Evolving Phytoplankton Stoichiometry Fueled Diversification of the Marine Biosphere
Abstract
:1. Introduction
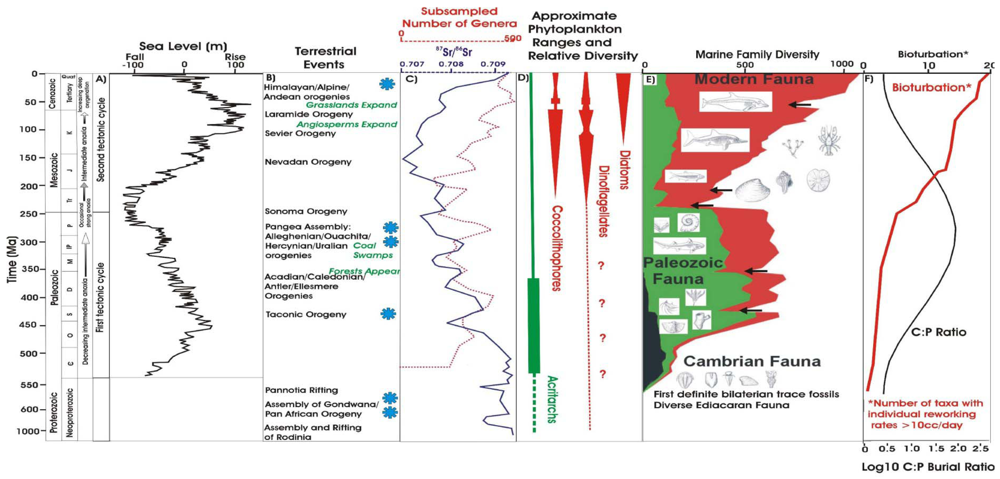
2. The Fossil Record of Plankton
3. Nutrients and the Evolution of Major Algal Lineages
4. The Shift from Green to Red Phytoplankton Lineages
5. Implications for the Phanerozoic Record of Marine Biodiversity
6. Food and the Emergence of Metazoans in the Fossil Record
7. Conclusions
Acknowledgements
References
- Sepkoski, J.J. A factor analytic description of the Phanerozoic marine fossil record. Paleobiology 1981, 7, 36–53. [Google Scholar]
- Alroy, J. The shifting balance of diversity among major marine animal groups. Science 2010, 329, 1191–1194. [Google Scholar] [CrossRef]
- Hannisdal, B.; Peters, S.E. Phanerozoic earth system evolution and marine biodiversity. Science 2011, 334, 1121–1124. [Google Scholar] [CrossRef]
- Benton, M.J. The Red Queen and the court jester: Species diversity and the role of biotic and abiotic factors through time. Science 2009, 323, 728–732. [Google Scholar] [CrossRef]
- Marshall, C.R. Marine biodiversity dynamics over deep time. Science 2010, 329, 1156–1157. [Google Scholar] [CrossRef]
- Tappan, H. Primary production, isotopes, extinctions and the atmosphere. Palaeogeog. Palaeoclimatol. Palaeoecol. 1968, 4, 187–210. [Google Scholar] [CrossRef]
- Tappan, H. Microplankton, Ecological Succession and Evolution; North American Paleontological Convention: Chicago, IL, USA, 1971; pp. 1058–1103, part H. [Google Scholar]
- Tappan, H. Phytoplankton: Below thesalt at theglobal table. J. Paleontol. 1986, 60, 545–554. [Google Scholar]
- Bambach, R.K. Energetics in the global marine fauna: A connection between terrestrial diversification and change in the marine biosphere. GeoBios 1999, 32, 131–144. [Google Scholar] [CrossRef]
- Bambach, R.K. Seafood through time: Changes in biomass, energetics, and productivity in the marine ecosystem. Paleobiology 1993, 19, 372–397. [Google Scholar]
- Martin, R.E. Secular increase in nutrient levels through the Phanerozoic: Implications for productivity, biomass, and diversity of the marine biosphere. Palaios 1996, 11, 209–219. [Google Scholar] [CrossRef]
- Kilham, P.; Kilham, S.S. The evolutionary ecology of phytoplankton. In The Physiological Ecology of Phytoplankton; Morris, I., Ed.; University of California Press: Berkeley, CA, USA, 1980; pp. 571–597. [Google Scholar]
- Bush, A.M.; Bambach, R.K. Paleoecologic megatrends in marine metazoa. Ann. Rev. Earth Planet. Sci. 2011, 39, 241–269. [Google Scholar] [CrossRef]
- Thayer, C.W. Sediment-mediated biological disturbance and the evolution of marine benthos. In Biotic Interactions in Recent and Fossil Benthic Communities; Tevesz, M.J.S., McCall, P.L., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 479–625. [Google Scholar]
