Effects of Dark Brooders on Behavior and Fearfulness in Layers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Animals and Housing Conditions
2.1.1. Brooder and Control Groups
2.1.2. The Four Brooder Treatments
2.1.3. Available Resources and Management
2.1.4. Ethical Note
2.2. Data Collection
2.2.1. Behavioral Time Budget
2.2.2. Novel Object Test
2.2.3. Open-Field Test
2.2.4. Tonic Immobility Test
2.3. Statistical Analysis
3. Results
| Behavioral measurements (%) 1 | SF2 (Day) | SM2 (Day) | LF2 (Day) | LM2 (Day) | C2 (Day) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Resting | 82.0 ± 1.6 b | 81.8 ± 1.6 b | 79.3 ± 2.3 b | 79.0 ± 2.6 b | 84.0 ± 1.8 b | 88.1 ± 1.9 a | 80.2 ± 2.7 b | 76.1 ± 3.0 b | 83.4 ± 1.6 b | 83.4 ± 1.6 b | 76.2 ± 2.3 b | 73.9 ± 2.6 b | 84.0 ± 1.6 b | 83.3 ± 1.6 b | 78.3 ± 2.3 b | 75.0 ± 2.6 b | 71.3 ± 1.3 c | 64.6 ± 1.3 d | 65.5 ± 1.9 d | 64.6 ± 2.1 d |
| Resting under the brooder | 73.7 ± 0.9 b | 77.0 ± 1.2 b | 75.9 ± 2.5 b | 76.6 ± 2.0 b | 75.4 ± 0.9 b | 84.3 ± 1.0 a | 76.4 ± 3.7 b | 73.7 ± 5.0 b | 74.2 ± 0.5 b | 78.9 ± 0.9 b | 73.9 ± 0.8 b | 71.6 ± 2.7 b | 77.4 ± 2.1 b | 77.6 ± 1.0 b | 74.8 ± 1.9 b | 70.6 ± 2.3 b | ||||
| Resting outside the brooder | 8.3 ± 1.6 a | 4.7 ± 0.7 b | 3.3 ± 0.8 b | 2.4 ± 0.3 c | 8.6 ± 1.5 a | 3.8 ± 0.6 b | 3.8 ± 0.4 b | 2.4 ± 0.1 c | 9.1 ± 1.0 a | 4.4 ± 0.9 b | 2.3 ± 0.5 c | 2.3 ± 0.2 c | 6.5 ± 0.6 a | 5.6 ± 0.9 a | 3.6 ± 0.6 b | 4.5 ± 0.3 b | ||||
| Feeding | 5.6 ± 0.3 c | 6.0 ± 1.0 c | 7.0 ± 1.6 c | 4.0 ± 0.7 d | 5.2 ± 0.4 c | 3.7 ± 0.3 d | 5.1 ± 0.6 c | 8.8 ± 2.6 b | 5.5 ± 0.5 c | 6.0 ± 0.5 c | 9.1 ± 0.6 b | 6.4 ± 1.1 c | 6.5 ± 0.9 c | 6.0 ± 0.5 c | 7.6 ± 1.7 c | 6.5 ± 0.9 c | 8.7 ± 0.5 b | 12.7 ± 1.1 a | 10.4 ± 1.4 b | 6.4 ± 0.8 c |
| Comfort | 0.5 ± 0.2 c | 0.0 ± 0.2 c | 1.1 ± 0.2 b | 1.4 ± 0.2 b | 0.2 ± 0.1 c | 0.0 ± 0.1 c | 0.6 ± 0.1 c | 1.4 ± 0.1 b | 0.1 ± 0.2 c | 0.1 ± 0.2 c | 1.5 ± 0.2 b | 1.9 ± 0.2 b | 0.4 ± 0.3 c | 0.1 ± 0.3 c | 1.1 ± 0.3 b | 1.5 ± 0.3 b | 1.3 ± 0.5 b | 2.2 ± 0.5 b | 4.3 ± 0.5 a | 5.0 ± 0.5 a |
| Drinking | 3.1 ± 0.3 b | 3.0 ± 0.4 b | 3.0 ± 0.4 b | 3.5 ± 0.4 b | 4.6 ± 0.3 a | |||||||||||||||
| Locomotion | 6.1 ± 0.5 b | 5.5 ± 0.6 b | 5.8 ± 0.3 b | 4.8 ± 0.3 b | 9.7 ± 0.4 a | |||||||||||||||
