Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Voluntary Animal Approach Test (VAA)
2.3. Restraint
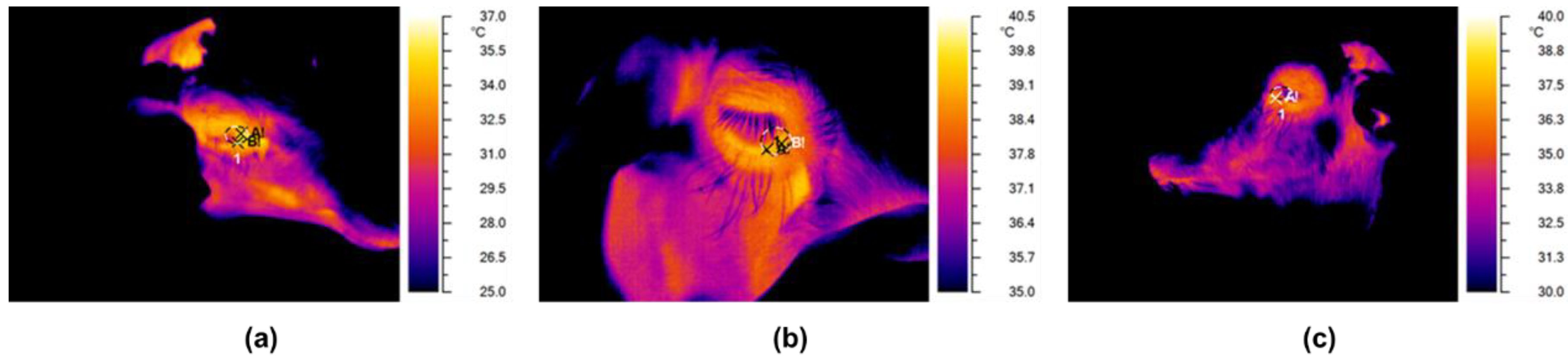
2.4. Infrared Thermography
2.5. Statistics
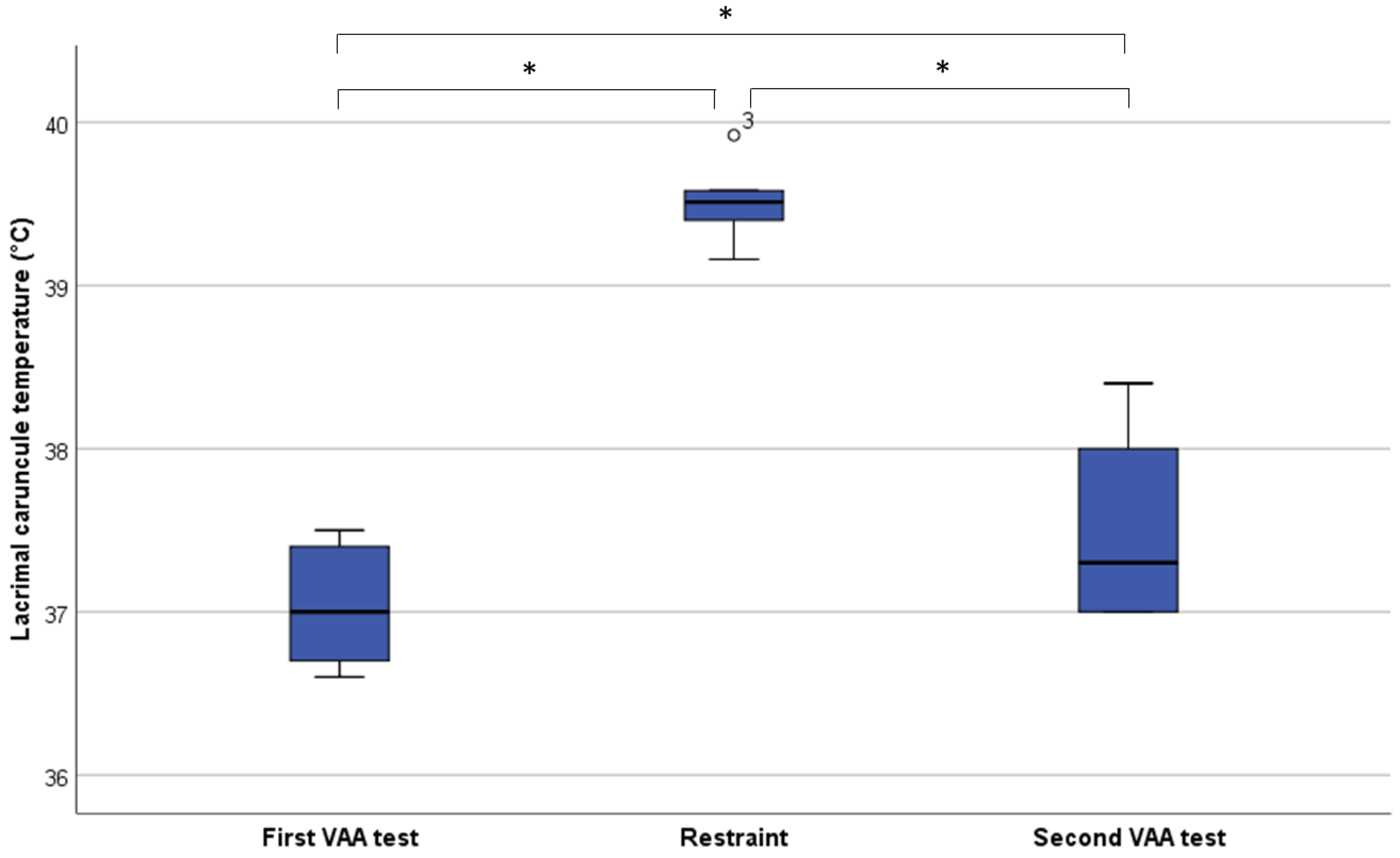
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boissy, A. Fear and fearfulness in determining behavior. In Genetics and the Behaviour of Domestic Animals; Grandin, T., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 67–111. [Google Scholar]
- Dwyer, C.M. How has the risk of predation shaped the behavioural responses of sheep to fear and distress? Anim. Welf. 2004, 13, 269–281. [Google Scholar]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.-C.C.; Canali, E.; Jones, R.B.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemsworth, P.; Barnett, J. Human-Animal Interactions. Vet. Clin. N. Am. Food Anim. Pract. 1987, 3, 339–356. [Google Scholar] [CrossRef]
- Webster, A.J.F. Farm Animal Welfare: The Five Freedoms and the Free Market. Vet. J. 2001, 161, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Beausoleil, N.J. Extending the “Five Domains” model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Rushen, J.; Taylor, A.A.; Passille, A.M. De Domestic animals’ fear of humans and its effect on their welfare. Appl. Anim. Behav. Sci. 1999, 65, 285–303. [Google Scholar] [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the human-animal relationship in farmed species: A critical review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Hild, S.; Coulon, M.; Schroeer, A.; Andersen, I.L.; Zanella, A.J. Gentle vs. aversive handling of pregnant ewes: I. Maternal cortisol and behavior. Physiol. Behav. 2011, 104, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Coleman, G.J. Human-Livestock Interactions: The Stockperson and the Productivity and Welfare of Intensively Farmed Animals, 2nd ed.; Hemsworth, P.H., Coleman, G.J., Eds.; CABI: Wallingford, UK, 2011. [Google Scholar]
- Geist, V. Mountain Sheep: A Study in Behaviour and Evolution; The University of Chicago Press: London, UK, 1971. [Google Scholar]
- Lynch, J.J.; Hinch, G.N.; Adams, D.B. The Behaviour of Sheep: Biological Principles and Implications for Production; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Romeyer, A.; Bouissou, M.F. Assessment of fear reactions in domestic sheep, and influence of breed and rearing conditions. Appl. Anim. Behav. Sci. 1992, 34, 93–119. [Google Scholar] [CrossRef]
- Boissy, A.; Bouissou, M.F. Assessment of individual differences in behavioural reactions of heifers exposed to various fear-eliciting situations. Appl. Anim. Behav. Sci. 1995, 46, 17–31. [Google Scholar] [CrossRef]
- Bouissou, M.F.; Vandenheede, M. Fear reactions of domestic sheep confronted with either a human or a human-like model. Behav. Process. 1995, 34, 81–92. [Google Scholar] [CrossRef]
