 | 1.82 | 11.0 | 5.47–70.4 | |
| Y | 2.19 | 5.91 | 13.1 | - | - | - | - | - | 11.3 | 20.3 | 157 | 56.6 | 26.1–76.5 | |
| Ho | <0.1 | 0.18 | 0.506 | 0.08–0.43 | 0.21–1.03 | 1.33 | 0.20–1.86 | 0.12–0.61 | 0.38 | 0.7 | 0.343 | 2.09 | 1.08–8.97 | |
| Er | 0.19 | 0.48 | 1.42 | 0.19–1.2 | 0.53–2.83 | 3.48 | 0.65–5.93 | 0.38–1.78 | 0.97 | 2.0 | 1.06 | 6.03 | 3.20–23.8 | |
| Tm | <0.1 | - | 0.179 | 0.039–0.18 | 0.08–0.39 | 0.54 | 0.09–0.83 | 0.05–0.29 | 0.11 | 0.3 | 0.157 | 0.918 | 0.521–4.61 | |
| Yb | 0.15 | 0.32 | 1.31 | 0.19–1.2 | 0.5–2.34 | 3.66 | 0.72–5.35 | 0.33–1.57 | 0.71 | 1.4 | 1.09 | 5.91 | 3.47–34.6 | |
| Lu | <0.1 | - | 0.245 | 0.039–0.19 | 0.08–0.36 | 0.56 | 0.10–0.96 | 0.04–0.23 | - | 0.2 | 0.156 | 0.885 | 0.549–4.93 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiket, Ž.; Mlakar, M.; Kniewald, G. Distribution of Rare Earth Elements in Sediments of the Marine Lake Mir (Dugi Otok, Croatia). Geosciences 2018, 8, 301. https://doi.org/10.3390/geosciences8080301
Fiket Ž, Mlakar M, Kniewald G. Distribution of Rare Earth Elements in Sediments of the Marine Lake Mir (Dugi Otok, Croatia). Geosciences. 2018; 8(8):301. https://doi.org/10.3390/geosciences8080301
Chicago/Turabian StyleFiket, Željka, Marina Mlakar, and Goran Kniewald. 2018. "Distribution of Rare Earth Elements in Sediments of the Marine Lake Mir (Dugi Otok, Croatia)" Geosciences 8, no. 8: 301. https://doi.org/10.3390/geosciences8080301