| Feather pecking | 0.1 ± 0.4 b | 0.1 ± 0.4 b | 0.1 ± 0.4 b | 0.2 ± 0.1 b | 0.9 ± 0.1 a | |||||||||||||||
| Behavioral measurements (%) 1 | SF2 (Day) | SM2 (Day) | LF2 (Day) | LM2 (Day) | C2 (Day) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 16 | 23 | 30 | 42 | 9 | 16 | 23 | 30 | 42 | 9 | 16 | 23 | 30 | 42 | 9 | 16 | 23 | 30 | 42 | 9 | 16 | 23 | 30 | 42 | |
| Resting | 69.6 ± 1.2 a | 55.9 ± 2.3 c | 49.6 ± 2.3 c | 48.9 ± 2.3 c | 36.1 ± 1.3 e | 73.9 ± 2.8 a | 58.4 ± 2.2 c | 50.7 ± 2.9 c | 43.7 ± 1.5 d | 38.6 ± 1.4 e | 64.8 ± 1.7 b | 53.2 ± 1.2 c | 46.7 ± 3.9 d | 44.8 ± 2.9 d | 37.4 ± 0.9 e | 64.9 ± 2.0 b | 58.1 ± 1.4 c | 53.2 ± 2.2 c | 45.9 ± 2.1 d | 40.4 ± 1.7 d | 62,9 ± 1.5 b | 46.7 ± 1.7 d | 42.0 ± 1.9 d | 41.9 ± 2.0 d | 38.1 ± 1.2 e |
| Resting under the brooder | 61.5 ± 2.0 a | 41.3 ± 2.0 c | 35.6 ± 2.7 d | 33.3 ± 1.9 d | 65.6 ± 2.3 a | 42.2 ± 2.3 c | 37.4 ± 3.2 d | 28.5 ± 2.2 d | 56.3 ± 2.0 b | 41.4 ± 2.0 c | 37.7 ± 2.7 d | 32.0 ± 1.9 d | 56.3 ± 2.0 b | 41.4 ± 2.0 c | 37.7 ± 2.7 d | 32.0 ± 1.9 d | |||||||||
| Resting outside the brooder | 8.1 ± 0.1 a | 12.5 ± 1.6 a | 14.0 ± 1.6 a | 15.5 ± 1.4 a | 7.4 ± 1.2 a | 16.1 ± 1.8 a | 13.3 ± 1.8 a | 15.1 ± 1.6 a | 8.6 ± 1.0 a | 12.5 ± 1.6 a | 12.7 ± 1.6 a | 16.0 ± 1.4 a | 8.6 ± 1.0 a | 16.7 ± 1.6 a | 15.5 ± 1.6 a | 13.9 ± 1.4 a | |||||||||
| Resting on the brooder | 0.7 ± 0.4 c | 10.3 ± 1.9 b | 12.6 ± 0.8 b | 15.2 ± 1.2 a | 0.1 ± 0.1 c | 14.7 ± 2.3 a | 12.3 ± 0.1 b | 16.3 ± 2.2 a | 2.1 ± 1.5 c | 9.9 ± 0.8 b | 12.8 ± 0.6 b | 14.8 ± 1.7 a | 3.8 ± 2.6 c | 17.3 ± 0.8 a | 10.2 ± 0.9 b | 12.9 ± 2.2 b | |||||||||
| Feeding | 5.8 ± 1.0 c | 9.1 ± 0.8 b | 11.9 ± 0.9 a | 11.8 ± 1.4 a | 12.2 ± 0.5 a | 5.0 ± 0.6 c | 11.3 ± 1.0 a | 13.6 ± 0.5 a | 11.9 ± 1.9 a | 14.9 ± 2.3 a | 8.0 ± 1.5 b | 9.5 ± 0.5 b | 13.2 ± 1.0 a | 15.4 ± 0.9 a | 13.6 ± 2.0 a | 5.5 ± 0.6 c | 10.2 ± 1.2 a | 14.3 ± 1.1 a | 12.2 ± 1.0 a | 12.1 ± 0.8 a | 6.3 ± 0.8 c | 11.7 ± 1.2 a | 14.3 ± 1.9 a | 10.8 ± 0.9 a | 9.2 ± 0.6 b |
| Foraging | 10.4 ± 1.3 c | 11.2 ± 1.1 c | 12.3 ± 0.7 a | 12.9 ± 1.0 c | 18.6 ± 1.0 a | 6.6 ± 0.2 e | 10.3 ± 0.6 c | 12.6 ± 0.8 c | 12.2 ± 0.9 c | 15.9 ± 1.3 b | 8.3 ± 1.0 d | 14.4 ± 1.2 b | 12.9 ± 1.0 c | 13.1 ± 0.8 c | 14.6 ± 0.9 b | 9.8 ± 1.2 c | 11.8 ± 0.5 c | 10.0 ± 0.9 c | 16.0 ± 2.2 b | 14.9 ± 0.7 b | 8.6 ± 0.5 d | 10.6 ± 0.8 c | 10.9 ± 0.5 c | 11.4 ± 1.2 c | 9.7 ± 1.0 c |
