Spatial Evolution of the Chromium Contamination in Soils from the Assopos to Thiva Basin and C. Evia (Greece) and Potential Source(s): Anthropogenic versus Natural Processes
Abstract
:1. Introduction
2. Geological and Hydrogeologic Outline

3. Analytical Methods
4. Results
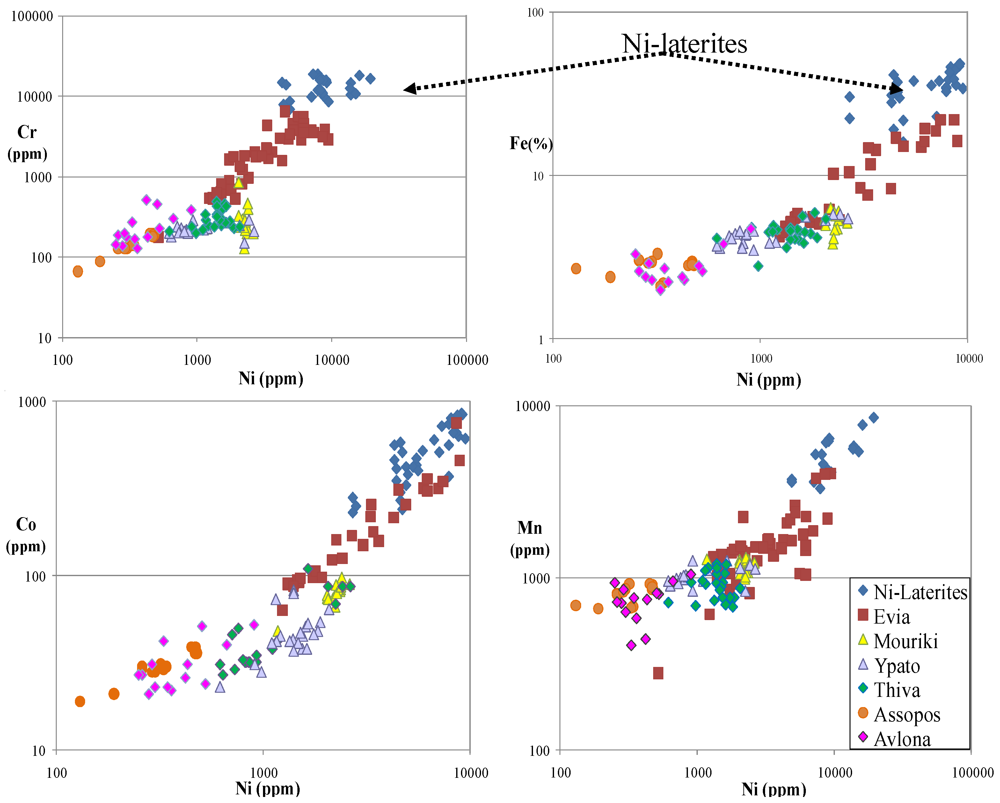
4.1.Soil Contamination and Its Spatial Evolution
| Location | Range | Cr (ppm) | Ni (ppm) | Mn (ppm) | Fe (wt %) |
|---|---|---|---|---|---|
| Evia | min | 540 | 1200 | 600 | 4.2 |
| max | 3800 | 8900 | 2200 | 19 | |
| median | 830 | 1800 | 1300 | 5.6 | |
| n = 16 | |||||
| Avlona | min | 130 | 250 | 405 | 2 |
| max | 520 | 900 | 1040 | 4.7 | |
| median | 200 | 350 | 750 | 2.6 | |
| n = 14 | |||||
| Oropos | min | 50 | 90 | 260 | 1.1 |
| max | 160 | 420 | 550 | 2.1 | |
| median | 60 | 120 | 330 | 1.3 | |
| n = 12 | |||||
| Thiva | min | 230 | 710 | 990 | 4.2 |
| max | 340 | 1650 | 1260 | 5.6 | |
| median | 220 | 1100 | 960 | 4.10 | |
| n = 6 | |||||
| Detection limits | 1 | 0.1 | 1 | 0.01 | |
| STD DS7 | 194 | 50 | 618 | 2.4 |
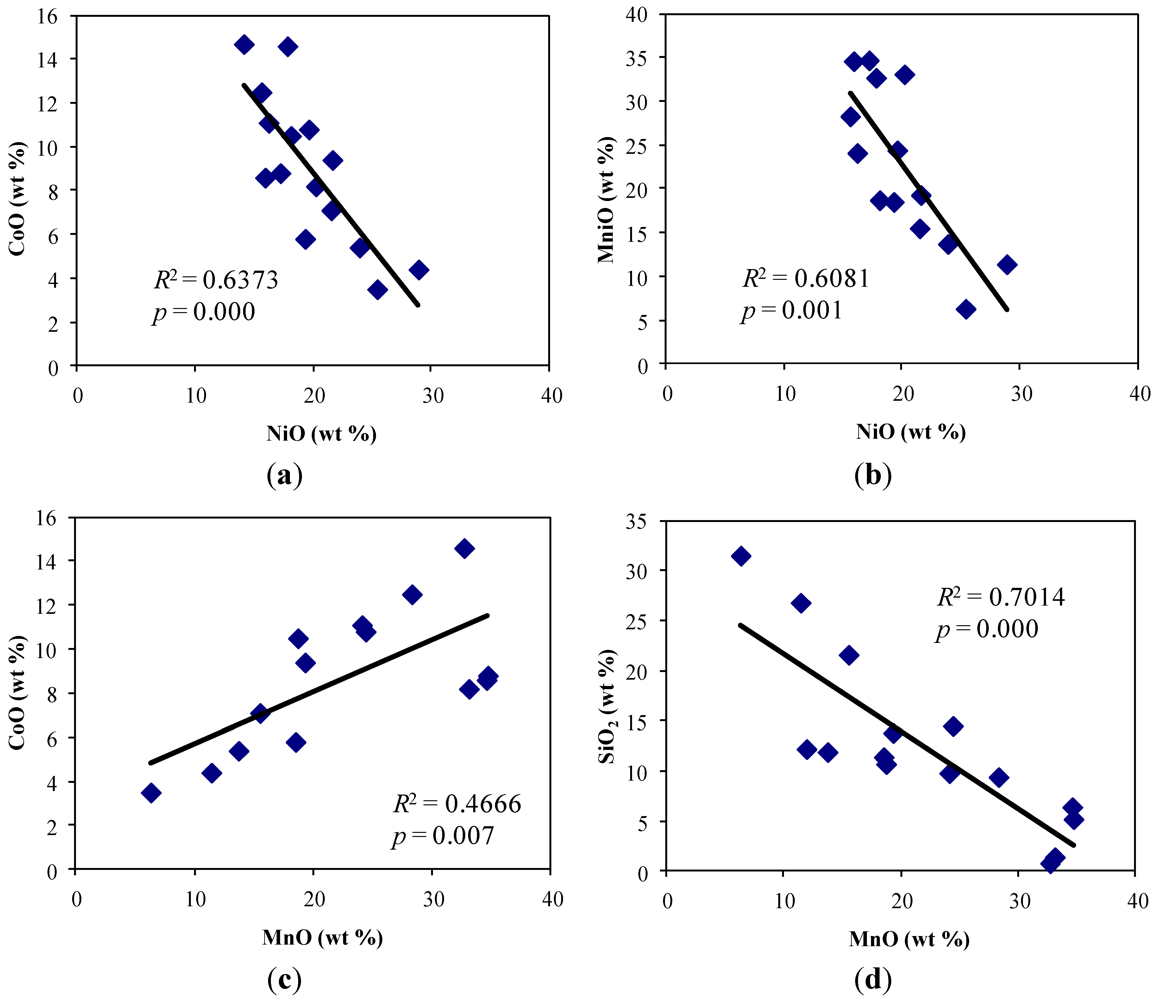
4.2. Mineralogical Composition of Soils and Mineral-Hosts of Heavy Metals
| (wt %) | Chromite | Goethite | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 0.5 | 0.3 | 0.3 | 1.1 | 0.4 | 0.8 | 1.4 | 1.7 | 2.1 | 2.2 |
| Al2O3 | 12.9 | 6.6 | 18.6 | 37.7 | 43.1 | 1.4 | 1.6 | 1.6 | 0.8 | 2.5 |
| Cr2O3 | 53.4 | 58.2 | 51.6 | 30.9 | 24.8 | 1.6 | 0.8 | 1.0 | 1.7 | 1.6 |
| Fe2O3 | 2.1 | 3.3 | 0.3 | 0.2 | 2.0 | 82.2 | 80.5 | 80.8 | 82.5 | 75.6 |
| FeO | 25.6 | 8.4 | 14.5 | 14.2 | 13.4 | n.d. | n.d. | n.d. | n.d. | n.d. |
| MgO | 5.4 | 3.5 | 11.0 | 15.4 | 16.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| MnO | n.d. | 13.0 | 1.3 | n.d. | n.d. | n.d. | 1.18 | 1.21 | 0.3 | 2.3 |