- Fox, R. Energy and the Evolution of Life; W.H. Freeman: New York, NY, USA, 2008; p. 182. [Google Scholar]
- Quigg, A.; Finkel, Z.V.; Irwin, A.J.; Rosenthal, Y.; Ho, T.-Y.; Reinfelder, J.R.; Schofield, O.; Morel, F.M.M.; Falkowski, P.G. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 2003, 425, 291–294. [Google Scholar]
- Quigg, A.; Irwin, A.J.; Finkel, Z.V. Evolutionary imprint of endosymbiosis of elemental stoichiometry: Testing inheritance hypotheses. Proc. R. Soc. London Ser. B 2011, 278, 526–534. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; p. 439. [Google Scholar]
- Martin, R.E.; Quigg, A.; Podkovyrov, V. Marine biodiversification in response to evolving phytoplankton stoichiometry. Palaeogeog. Palaeoclimatol. Palaeoecol. 2008, 258, 277–291. [Google Scholar] [CrossRef]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.F.; Diener, A.; Ebneth, S.; Godderis, Y.; et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef]
- Montañez, I.P.; Osleger, D.A.; Banner, J.L.; Mack, L.E. Evolution of the Sr and C isotope composition of Cambrian oceans. GSA Today 2000, 10, 1–7. [Google Scholar]
- Tardy, Y.; N’Kounkou, R.; Probst, J.-L. The global water cycle and continental erosion during Phanerozoic time (570 my). Am. J. Sci. 1989, 289, 455–483. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.; Froelich, P.N. Lithium isotope history of Cenozoic seawater: Changes in silicate weathering and reverse weathering. Science 2012, 335, 818–823. [Google Scholar]
- Egge, J.K.; Aksnes, D.L. Silica as regulating nutrient in phytoplankton competition. Mar. Ecol. Progr. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Aubry, M.-P. Early paleogene calcareous nannoplankton evolution: A tale of climatic amelioration. In Late Paleocene-Early Eocene Climatic and Biotic Events in the Marine and Terrestrial Records; Aubry, M.-P., Lucas, S., Berggren, W.A., Eds.; Columbia University Press: New York, NY, USA, 1998; pp. 158–203. [Google Scholar]
- Riegman, R.; Stolte, W.; Noordeloos, A.A.M.; Slezak, D. Nutrient uptake and alkaline phosphatase (ec 3:1:3:1) activity of Emiliania huxleyi (Prymnesiophyceae) during growth under N and P limitation in continuous cultures. J. Phycol. 2000, 36, 87–96. [Google Scholar] [CrossRef]
- Rabosky, D.L.; Sorhannus, U. Diversitydy namics of marine planktonic diatoms across the Cenozoic. Nature 2009, 557, 183–186. [Google Scholar]
- Pirini-Radrazzani, C. Coccoliths from Permian deposits of Eastern Turkey. In Proceedings of the II Planktonic Conference, Rome, Italy, 1971; Farinacci, A., Ed.; Edizioni Tecnoscienza: Rome, Italy; 2, pp. 993–1001.