| Comfort | 1.8 ± 0.3 c | 2.9 ± 0.7 b | 4.7 ± 0.1 b | 4.4 ± 0.7 b | 7.4 ± 0.9 a | 1.5 ± 0.2 c | 2.2 ± 0.7 c | 3.8 ± 1.5 b | 6.0 ± 0.9 b | 5.9 ± 1.5 b | 1.5 ± 0.5 c | 2.7 ± 1.0 b | 3.0 ± 0.3 b | 5.2 ± 0.5 b | 7.6 ± 0.8 a | 1.8 ± 0.3 c | 2.3 ± 0.2 c | 3.1 ± 0.7 b | 4.4 ± 0.9 b | 7.0 ± 1.6 a | 3.4 ± 0.6 b | 3.6 ± 0.7 b | 3.8 ± 0.4 b | 4.4 ± 0.6 b | 3.8 ± 0.5 b |
| Drinking | 2.7 ± 0.8 b | 3.1 ± 0.3 b | 3.6 ± 0.8 b | 4.2 ± 0.8 b | 5.8 ± 0.3 a | 1.9 ± 0.9 b | 2.4 ± 0.4 b | 4.6 ± 0.3 b | 5.9 ± 0.3 a | 3.5 ± 0.7 b | 2.8 ± 0.4 b | 3.1 ± 0.6 b | 4.1 ± 0.5 b | 3.6 ± 0.3 b | 4.2 ± 0.4 b | 2.9 ± 0.5 b | 2.6 ± 0.3 b | 3.5 ± 0.5 b | 4.1 ± 0.8 b | 4.1 ± 0.5 b | 2.8 ± 0.4 b | 3.3 ± 0.9 b | 3.3 ± 0.3 b | 2.8 ± 0.5 b | 3.7 ± 0.5 b |
| Locomotion | 8.7 ± 0.6 e | 13.8 ± 1.5 d | 14.3 ± 2.0 d | 10.1 ± 1.0 d | 14.9 ± 1.1 d | 11.0 ± 1.5 d | 12.2 ± 1.7 d | 11.0 ± 0.8 d | 11.3 ± 0.8 d | 15.6 ± 1.2 d | 12.9 ± 2.8 d | 13.7 ± 0.3 d | 14.2 ± 2.1 d | 12.8 ± 0.8 d | 17.9 ± 0.9 c | 12.8 ± 1.5 d | 12.7 ± 0.3 d | 12.1 ± 1.0 d | 11.2 ± 0.9 d | 15.2 ± 1.4 d | 13.2 ± 1.1 d | 18.7 ± 1.2 c | 19.1 ± 1.2 c | 23.2 ± 1.3 b | 27.9 ± 0.9 a |
| Feather pecking | 0.4 ± 0.2 b | 1.6 ± 0.2 b | 1.2 ± 0.6 b | 1.5 ± 0.6 b | 1.0 ± 0.5 b | 0.1 ± 0.1 c | 1.3 ± 0.6 b | 0.9 ± 0.4 b | 0.4 ± 0.2 b | 1.4 ± 0.6 b | 0.0 ± 0.1 c | 0.7 ± 0.3 b | 1.1 ± 0.3 b | 0.9 ± 0.7 b | 1.4 ± 0.8 b | 0.0 ± 0.1 c | 0.6 ± 0.2 b | 0.7 ± 0.4 b | 0.7 ± 0.2 b | 1.0 ± 0.3 b | 1.2 ± 0.1 b | 2.6 ± 0.6 a | 3.2 ± 0.8 a | 2.5 ± 0.7 a | 2.5 ± 0.3 a |
| Fleeing | 0.1 ± 0.2 c | 0.7 ± 0.2 c | 0.3 ± 0.2 c | 0.2 ± 0.2 c | 0.3 ± 0.2 c | 0.1 ± 0.2 c | 0.8 ± 0.2 c | 0.0 ± 0.2 c | 0.3 ± 0.2 c | 0.6 ± 0.2 c | 0.2 ± 0.3 c | 0.5 ± 0.3 c | 0.3 ± 0.3 c | 0.6 ± 0.3 c | 0.5 ± 0.3 c | 0.4 ± 0.2 c | 0.8 ± 0.2 c | 0.0 ± 0.2 c | 0.3 ± 0.2 c | 0.4 ± 0.2 c | 0.8 ± 0.4 c | 1.6 ± 0.4 b | 2.1 ± 0.4 b | 1.7 ± 0.4 b | 3.5 ± 0.4 a |
| Exploration | 0.5 ± 0.2 b | 1.5 ± 0.4 b | 1.9 ± 0.5 b | 5.1 ± 0.5 a | 2.5 ± 0.9 b | 0.4 ± 0.2 b | 0.9 ± 0.5 b | 2.5 ± 0.6 b | 7.4 ± 1.6 a | 3.5 ± 0.7 a | 1.0 ± 0.4 b | 1.7 ± 0.4 b | 3.9 ± 1.3 a | 3.1 ± 1.1 a | 2.1 ± 0.7 b | 1.0 ± 0.3 b | 0.6 ± 0.1 c | 2.3 ± 0.6 b | 4.5 ± 1.0 a | 3.9 ± 0.8 a | 0.6 ± 0.3 b | 0.8 ± 0.3 b | 0.7 ± 0.3 b | 0.8 ± 0.2 b | 1.3 ± 0.4 b |