| ZnO | n.d. | 2.1 | 0.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| CoO | n.d. | 4.1 | 2.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| NiO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.7 | n.d. | 0.5 | 1.5 |
| Total | 99.9 | 99.6 | 100.7 | 99.5 | 100.2 | 86.03 | 88.2 | 86.3 | 87.9 | 85.7 |
| Cr# | 0.73 | 0.86 | 0.65 | 0.36 | 0.28 | |||||
| Mg# | 0.27 | 0.19 | 0.53 | 0.66 | 0.68 | |||||
| (Mn-Co-Ni)-asbolane and silicates | ||||||||||
| SiO2 | 1.4 | 0.8 | 5.2 | 9.4 | 10.7 | 14.5 | 6.4 | 21.6 | 11.9 | |
| Al2O3 | 4.7 | 2.7 | n.d. | n.d. | 11.3 | 0.7 | n.d. | 0.5 | 16.2 | |
| Cr2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| FeOt | 2.1 | 0.6 | 0.5 | n.d. | 4.3 | 1.3 | n.d. | 2.1 | n.d. | |
| MgO | 0.5 | n.d. | 2.2 | 4.7 | 0.4 | 5.9 | 3.8 | 11.1 | n.d. | |
| MnO | 33.1 | 32.7 | 34.7 | 28.3 | 18.7 | 24.4 | 34.6 | 15.5 | 13.7 | |
| CaO | 1.2 | 1.5 | 1.6 | 1.1 | 0.7 | 0.8 | 1.8 | 0.7 | 0.4 | |
| CoO | 8.2 | 14.6 | 8.8 | 12.5 | 10.5 | 10.8 | 8.6 | 7.1 | 5.4 | |
| NiO | 20.2 | 17.8 | 17.2 | 15.6 | 18.1 | 19.6 | 15.9 | 21.5 | 23.9 | |
| Total | 71.4 | 70.7 | 70.2 | 71.6 | 74.7 | 78 | 80.1 | 71.1 | 71.5 | |
| (wt %) | Chlorite within goethite | Serpentine | ||||||||
| SiO2 | 21.23 | 31.38 | 31.92 | 31.45 | 41.52 | 34.3 | 38.51 | 33.98 | 38.9 | |
| Al2O3 | 10.55 | 14.21 | 15.94 | 15.41 | n.d. | 11.6 | 0.73 | 2.22 | 1.2 | |
| Cr2O3 | 0.31 | 1.25 | 0.81 | 0.3 | n.d. | n.d. | n.d. | n.d. | 0.3 | |
| FeOt | 32.42 | 14.12 | 17.82 | 13.2 | 6.72 | 11.6 | 3.48 | 14.5 | 8.6 | |
| MgO | 14.04 | 19.58 | 14.74 | 14.7 | 38.4 | 29.3 | 1.51 | 3.25 | 37.1 | |
| MnO | 0.49 | 0.32 | 0.28 | n.d. | n.d. | n.d. | 0.53 | n.d. | n.d. | |
| CaO | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 1.1 | n.d. | |
| CoO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.52 | n.d. | n.d. | |