- Gartner, S.; Gentile, R. Problematic Pennsylvanian coccoliths from Missouri. Micropaleontology 1972, 18, 401–404. [Google Scholar] [CrossRef]
- Minoura, N.; Chitoku, T. Calcareous nannofossil and problematic microorganisms found in the late Paleozoic limestones. J. Fac. Sci. Hokkaido Univ. Series IV Geol. Miner. 1972, 19, 199–212. [Google Scholar]
- Munnecke, A.; Samtleben, C.; Servais, T.; Vachard, D. SEM-observation of calcareous micro- and nannofossils incertae sedis from the Silurian of Gotland, Sweden: Preliminary results. Geobios 1998, 32, 307–314. [Google Scholar]
- Munnecke, A.; Servais, T.; Vachard, D. A new familyof calcareous microfossils from theSilurian ofGotland, Sweden. Palaeontology 2000, 43, 1153–1172. [Google Scholar]
- Flügel, E.H. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Springer Verlag: Berlin, Germany, 2010; p. 984. [Google Scholar]
- Bown, P.R.; Lees, J.A.; Young, J.R. Calcareous nannoplankton evolution and diversity through time. In Coccolithophores: From Molecular Processes to Global Impact; Thierstein, H., Young, J.R., Eds.; Springer-Verlag: Berlin, Germany, 2004; pp. 481–508. [Google Scholar]
- De Vargas, C.; Aubry, M.-P.; Probert, I.; Young, J. Origin and evolution of coccolithophores: From coastal hunters to oceanic farmers. In Evolution of Primary Producers in the Sea; Falkowski, P., Knoll, A.H., Eds.; Academic Press: New York, NY, USA, 2007; pp. 251–285. [Google Scholar]
- Moldowan, J.M.; Dahl, J.; Jacobson, S.R.; Huizinga, B.J.; Fago, F.J.; Shetty, R.; Watt, D.S.; Peters, K.E. Chemostratigraphic reconstruction of biofacies: Molecular evidence linking cyst-forming dinoflagellates with pre-Triassic ancestors. Geology 1996, 24, 159–162. [Google Scholar] [CrossRef]
- Grantham, P.J.; Wakefield, L.L. Variations in the sterane carbon number distributions of marine source rock derived crude oils through geological time. Org. Geochem. 1988, 12, 61–73. [Google Scholar] [CrossRef]
- Schwark, L.; Empt, P. Sterane biomarkers as indicators of Palaeozoic algal evolution and extinction events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 240, 225–236. [Google Scholar] [CrossRef]
- Perch-Nielsen, K. Mesozoic calcareous nannofossils. In Plankton Stratigraphy; Bolli, H.M., Saunders, J.B., Perch-Nielsen, K., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 329–426. [Google Scholar]
- Boss, S.K.; Wilkinson, B.H. Planktogenic/eustatic control on cratonic oceanic carbonate accumulation. J. Geol. 1991, 99, 497–513. [Google Scholar]
- Van Andel, T.H. Mesozoic/Cenozoic calcite compensation depth and the global distribution of calcareous sediments. Earth Planet Sci. Lett. 1975, 26, 187–195. [Google Scholar] [CrossRef]
- Hüneke, H.; Henrich, R. Pelagic sedimentation in modern and ancient oceans. In Deep-Sea Sediments; Hüneke, H., Mulder, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 215–351. [Google Scholar]
- Bown, P.R.; Dunkley Jones, T.; Lees, J.A.; Randell, R.D.; Mizzi, J.A.; Pearson, P.N.; Coxall, H.K.; Young, J.R.; Nicholas, C.J.; Karega, A.; et al. A Paleogene calcareous microfossil Konservat-Lagerstätte from the Kilwa Group of coastal Tanzania. Geol. Soc. Am. Bull. 2008, 120, 3–12. [Google Scholar] [CrossRef]
- Stanley, S.M.; Hardie, L.A. Hypercalcification: Paleontology links plate tectonics and geochemistry to sedimentology. GSA Today 1999, 9, 2–7. [Google Scholar]
- Stanley, S.M.; Ries, J.B.; Hardie, L.A. Seawater chemistry, coccolithophore population growth, and the origin of Cretaceous chalk. Geology 2005, 33, 593–596. [Google Scholar] [CrossRef]