| Dustbathing | 0.0 ± 0.2 b | 0.1 ± 0.2 b | 0.1 ± 0.2 b | 0.7 ± 0.2 a | 0.9 ± 0.2 a | 0.1 ± 0.2 b | 0.1 ± 0.2 b | 0.3 ± 0.2 b | 0.9 ± 0.2 a | 0.1 ± 0.2 b | 0.4 ± 0.2 b | 0.5 ± 0.2 a | 0.5 ± 0.2 a | 0.3 ± 0.2 b | 0.6 ± 0.2 a | 0.6 ± 0.2 a | 0.2 ± 0.2 b | 0.6 ± 0.2 a | 0.7 ± 0.2 a | 1.0 ± 0.2 a | 0.1 ± 0.1 b | 0.4 ± 0.1 b | 0.3 ± 0.1 b | 0.3 ± 0.1 b | 0.4 ± 0.1 b |
| Behavioral measurements 1 | SF2 (Days) | SM2 (Days) | LF2 (Days) | LM2 (Days) | C2 (Days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | 96 | 181 | 26 | 96 | 181 | 26 | 96 | 181 | 26 | 96 | 181 | 26 | 96 | 181 | |
| Inductions (n) | 2.4 ± 0.2 a | 2.6 ± 0.2 a | 2.3 ± 0.2 a | 2.6 ± 0.2 a | 2.6 ± 0.2 a | 1.5 ± 0.2 b | 2.7 ± 0.2 a | 2.5 ± 0.2 a | 2.1 ± 0.2 a | 2.6 ± 0.2 a | 2.5 ± 0.2 a | 2.1 ± 0.2 a | 2.1 ± 0.2 a | 1.8 ± 0.1 b | 1.9 ± 0.1 b |
| Latency to first head movement (s) | 69.1 ± 10.0 a | 57.0 ± 10.9 a | 49.3 ± 6.4 a | 80.7 ± 11.4 a | 116.0 ± 10.8 b | ||||||||||
| TI duration (s) | 105.3 ± 13.2 a | 93.2 ± 12.5 a | 76.6 ± 10.1 a | 112.6 ± 13.0 a | 154.9 ± 12.5 b | ||||||||||
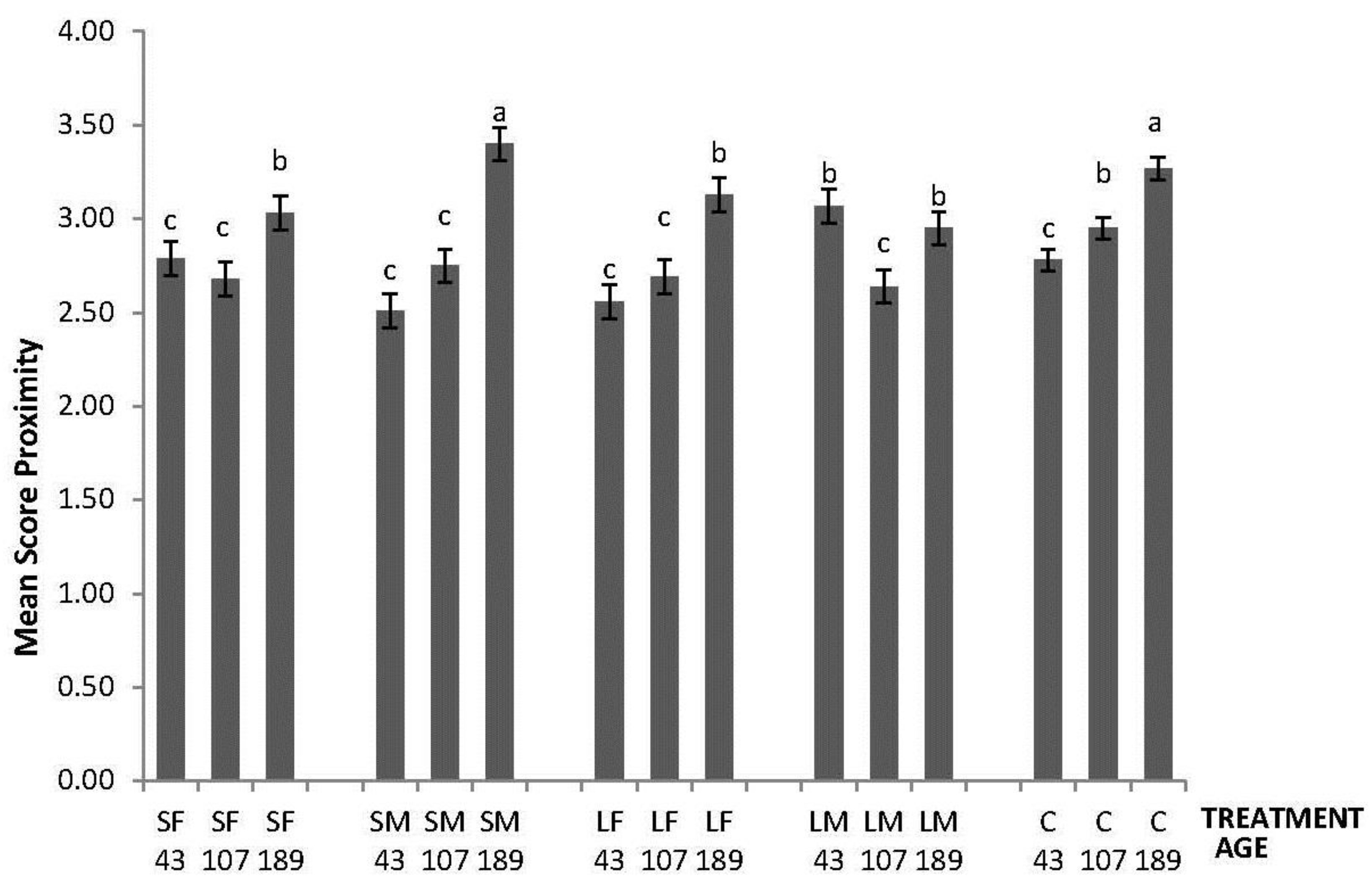

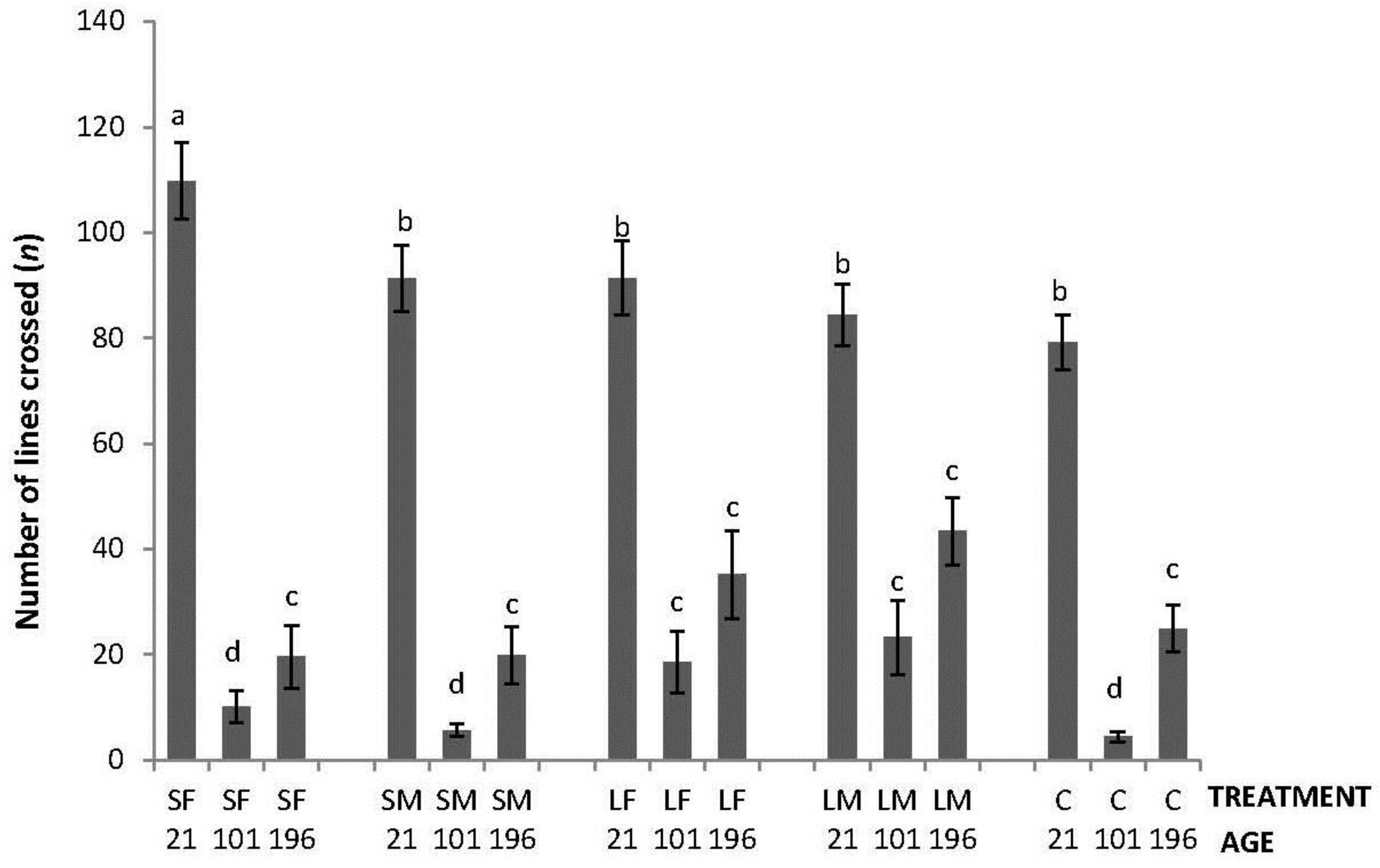
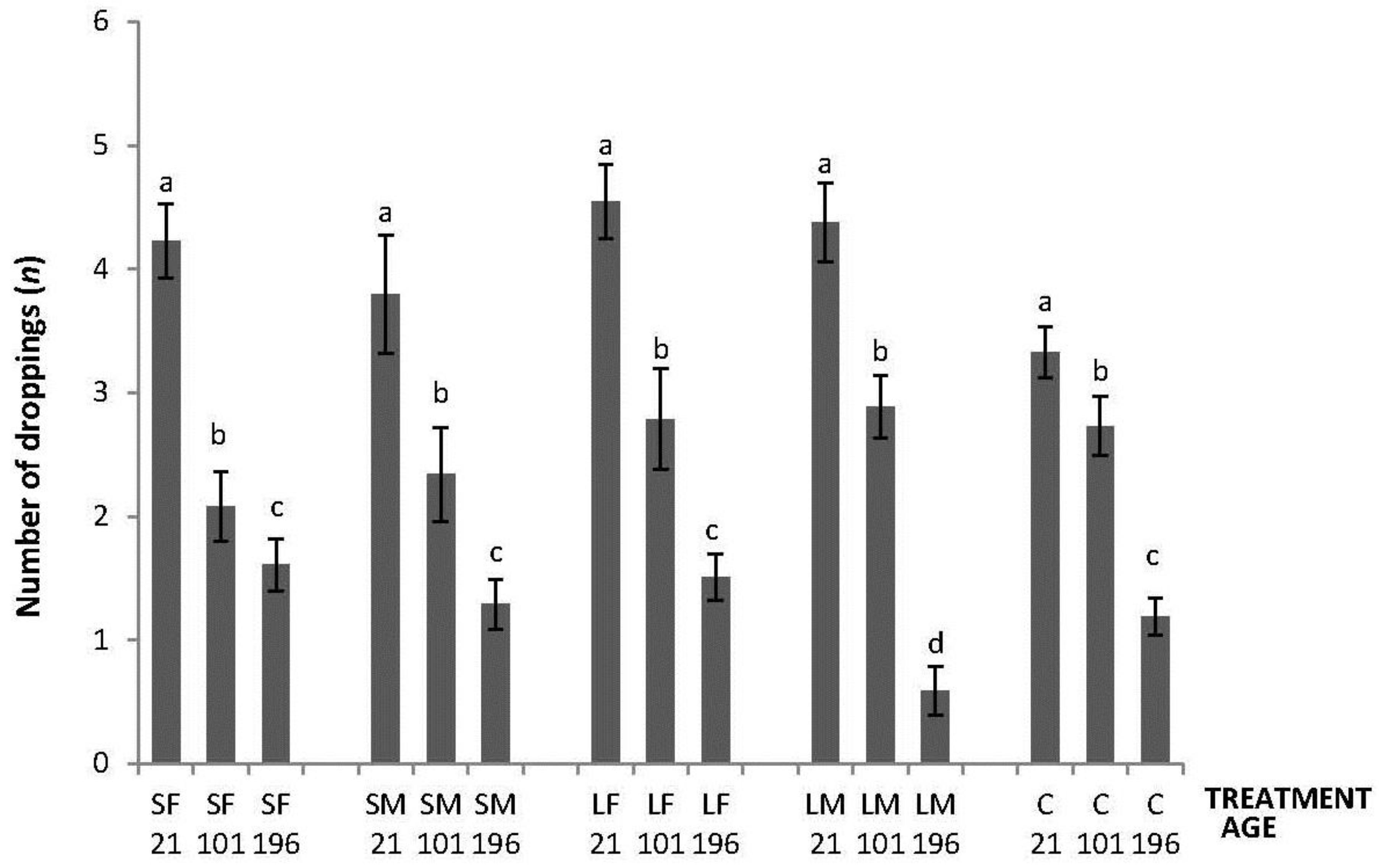
4. Discussion
4.1. Effects of Dark Brooders on Time Budget: Control vs. Brooder Chicks
4.1.1. Phase I
4.1.2. Phase II
4.2. Effects of Dark Brooders on Time Budget: Differences between the Brooder Treatments
4.3. Effect of Brooders on Fearfulness: Control vs. Brooder Birds
4.4. Effect of Brooders on Fearfulness: Differences between Brooder Treatments
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weekstein, D.R.; Zolman, J.F. Ontogeny of heat production in chicks. FASEB J. 1969, 28, 1023–1027. [Google Scholar]
- Gilani, A.M.; Knowles, T.G.; Nicol, C.J. The effect of dark brooders on feather pecking on commercial farms. Appl. Anim. Behav. Sci. 2012, 142, 42–50. [Google Scholar] [CrossRef]
- Jensen, A.B.; Palme, R.; Forkman, B. Effect of brooders on feather pecking and cannibalism in domestic fowl (gallus gallus domesticus). Appl. Anim. Behav. Sci. 2006, 99, 287–300. [Google Scholar] [CrossRef]
- Sherry, D.F. Parental care and the development of thermoregulation in red junglefowl. Behaviour 1981, 76, 250–279. [Google Scholar] [CrossRef]