| NiO | 6.64 | 5.08 | 3.51 | 9.9 | n.d. | 0.7 | 39.31 | 26.8 | 0.3 | |
| Total | 85.68 | 85.94 | 85.02 | 84.96 | 86.64 | 87.5 | 84.59 | 81.85 | 86.4 | |

| Location | Range | Cr (ppm) | Cr(VI) (ppm) | Ni (ppm) | Mn (ppm) | Fe (ppm) | |
|---|---|---|---|---|---|---|---|
| Central Evia | min | 0.2 | 0.5 | 2 | 9 | 20 | |
| max | 69 | 1.8 | 315 | 300 | 6090 | ||
| median | 16 | 0.7 | 36 | 44 | 550 | ||
| n | 95 | 8 | 95 | 95 | 95 | ||
| Avlona | min | 0.23 | 0.1 | 0.2 | 3 | 25 | |
| max | 11 | 1.8 | 52 | 105 | 1170 | ||
| median | 2.3 | 0.5 | 5.2 | 20 | 180 | ||
| n | 69 | 5 | 69 | 69 | 69 | ||
| Oropos | min | 0.4 | 0.2 | 0.3 | 9 | 78 | |
| max | 4.5 | 0.4 | 9.2 | 115 | 1170 | ||
| median | 0.9 | 0.2 | 1.5 | 56 | 144 | ||
| n | 21 | 5 | 21 | 21 | 21 | ||
| Thiva | Ypato | carrot | 2 | 0.14 | 3.3 | 8 | 40 |
| Ypato-Eleonas | potatoes | 2.1 | 0.23 | 6.2 | 9 | 90 | |
| potatoes | 2.1 | 0.22 | 5.7 | 6 | 50 | ||
| Charaidini | carrot | 2.4 | 0.28 | 5.2 | 7 | 40 | |
| potatoes | 2.3 | 0.22 | 6.3 | 7.5 | 40 | ||
| Detection Limits | 0.1 | 0.1 | 1 | 10 | |||
| STD DS8 | 114 | 36.4 | 594 | 2.35 | |||
| Normal values | 0.1–0.5 | 0.1–5 | 30–300 | ||||
| Excessive values | 5–30 | 10–100 | 400–1000 | ||||
4.3. Heavy Metal Contents in Plants-Crops
| Description | Crtotal (ppm) | Ni (ppm) | Mn (ppm) | Fe (wt %) |
|---|---|---|---|---|
| C. Evia | ||||
| soil | 1300 | 2800 | 1300 | 7.9 |
| plant | 10 | 31 | 63 | 0.07 |
| % mp/ms | 0.8 | 1.1 | 4.8 | 8.6 |
| Avlona | ||||
| soil | 250 | 460 | 830 | 3.4 |
| plant | 3 | 7 | 30 | 0.023 |
| % mp/ms | 1.2 | 1.5 | 3.6 | 0.7 |
| Oropos | ||||
| soil | 73 | 157 | 363 | 1.4 |
| plant | 1.7 | 2.8 | 51 | 0.03 |
| % mp/ms | 2.3 | 1.8 | 14 | 2.1 |
| Thiva | ||||
| soil | 230 | 760 | 1000 | 4.39 |
| carrot | 2.2 | 3.5 | 8.8 | 0.004 |
| % mp/ms | 0.96 | 0.5 | 0.9 | 0.09 |