- Paasche, E. Roles of nitrogen and phosphorus in coccolith formation in Emiliania huxleyi (Prymnesiophyceae). Eur. J. Phycol. 1998, 33, 33–42. [Google Scholar]
- Riebesell, U. Effects of CO2 enrichment on marine phytoplankton. J. Oceanogr. 2004, 60, 719–729. [Google Scholar] [CrossRef]
- Walker, K.R.; Diehl, W.W. The role of marine cementation in the preservation of Lower Paleozoic assemblages. In Extraordinary Fossil Biotas: Their Ecological and Evolutionary Significance; Whittington, H.B., Conway Morris, S., Eds.; Scholium International, Inc.: Washington, NY, USA, 1985; Volume B311, pp. 143–153. [Google Scholar]
- Young, J.R.; Geisen, M.; Probert, I. Review of selected aspects of coccolithophore biology with implications for paleobiodiversity estimation. Micropaleontology 2005, 51, 267–288. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Katz, M.E.; Knoll, A.H.; Quigg, A.; Raven, J.A.; Schofield, O.; Taylor, F.J.R. The evolution of modern eukaryotic phytoplankton. Science 2004, 305, 354–360. [Google Scholar]
- Hackett, J.D.; Yoon, H.S.; Butterfield, N.J.; Sanderson, M.J.; Bhattacharya, D. Plastid endosvmbiosis: Sources and timing of the major events. In Evolution of Primary Producers in the Sea; Falkowski, P., Knoll, A.H., Eds.; Academic Press: New York, NY, USA, 2007; pp. 109–132. [Google Scholar]
- Wilde, P.; Lyons, T.W.; Quinby-Hunt, M.S. Organic proxies in black shales: Molybdenum. Chem. Geol. 2004, 206, 167–176. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hackett, J.D.; Bhattacharya, D. A single origin of the peridinin- and fucoxanthin- containing plastids in dinoflagellates through tertiary endosymbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 11724–11729. [Google Scholar]
- Yoon, H.S.; Hackett, J.D.; Pinto, G.; Bhattacharya, D. Thesingle, ancient origin of chromist plastids. Proc. Natl. Acad. Sci. USA 2002, 99, 15507–15512. [Google Scholar]
- Butterfield, N.J. A vaucheriacean alga from the middle Neoproterozoic of Spitsbergen: Implications for the evolution of Proterozoic eukaryotes and the Cambrian explosion. Paleobiology 2004, 30, 231–252. [Google Scholar] [CrossRef]
- Cohen, P.A.; Knoll, A.H.; Kodner, R.B. Large spinose microfossils in Ediacaran rocks as resting stages of early animals. Proc. Natl. Acad. Sci. USA 2009, 106, 6519–6524. [Google Scholar]
- Van de Schootbrugge, B.; Bailey, T.; Rosenthal, Y.; Katz, M.E.; Wright, J.D.; Feist-Burkhardt, S.; Miller, K.G.; Falkowski, P.G. Early Jurassic climate change and the radiation of organic-walled phytoplankton in the Tethys Ocean. Paleobiology 2005, 31, 73–97. [Google Scholar] [CrossRef]
- Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 1999, 400, 525–531. [Google Scholar] [CrossRef]
- Howarth, R.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine environments: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar]
- Elser, J.J.; Dobberfuhl, D.R.; MacKay, N.A.; Schampel, J.H. Organism size, life history, and N:P stoichiometry. BioScience 1996, 46, 674–684. [Google Scholar] [CrossRef]
- Main, T.M.; Dobberfuhl, D.R.; Elser, J.J. N:P stoichiometry and ontogeny of crustacean zooplankton: A test of the growth rate hypothesis. Limnol. Oceanogr. 1997, 42, 1474–1478. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Charnov, E.L.; West, G.B.; Savage, V.M.; Brown, J.H. Effects of size and temperature on developmental time. Nature 2002, 417, 70–73. [Google Scholar]
- Mulder, C.; Elser, J.J. Soil acidity, ecological stoichiometry and allometric scaling in grassland food webs. Glob. Chang. Biol. 2009, 15, 2730–2738. [Google Scholar] [CrossRef]