- Cherfas, J.J. Simultaneous color discrimination in chicks is improved by brief exposure to light. Anim. Behav. 1978, 26, 1–5. [Google Scholar] [CrossRef]
- Zolman, J.F.; Pursley, D.G.; Hall, J.A.; Sahley, C.L. Form preferences in successive discrimination-learning of young chicks. J. Comp. Physiol. Psychol. 1975, 89, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, E.A.; Hunter, N.B.; Gutowski, K.A.; Bader, S.A. Autoshaping chicks with heat reinforcement—Role of stimulus-reinforcer and response-reinforcer relations. J. Exp. Psychol. Anim. Behav. Process. 1975, 1, 158–169. [Google Scholar] [CrossRef]
- Riber, A.B.; Nielsen, B.L.; Ritz, C.; Forkman, B. Diurnal activity cycles and synchrony in layer hen chicks (gallus gallus domesticus). Appl. Anim. Behav. Sci. 2007, 108, 276–287. [Google Scholar] [CrossRef]
- Sherry, D.F. Parental food-calling and the role of the young in burmese red junglefowl (gallus gallus spadiceus). Anim. Behav. 1977, 25, 594–601. [Google Scholar] [CrossRef]
- Stokes, A.W. Parental and courtship feeding in red jungle fowl. Auk 1971, 88, 21–29. [Google Scholar] [CrossRef]
- Wauters, A.M.; Richard-Yris, M.A.; Talec, N. Maternal influences on feeding and general activity in domestic chicks. Ethology 2002, 108, 529–540. [Google Scholar] [CrossRef]
- Riber, A.B.; Wichman, A.; Braastad, B.O.; Forkman, B. Effects of broody hens on perch use, ground pecking, feather pecking and cannibalism in domestic fowl (gallus gallus domesticus). Appl. Anim. Behav. Sci. 2007, 106, 39–51. [Google Scholar] [CrossRef]
- Shimmura, T.; Kamimura, E.; Azuma, T.; Kansaku, N.; Uetake, K.; Tanaka, T. Effect of broody hens on behaviour of chicks. Appl. Anim. Behav. Sci. 2010, 126, 125–133. [Google Scholar] [CrossRef]
- Perre, Y.; Wauters, A.M.; Richard-Yris, M.A. Influence of mothering on emotional and social reactivity of domestic pullets. Appl. Anim. Behav. Sci. 2002, 75, 133–146. [Google Scholar] [CrossRef]
- Roden, C.; Wechsler, B. A comparison of the behaviour of domestic chicks reared with or without a hen in enriched pens. Appl. Anim. Behav. Sci. 1998, 55, 317–326. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; Uitdehaag, K.A.; Ellen, E.D.; Komen, J. The effects of selection on low mortality and brooding by a mother hen on open-field response, feather pecking and cannibalism in laying hens. Anim. Welf. 2009, 18, 427–432. [Google Scholar]