| Thiva | ||||
| soil | 290 | 930 | 1000 | 4.4 |
| potatoe | 2.2 | 5.8 | 7.5 | 0.006 |
| % mp/ms | 0.88 | 0.8 | 0.8 | 0.14 |
| Thiva | ||||
| soil | 320 | 1400 | 1140 | 4.58 |
| onion | 3.8 | 9.6 | 15 | 0.014 |
| % mp/ms | 1.2 | 0.7 | 1.3 | 0.31 |
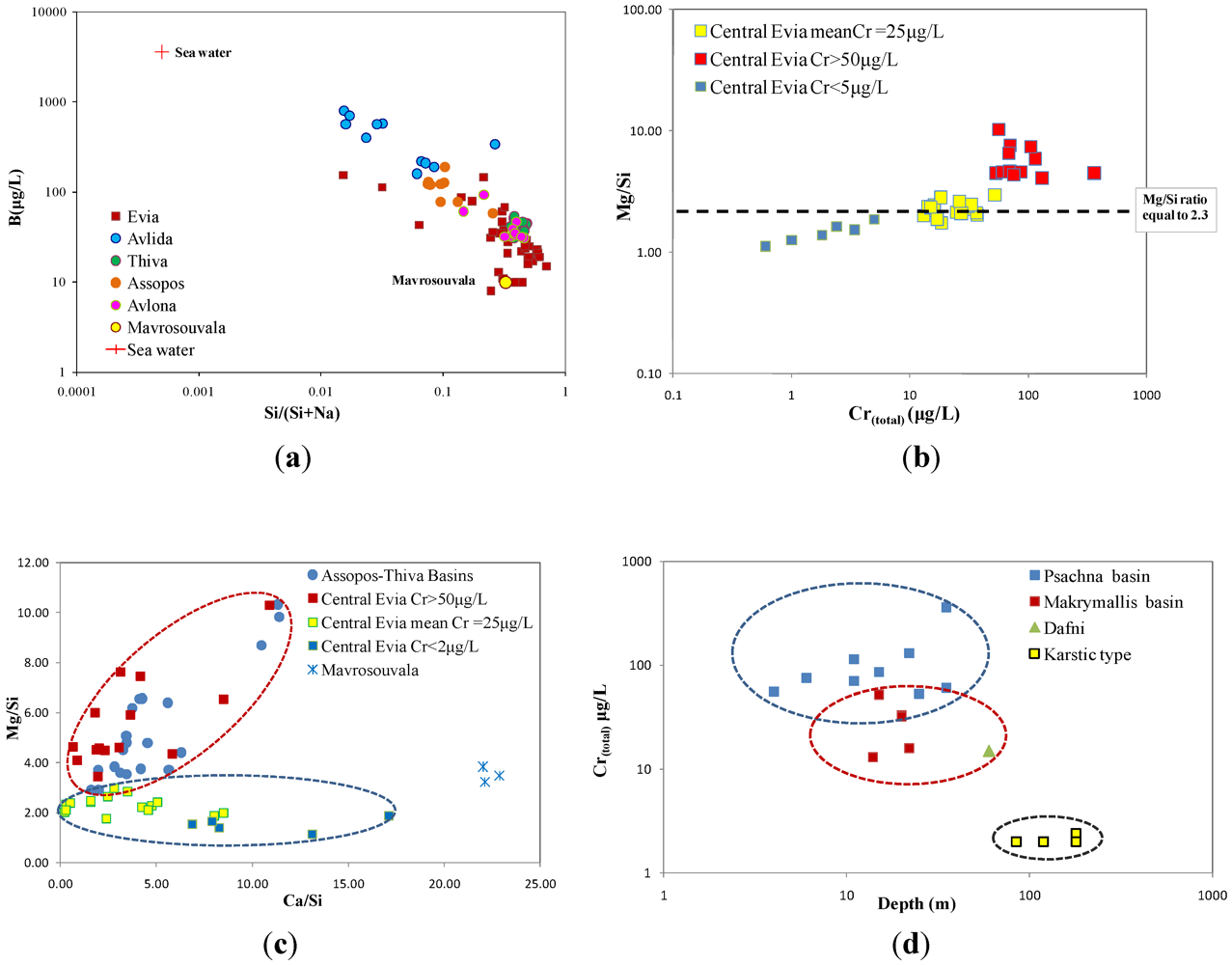
4.4. Distribution of Heavy Metals in Groundwater
| Location | Range | Cr(total) (ppm) | Cr(VI) (ppm) | Ca (ppm) | Mg (ppm) | Si (ppm) | Na (ppm) | B (ppm) | pH | Eh (mV) |
|---|---|---|---|---|---|---|---|---|---|---|
| C. Evia | min | 2 | 4 | 7,400 | 4,400 | 3,900 | 10,800 | 8 | 7.06 | −62 |
| max | 360 | 360 | 141.500 | 420,000 | 34,000 | 98,000 | 146 | 7.88 | 16 | |
| median | 27 | 26 | 63,700 | 59,000 | 17,400 | 21,700 | 23 | 7.43 | −34 | |
| n = 41 | ||||||||||
| Avlona | min | 2 | 4 | 25,000 | 11,000 | 5,000 | 7,000 | 9 | 7.10 | −36 |
| max | 90 | 85 | 62,000 | 132,000 | 23,000 | 104,000 | 420 | 7.54 | −11 | |
| median | 50 | 48 | 40,000 | 61,000 | 19,000 | 32,500 | 30 | 7.3 | −19 | |
| n = 12 | ||||||||||
| Oropos | min | 10 | 4 | 32,400 | 46,600 | 8,900 | 55,000 | 58 | 7.31 | −39 |
| max | 140 | 120 | 202,600 | 185,500 | 19,300 | 168,700 | 200 | 7.66 | −14 | |
| median | 53 | 46 | 60,250 | 84,575 | 12,400 | 114,950 | 120 | 7.5 | −25 | |
| n =16 | ||||||||||
| Avlida | min | 13 | 9 | 12,000 | 8,000 | 7,100 | 10,000 | 160 | 7.2 | −47 |
| max | 120 | 110 | 75,000 | 190,000 | 12,000 | 700,000 | 800 | 7.7 | −12 | |
| median | 53 | 44 | 36,000 | 91,000 | 10,500 | 200,500 | 455 | 7.4 | −24 | |
| n = 11 | ||||||||||
| Thiva | min | 8 | 6 | 13,000 | 44,000 | 6,700 | 9,000 | 31 | 7.12 | −78 |
| max | 37 | 33 | 42,000 | 88,000 | 15,000 | 16,000 | 54 | 8.09 | −25 | |
| median | 27 | 24 | 25,000 | 58,500 | 9,250 | 12,500 | 43 | 7.6 | −49 | |
| n = 13 | ||||||||||