- Allmon, W.D.; Ross, R.M. Nutrients and evolution in the marine realm. In Evolutionary Paleoecology: The Ecological Context of Evolutionary Change; Allmon, W.D., Bottjer, D.J., Eds.; Columbia University Press: New York, NY, 2001; pp. 105–148. [Google Scholar]
- Falkowski, P.G.; Rosenthal, Y. Biological diversity and resource plunder in the geological record: Casual correlations or causal relationships? Proc. Natl. Acad. Sci. USA 2001, 98, 4290–4292. [Google Scholar] [CrossRef]
- Ingall, E.; Jahnke, R. Influence of water-column anoxia on the elemental fractionation of carbon and phosphorus during sediment diagenesis. Mar. Geol. 1997, 139, 219–229. [Google Scholar] [CrossRef]
- Goldhammer, T.; Brüchert, V.; Ferdelman, T.G.; Zabel, M. Microbial sequestration of phosphorus in anoxic upwelling sediments. Nat. Geosci. 2010, 3, 557–561. [Google Scholar] [CrossRef]
- Williams, R.J.P.; Frausto Da Silva, J.J.R. The Natural Selection of the Chemical Elements; Bath Press Ltd.: Bath, UK, 1996; p. 646. [Google Scholar]
- Riegel, W. The Late Palaeozoic phytoplankton blackout—Artefact or evidence of global change? Rev. Palaeobot. Palynol. 2008, 148, 73–90. [Google Scholar] [CrossRef]
- Pitrat, C.W. Phytoplankton and the late Paleozoic wave of extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1970, 8, 49–55. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Schofield, O.; Katz, M.E.; van de Schootbrugge, B.; Knoll, A. Why is the land green and the ocean red? In Coccolithophores: From Molecular Processes to Global Impact; Thierstein, H., Young, J.R., Eds.; Springer-Verlag: Berlin, Germany, 2004; pp. 429–453. [Google Scholar]
- Tyrrell, T.; Taylor, A.H. A modelling study of Emiliania huxleyi in the NE Atlantic. J. Mar. Syst. 1996, 9, 83–112. [Google Scholar] [CrossRef]
- Andrusevich, V.E.; Engel, M.H.; Zumberge, J.E.; Brothers, L.A. Secular, episodic changes in stable carbon isotope composition of crude oils. Chem. Geol. 1998, 152, 59–72. [Google Scholar] [CrossRef]
- Cárdenas, A.; Harries, P.J. Effect of nutrient availability on marine origination rates throughout the Phanerozoic eon. Nat. Geosci. 2010, 3, 430–434. [Google Scholar]
- Martin, R.E. Cyclic and secular variation in microfossil biomineralization: Clues to the biogeochemical evolution of Phanerozoic oceans. Glob. Planet. Chang. 1995, 11, 1–23. [Google Scholar] [CrossRef]
- Martin, R.E. Cyclic and secular trends in preservation through geologic time: Implications for the evolution of biogeochemical cycles. In Proceedings of International Conference Taphos 2002, Third Meeting on Taphonomy and Fossilization, Valencia, Spain, 14–16 February 2002; De Renzi, M., Alonso, M.V.P., Belinchón, M., Peñalver, E., Montoya, P., Márquez-Aliaga, A., Eds.; Gráficas Ronda, S.L.: Valencia, Spain, 2002; pp. 67–76. [Google Scholar]
- Martin, R.E. The fossil record of biodiversity: Nutrients, productivity, habitat area and differential preservation. Lethaia 2003, 36, 179–193. [Google Scholar] [CrossRef]
- Jones, N. Cambrian’s fiercest hunter defanged. Nature 2009. [Google Scholar]
- Signor, P.; Brett, C.B. The mid-Paleozoic precursor to the Mesozoic marine revolution. Paleobiology 1984, 10, 229–245. [Google Scholar]
- Ausich, W.I.; Bottjer, D.J. History of tiering among suspension feeders in the benthic marineecosystem. J. Geol. Educ. 1991, 39, 313–318. [Google Scholar]
- Solan, M.; Batty, P.; Bulling, M.T.; Godbold, J.A. How biodiversity affects ecosystem processes: Implications for ecological revolutions and benthic ecosystem function. Aquat. Biol. 2008, 2, 289–301. [Google Scholar]
- Teal, L.R.; Parker, E.R.; Solan, M. Sediment mixed layer as a proxy for benthic ecosystem process and function. Mar. Ecol. Progr. Ser. 2010, 414, 27–40. [Google Scholar] [CrossRef]