- Shimmura, T.; Maruyama, Y.; Fujino, S.; Kamimura, E.; Uetake, K.; Tanaka, T. Persistent effect of broody hens on behaviour of chickens. Anim. Sci. J. 2015, 86, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Fält, B. Differences in agressiveness between brooded and non-brooded domestic chicks. Appl. Anim. Ethol. 1978, 4, 211–221. [Google Scholar] [CrossRef]
- Johnsen, P.F.; Kristensen, H.H. Effect of Brooder Quality on the Early Development of Feather Pecking Behaviour in Domestic Chicks. In Proceedings of the 6th European Symposium on Poultry Welfare, Zollikofen, Switzerland, 2001; Oester, H., Wyss, C., Eds.; Swiss Branch of the World's Poultry Science Association (WPSA): Zollikofen, Switzerland, 2001; pp. 209–212. [Google Scholar]
- Riber, A.B. Effect of Dark Brooders on Fearfulness in Layers; Aarhus University: Aarhus, Denmark, 2015. [Google Scholar]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Daigle, C.L.; Siegford, J.M. When continuous observations just won’t do: Developing accurate and efficient sampling strategies for the laying hen. Behav. Process. 2014, 103, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.M.; Channing, C.E.; Waddington, D.; Jones, R.B. Age-related changes in fear, sociality and pecking behaviours in two strains of laying hen. Br. Poult. Sci. 2001, 42, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B. Fear and adaptability in poultry: Insights, implications and imperatives. Worlds Poult. Sci. J. 1996, 52, 131–174. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. Infostat, InfoStat versión 2015; InfoStat Group, National University of Córdoba: Córdoba, Argentina, 2015. [Google Scholar]
- Di Rienzo, J.A.; Guzman, A.W.; Casanoves, F. A multiple-comparisons method based on the distribution of the root node distance of a binary tree. JABES 2002, 7, 129–142. [Google Scholar] [CrossRef]
- Riber, A.B. Ontogeny of Behaviour in Domestic Fowl—With Emphasis on Feather Pecking. Ph.D. Thesis, University of Copenhagen, København, Denmark, 2007; p. 55. [Google Scholar]