| Mavrosouvala | 2 | <4 | 90,000 | 15,000 | 4,200 | 8,700 | 10 | 7.38 | −18 | |
| Sea water Evia gulf | <10 | 370,000 | 1,300,000 | 3,100 | 6,200,000 | 3,600 | ||||
| Detection Limits | <2 | <4 | 50 | 50 | 1 | 50 | 5 | |||
| Parameter values | 50 μg/L | 200 mg/L | 1.0 mg/L | 6.5–9.5 |
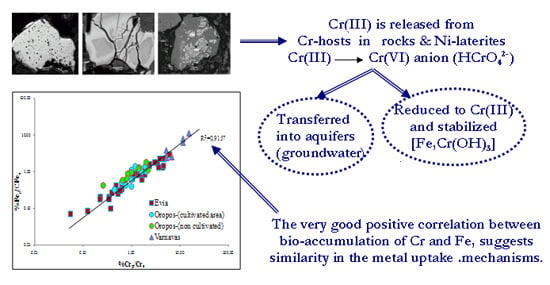
5. Discussion
5.1. Contamination of Soils by Cr, Ni, Co, Mn and Fe and Their Bioaccumulation
5.2. Trace Element Concentrations in Groundwater
6. Conclusions
Acknowledgments
References
- Jardine, P.M.; Mehlhorn, T.L.; Bailey, W.B.; Brooks, S.C.; Fendorf, S.; Gentry, R.W.; Phelps, T.J.; Saiers, J.E. Geochemical processes governing the fate and transport of chromium (III) and chromium (VI). Vadose Zone J. 2011, 10, 1058–1070. [Google Scholar] [CrossRef]
- Cross, H.J.; Faux, S.P.; Sadhra, S.; Sorahan, T.; Levy, L.S.; Braithwaite, R.; McRoy, C.; Hamilton, L.; Calvert, I. Criteria Document for Hexavalent Chromium; International Chromium Development Association (ICDA): Paris, France, 1997. [Google Scholar]
- Commission of the European Communities. Council Directive (98/83/EC) of 3 November 1998 on the quality of water intended for human consumption. Offic. J. Eur. Commun. 1998, L330, 32–54.
- Fantoni, D.; Brozzo, G.; Canepa, M.; Cipolli, F.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environ. Geol. 2002, 42, 871–882. [Google Scholar] [CrossRef]
- Ganas, A.; Aerts, J.; Astaras, T.; de Vente, J.; Frogoudakis, E.; Lambrinos, N.; Riskakisi, C.; Oikonomidis, D.; Filippidis, A.; Kassoli-Fournaraki, A. The use of Earth observation and decision support systems in the restoration of open-cast nickel mines in Evia, central Greece. Int.J. Remote Sens. 2004, 25, 3261–3274. [Google Scholar] [CrossRef]