- Vandenheede, M.; Bouissou, M.F.; Picard, M. Interpretation of behavioural reactions of sheep towards fear-eliciting situations. Appl. Anim. Behav. Sci. 1998, 58, 293–310. [Google Scholar] [CrossRef]
- Vierin, M.; Bouissou, M. Responses of Weaned Lambs to Fear-Eliciting Situations: Origin of Individual Differences. Dev. Psychobiol. 2001, 42, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, N.J.; Stafford, K.J.; Mellor, D.J. Sheep show more aversion to a dog than to a human in an arena test. Appl. Anim. Behav. Sci. 2005, 91, 219–232. [Google Scholar] [CrossRef]
- Dodd, C.L.; Pitchford, W.S.; Hocking, J.E.; Hazel, S.J. Measures of behavioural reactivity and their relationships with production traits in sheep: A review. Appl. Anim. Behav. Sci. 2012, 140, 1–15. [Google Scholar] [CrossRef]
- Waiblinger, S.; Menke, C.; Fölsch, D.W. Influences on the avoidance and approach behaviour of dairy cows towards humans on 35 farms. Appl. Anim. Behav. Sci. 2003, 84, 23–39. [Google Scholar] [CrossRef]
- Cook, N.J.; Church, J.S.; Schaefer, A.L.; Webster, J.R.; Matthews, L.R.; Suttie, J.M. Stress and pain assessment of velvet antler removal from elk (Cervus elaphus canadensis) and reindeer (Rangifer tarandus). Online J. Vet. Res. 2005, 9, 13–25. [Google Scholar]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.; Eberhardt, N.L.; Levine, J.A. Human behaviour: Seeing through the face of deception. Nature 2002, 415, 35. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.L.; Stewart, M.; Webster, J.R.; Cook, N.J.; Colyn, J.J.; Lepage, P.; Church, J.S.; Haley, D.B. Objective measurement of pain and fear in cattle using infrared thermography. In Proceeding of International Society of Applied Ethology, North American Regional Meeting; The University of British Columbia: Vancouver, BC, Canada, 2006; p. 55. [Google Scholar]
- Dai, F.; Cogi, N.H.; Heinzl, E.U.L.; Dalla Costa, E.; Canali, E.; Minero, M. Validation of a fear test in sport horses using infrared thermography. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 128–136. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Dumont, B.; Boissy, A. Grazing behaviour of sheep in a situation of conflict between feeding and social motivations. Behav. Process. 2000, 49, 131–138. [Google Scholar] [CrossRef]
- Bartolomé, E.; Sánchez, M.J.; Molina, A.; Schaefer, A.L.; Cervantes, I.; Valera, M. Using eye temperature and heart rate for stress assessment in young horses competing in jumping competitions and its possible in fluence on sport performance. Animal 2013, 7, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.P.; Zickler, T.E.; Kriegman, D.J.; Belhumeur, P.N. Beyond Lambert: Reconstructing specular surfaces using color. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Diego, CA, USA, 20–25 June 2005; Volume 2, pp. 619–626. [Google Scholar]