- Brown, L.T. A critical period in learning of motionless stimulus properties in chicks. Anim. Behav. 1964, 12, 353–361. [Google Scholar] [CrossRef]
- Hess, E.H. Imprinting in birds—Research has borne out concept of imprinting as type of learning different from association learning. Science 1964, 146, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Sanotra, G.S.; Vestergaard, K.S.; Agger, J.F.; Lawson, L.G. The relative preferences for feathers, straw, wood-shavings and sand for dustbathing, pecking and scratching in domestic chicks. Appl. Anim. Behav. Sci. 1995, 43, 263–277. [Google Scholar] [CrossRef]
- Vestergaard, K.S.; Baranyiova, E. Pecking and scratching in the development of dust perception in young chicks. Acta Vet. Brno 1996, 65, 133–142. [Google Scholar] [CrossRef]
- Jones, R.B.; Satterlee, D.G.; Ryder, F.H. Fear of humans in japanese quail selected for low or high adrenocortical response. Physiol. Behav. 1994, 56, 379–383. [Google Scholar] [CrossRef]
- Kruijt, J.P. Ontogeny of social behaviour in burmase red junglefowl (gallus gallus spadiceus) bonnaterre. Behaviour 1964, Suppl XII, 1–201. [Google Scholar]
- Malleau, A.E.; Duncan, I.J.H.; Widowski, T.M.; Atkinson, J.L. The importance of rest in young domestic fowl. Appl. Anim. Behav. Sci. 2007, 106, 52–69. [Google Scholar] [CrossRef]
- Alvino, G.M.; Archer, G.S.; Mench, J.A. Behavioural time budgets of broiler chickens reared in varying light intensities. Appl. Anim. Behav. Sci. 2009, 118, 54–61. [Google Scholar] [CrossRef]
- Blatchford, R.A.; Klasing, K.C.; Shivaprasad, H.L.; Wakenell, P.S.; Archer, G.S.; Mench, J.A. The effect of light intensity on the behavior, eye and leg health, and immune function of broiler chickens. Poult. Sci. 2009, 88, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Cornetto, T.; Estevez, I. Behavior of the domestic fowl in the presence of vertical panels. Poult. Sci. 2001, 80, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S. Domestic fowl - strategies to confront environmental conditions. Avian Poult. Biol. Rev. 2000, 11, 81–95. [Google Scholar]