- Papastergios, G.; Filippidis, A.; Fernandez-Turiel, J.L.; Gimeno, D.; Sikalidis, C. Distribution of potentially toxic elements in sediments of an industrialized coastal zone of the Northern Aegean Sea. Environ. Forensics 2010, 11, 282–292. [Google Scholar] [CrossRef]
- Linos, A.; Petralias, A.; Christophi, C.; Christoforidou, E.; Kouroutou, P.; Stoltidis, M.; Veloudaki, A.; Tzala, E.; Makris, K.; Karagas, M. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece—An ecological study. Environ. Health 2011, 10, 50:1–50:8. [Google Scholar]
- Papastergios, G.; Filippidis, A.; Fernandez-Turiel, J.L.; Gimeno, D.; Sikalidis, C. Surface soil geochemistry for environmental assessment in Kavala Area, Northern Greece. Water Air Soil Pollut. 2011, 216, 141–152. [Google Scholar] [CrossRef]
- Vasilatos, Ch.; Megremi, I.; Economou-Eliopoulos, M. Geochemical Characteristics of Natural Waters Contaminated by Hexavalent Chromium, in Eastern Sterea Hellas, Greece. In Proceedings of the XIX Congress of the Carpathian Balkan Geological Association; Christofides, G., Kantiranis, N., Kostopoulos, D.S., Chatzipetros, A.A., Eds.; Scientific Annales, School of Geology, Aristotle University of Thessaloniki: Thessaloniki, Greece, 2010; Volume 99, pp. 347–353. [Google Scholar]
- Petrotou, A.; Skordas, K.; Papastergios, G.; Filippidis, A. Factors affecting the distribution of potentially toxic elements in surface soils around an industrialized area of northwestern Greece. Environ. Earth Sci. 2012, 65, 823–833. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Megremi, I.; Atsarou, C.; Theodoratou, Ch.; Vasilatos, Ch. Contamination at the Assopos—Thiva Basins and C. Evia, Greece: An Integrated Approach on the Soil, Plant-Crops and Graoundwater System. In Proceedings of 3rd International Conference on Industrial and Hazardous Waste Management, Chania, Greece, 12–14 September 2012.