- Lehnert, O.; Vecoli, M.; Servasis, T.; Nützel, A. Did plankton evolution trigger the Ordovician diversifications? Acta Palaeontol. Sinica 2007, 46, 262–268. [Google Scholar]
- Erwin, D.H.; Laflamme, M.; Tweedt, S.M.; Sperling, E.A.; Pisani, D.; Peterson, K.J. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science 2011, 334, 1091–1097. [Google Scholar]
- Sepkoski, J.J.; Miller, A.I. Evolutionary faunas and the distribution of Paleozoic marine communities in space and time. In Phanerozoic Diversity Patterns: Profiles in Macroevolution; Valentine, J.W., Ed.; Princeton University Press: Princeton, NJ, USA, 1985; pp. 153–190. [Google Scholar]
- Butterfield, N.J.; Knoll, A.H.; Swett, K. A bangiophyte red alga from the Proterozoic rocks of Arctic Canada. Science 1990, 250, 104–107. [Google Scholar]
- Filippelli, G.M. Phosphorus and thegust of fresh air. Nature 2010, 467, 1052–1053. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Rouxel, O.J.; Bekker, A.; Lalonde, S.V.; Konhauser, K.O.; Reinhard, C.T.; Lyons, T.W. The evolution of the marine phosphate reservoir. Nature 2010, 467, 1088–1090. [Google Scholar]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Krom, M.D.; Mantoura, R.F.C.; Flaten, G.A.F.; Groom, S. Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science 2005, 309, 1068–1071. [Google Scholar]
- Elser, J.J.; Watts, J.; Schampel, J.H.; Farmer, J. Early food-webs on a trophic knife-edge? Experimental data from a modern microbialite-based ecosystem. Ecol. Lett. 2006, 9, 295–303. [Google Scholar] [CrossRef]
- Zhuravlev, A.Y. Biotic diversity and structure during the Neoproterozoic-Ordovician transition. In The Ecology of the Cambrian Radiation; Zhuravlev, A.Y., Riding, R., Eds.; Columbia University Press: New York, NY, USA, 2001; pp. 173–199. [Google Scholar]
- Cook, P.J.; McElhinny, M.W. A reevaluation of the spatial and temporal distribution of sedimentary phosphate deposits in the light of plate tectonics. Econ. Geol. 1979, 74, 315–330. [Google Scholar]
- Huntley, J.W.; Xiao, S.; Kowalewski, M. 1.3 Billion years of acritarch history: An empirical morphospace approach. Precambrian Res. 2006, 144, 52–68. [Google Scholar] [CrossRef]
- Hayes, J.M.; Strauss, H.; Kaufman, A.J. The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem. Geol. 1999, 161, 103–125. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Katz, M.; Wright, J.; Schofield, O.; Falkowski, P.G. Climatically driven macroevolutionary patterns in the size of marine diatoms over the Cenozoic. Proc. Natl. Acad. Sci. USA 2005, 102, 8927–8932. [Google Scholar]
- Finkel, Z.V.; Sebbo, J.; Feist-Burkhardt, S.; Irwin, A.J.; Katz, M.E.; Schofield, O.; Young, J.R.; Falkowski, P.G. A universal driver of macroevolutionary change in the size of marine phytoplankton over the Cenozoic. Proc. Natl. Acad. Sci. USA 2007, 104, 20416–20420. [Google Scholar]
- Bottjer, D.J.; Hagadorn, J.W.; Dornbos, S.Q. The Cambrian substrate revolution. GSA Today 2000, 10, 1–7. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martin, R.; Quigg, A. Evolving Phytoplankton Stoichiometry Fueled Diversification of the Marine Biosphere. Geosciences 2012, 2, 130-146. https://doi.org/10.3390/geosciences2020130
Martin R, Quigg A. Evolving Phytoplankton Stoichiometry Fueled Diversification of the Marine Biosphere. Geosciences. 2012; 2(2):130-146. https://doi.org/10.3390/geosciences2020130
Chicago/Turabian StyleMartin, Ronald, and Antonietta Quigg. 2012. "Evolving Phytoplankton Stoichiometry Fueled Diversification of the Marine Biosphere" Geosciences 2, no. 2: 130-146. https://doi.org/10.3390/geosciences2020130