- GRAYESS. IRT Analyser Users’ Manual; GRAYESS, Inc.: Bradenton, FL, USA, 2007. [Google Scholar]
- Boissy, A.; Aubert, A.; Désiré, L.; Greiveldinger, L.; Delval, E.; Veissier, I.; Desire, L. Cognitive sciences to relate ear postures to emotions in sheep. Anim. Welf. 2011, 20, 47–56. [Google Scholar]
- Hargreaves, A.L.; Hutson, G.D. The effect of gentling on heart rate, flight distance and aversion of sheep to a handling procedure. Appl. Anim. Behav. Sci. 1990, 26, 243–252. [Google Scholar] [CrossRef]
- National Farm Animal Care Council. Code of Practice for the Care and Handling of Sheep; National Farm Animal Care Council: Calgary, AB, Canada, 2013. [Google Scholar]
- Nakayama, K.; Goto, S.; Kuraoka, K.; Nakamura, K. Decrease in nasal temperature of rhesus monkeys (Macaca mulatta) in negative emotional state. Physiol. Behav. 2005, 84, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Simoens, P. Anatomie Compare des Mammifères Domestiques; Tome 7 Neurologie II; Vigot: Paris, France, 2010. [Google Scholar]
- Špinka, M. Social dimension of emotions and its implication for animal welfare. Appl. Anim. Behav. Sci. 2012, 138, 170–181. [Google Scholar] [CrossRef]
- O’Hara, S.J.; Reeve, A.V. A test of the yawning contagion and emotional connectedness hypothesis in dogs, Canis familiaris. Anim. Behav. 2011, 81, 335–340. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Reefmann, N.; Bütikofer Kaszàs, F.; Wechsler, B.; Gygax, L. Ear and tail postures as indicators of emotional valence in sheep. Appl. Anim. Behav. Sci. 2009, 118, 199–207. [Google Scholar] [CrossRef]
- Reefmann, N.; Wechsler, B.; Gygax, L. Behavioural and physiological assessment of positive and negative emotion in sheep. Anim. Behav. 2009, 78, 651–659. [Google Scholar] [CrossRef]
- Napolitano, F.; De Rosa, G.; Girolami, A.; Scavone, M.; Braghieri, A. Avoidance distance in sheep: Test-retest reliability and relationship with stockmen attitude. Small Rumin. Res. 2011, 99, 81–86. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep. Animals 2018, 8, 146. https://doi.org/10.3390/ani8090146
Cannas S, Palestrini C, Canali E, Cozzi B, Ferri N, Heinzl E, Minero M, Chincarini M, Vignola G, Dalla Costa E. Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep. Animals. 2018; 8(9):146. https://doi.org/10.3390/ani8090146
Chicago/Turabian StyleCannas, Simona, Clara Palestrini, Elisabetta Canali, Bruno Cozzi, Nicola Ferri, Eugenio Heinzl, Michela Minero, Matteo Chincarini, Giorgio Vignola, and Emanuela Dalla Costa. 2018. "Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep" Animals 8, no. 9: 146. https://doi.org/10.3390/ani8090146