- Papanikolaou, D.; Mariolakos, I.; Lekkas, E.; Lozios, S. Morphotectonic observations at the Assopos basin and the coastal zone of Oropos. Contribution to the neotectonics of Northern Attiki. Bull. Geol. Soc. Greece 1988, 20, 251–267. [Google Scholar]
- Chatoupis, Th.; Fountoulis, I. The neotectonic deformation of N. Parnis Mt, (Attica, Greece). Bull. Geol. Soc. Greece 2004, 36, 1588–1597. [Google Scholar]
- Giannoulopoulos, P.A. Preliminary Hydrological—Hydrochemical Research, Contamination of Ground Water in the Assopos Basin, Boeotia Region; Institute of Geology and Mineral Exploration (IGME): Athens, Greece, 2009; Internal Report. [Google Scholar]
- American Public Health Association, American Water Rocks Association, Water Pollution; Standard Methods for the Examination of Water and Wastewater, 17th ed; Water Pollution Control Federation: Washington, DC, USA, 1989.
- American Society for Testing and Materials, Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils; Method D 2974-00; American Society for Testing and Materials: West Conshohocken, PA, USA, 2000.
- Megremi, I. Distribution and bioavailability of Cr in Central Evia, Greece. Cent. Eur. J. Geosci. 2009, 2, 103–123. [Google Scholar] [CrossRef]
- Megremi, I. Controlling Factors of the Mobility and Bioavailability of Cr and Other Metals at the Environment of Ni-Laterites. Ph.D. Thesis, University of Athens, Athens, Greece, 17 March 2010. [Google Scholar]
- Economou-Eliopoulos, M.; Megremi, I.; Vasilatos, Ch. Factors controlling the heterogeneous distribution of Cr(VI) in soil, plants and groundwater: Evidence from the Assopos basin, Greece. Chem. Erde 2011, 71, 39–52. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Antivach, D.; Vasilatos, Ch.; Megremi, I. Evaluation of the Cr(VI) and other toxic element contamination and their potential sources: The case of the Thiva basin (Greece). Geosci. Front. 2012, 3, 1–17. [Google Scholar] [CrossRef]
- Atsarou, C. Distribution of Chromium and Other Heavy Metals in Groundwater, Soil and Crops at the Avlona Area, Attica: Factors Controlling Their Bioavailability. Master’s Thesis, University of Athens, Athens, Greece, 17 June 2010. [Google Scholar]
- Theodoratou, Ch. Assessment of Contamination Due to Cr (VI) and Other Heavy Metals at the Oropos Area: Interaction in the System Soil-Plant-Water. Master’s Thesis, University of Athens, Athens, Greece, 26 May 2011. [Google Scholar]
- Papafilippaki, A.; Gasparatos, D.; Haidouti, C.; Stavroulakis, G. Total and bioavailable forms of Cu, Zn, Pb and Cr in agricultural soils: A study from the hydrological basin of Keritis, Chania, Greece. Glob. NEST J. 2007, 9, 201–206. [Google Scholar]
- Kabata-Pendlas, A. Trace Elements in Soils and Plants; CRC Press, Inc.: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kampouroglou, E. Investigation of the Contamination in the Carbonate Basin of Varnava (Attica) by Arsenic and Heavy Metals and Their Source. Master’s Thesis, University of Athens, Athens, Greece, 2 December 2012. [Google Scholar]
- Vasilatos, Ch.; Megremi, I.; Economou-Eliopoulos, M.; Mitsis, I. Hexavalent chromium and other toxic elements in natural waters in Thiva-Tanagra-Malakasa basin, Greece. Hell. J. Geosci. 2008, 43, 57–66. [Google Scholar]
- Moraki, A. Assessment of groundwater contamination by hexavalent chromium and its remediation at Avlida area, Central Greece. Hell. J. Geosci. 2010, 45, 175–183. [Google Scholar]
- Golightly, J.P. Nickeliferious laterite deposits. Econ. Geol. 1981, 75, 70–735. [Google Scholar]
- Navrotsky, A.; Mazeina, L.; Majlan, J. Size-driven structural and thermodynamic complexity in iron oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M. Apatite and Mn, Zn, Co-enriched chromite in Ni-laterites of northern Greece and their genetic significance. J. Geochem. Explor. 2003, 80, 41–54. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, J.; Payzant, A.; Paranthaman, M.P. Electrical conductivity of the manganese chromite spinel solid solution. J. Am. Ceram. Soc. 2005, 88, 1050–1053. [Google Scholar] [CrossRef]
- Princivalle, F.; Margignago, F.; Dal Negro, A. Kinetics of cation ordering in natural Mg (Al, Cr3+)2O4 spinels. Am. Mineral. 2006, 91, 313–318. [Google Scholar] [CrossRef]
- Masel, R.I. Chemical Kinetics and Catalysis; Wiley Interscience: New York, NY, USA, 2001. [Google Scholar]
- Asyminas, G. Assessment of the Environmental Impact by Chromium and Other Heavy Metals in Soil and Groundwater of the Assopos Basin: the Role of the Organic Matter to the Metal Bio-Availability. Master’s Thesis, University of Athens, Athens, Greece, 2 January 2012. [Google Scholar]
- Stock, E.L. Minerals as energy sources for microorganisms. Econ. Geol. 2009, 104, 1235–1248. [Google Scholar] [CrossRef]
- Zayed, A.M.; Terry, N. Chromium in the environment: factors affecting biological remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Manceau, A.; Schlegel, M.L.; Musso, M.; Sole, V.A.; Gauthier, C.; Petit, P.A.; Trolard, F. Crystal chemistry of trace elements in natural and synthetic goethite. Geochim. Cosmochim. Acta 2000, 64, 3643–3661. [Google Scholar] [CrossRef]
- Murray, J.W. The interaction of cobalt with hydrous manganese dioxide. Geochim. Cosmochim. Acta 1975, 39, 635–648. [Google Scholar] [CrossRef]
- Shanker, A.K.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Scott, D.M.; Grunthaner, P.J.; Tsaur, B.Y.; Nicolet, M.-A.; Mayer, J.W. The Effect of Oxygen on the Growth Kinetics of Nickel Silicides. In Proceedings 80-2, Symposium on Thin Film Interfaces and Interactions, Los Angeles, CA, USA, Fall 1979; Baglin, J.E.E., Poate, J.M., Eds.; 1980; p. 148. [Google Scholar]
- Liesack, W.; Schnell, S.; Revsbech, N.P. Microbiology of flooded rice paddies. FEMS Microbiol. Rev. 2000, 24, 625–645. [Google Scholar] [CrossRef]
- Hansel, C.M.; Wielinga, B.W.; Fendorf, S.R. Structural and compositional evolution of Cr/Fe solids after indirect chromate reduction by dissimilatory iron-reducing bacteria. Geochim. Cosmochim. Acta 2003, 67, 401–412. [Google Scholar]
- Bonet, A.; Poschenrieder, C.; Barcelo, J. Chromium III ion interaction in Fe deficient bean plants. I. Growth and Nutrient content. J. Plant Nutr. 1991, 14, 403–414. [Google Scholar] [CrossRef]
- Lemanceau, P.; Bauer, B.; Kraemer, K.; Stand Briat, J.-F. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 2009, 321, 513–535. [Google Scholar] [CrossRef]
- Tziritis, E.P. Assessment of NO3− contamination in a karstic aquifer, with the use of geochemical data and spatial analysis. Environ. Earth Sci. 2009, 60, 1381–1390. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Economou-Eliopoulos, M.; Megremi, I.; Atsarou, C.; Theodoratou, C.; Vasilatos, C. Spatial Evolution of the Chromium Contamination in Soils from the Assopos to Thiva Basin and C. Evia (Greece) and Potential Source(s): Anthropogenic versus Natural Processes. Geosciences 2013, 3, 140-158. https://doi.org/10.3390/geosciences3020140
Economou-Eliopoulos M, Megremi I, Atsarou C, Theodoratou C, Vasilatos C. Spatial Evolution of the Chromium Contamination in Soils from the Assopos to Thiva Basin and C. Evia (Greece) and Potential Source(s): Anthropogenic versus Natural Processes. Geosciences. 2013; 3(2):140-158. https://doi.org/10.3390/geosciences3020140
Chicago/Turabian StyleEconomou-Eliopoulos, Maria, Ifigeneia Megremi, Cathy Atsarou, Christina Theodoratou, and Charalambos Vasilatos. 2013. "Spatial Evolution of the Chromium Contamination in Soils from the Assopos to Thiva Basin and C. Evia (Greece) and Potential Source(s): Anthropogenic versus Natural Processes" Geosciences 3, no. 2: 140-158. https://doi.org/10.3390/geosciences3020140