- Lin, H.; Zhang, H.F.; Jiao, H.C.; Zhao, T.; Sui, S.J.; Gu, X.H.; Zhang, Z.Y.; Buyse, J.; Decuypere, E. Thermoregulation responses of broiler chickens to humidity at different ambient temperatures. I. One week of age. Poult. Sci. 2005, 84, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Nichelmann, M.; Tzschentke, B.; Burmeister, A. Evaporative heat-loss at high relative air humidity in poultry. Arch. Geflugelkd. 1991, 55, 111–115. [Google Scholar]
- Riber, A.B.; Guzman, D.A. Effects of rearing with dark brooders on welfare and production in layers. Manuscript in preparation.
- Blokhuis, H.J. Feather-pecking in poultry: Its relation with ground-pecking. Appl. Anim. Behav. Sci. 1986, 16, 63–67. [Google Scholar] [CrossRef]
- Blokhuis, H.J. The effect of a sudden change in floor type on pecking behaviour in chicks. Appl. Anim. Behav. Sci. 1989, 22, 65–73. [Google Scholar] [CrossRef]
- Huber-Eicher, B.; Wechsler, B. Feather pecking in domestic chicks: Its relation to dustbathing and foraging. Anim. Behav. 1997, 54, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Huber-Eicher, B.; Wechsler, B. The effect of quality and availability of foraging materials on feather pecking in laying hen chicks. Anim. Behav. 1998, 55, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, K.S.; Kruijt, J.P.; Hogan, J.A. Feather pecking and chronic fear in groups of red junglefowl: Their relation to dustbathing, rearing environment and social status. Anim. Behav. 1993, 45, 1127–1140. [Google Scholar] [CrossRef]
- Vestergaard, K.S.; Lisborg, L. A model of feather pecking development which relates to dustbathing in the fowl. Behaviour 1993, 126, 89–105. [Google Scholar] [CrossRef]
- Vestergaard, K. Dust-bathing in the domestic fowl—Diurnal rhytm and dust deprivation. Appl. Anim. Ethol. 1982, 8, 487–495. [Google Scholar] [CrossRef]
- Schütz, K.E.; Jensen, P. Effects of resource allocation on behavioural strategies: A comparison of red junglefowl (gallus gallus) and two domesticated breeds of poultry. Ethology 2001, 107, 753–765. [Google Scholar] [CrossRef]
- Hansen, I. Behavioral expression of laying hens in aviaries and cages—Frequencies, time budgets and facility utilization. Br. Poult. Sci. 1994, 35, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Blokhuis, H.J.; Beuving, G. Open-field and tonic immobility responses in domestic chicks of two genetic lines differing in their propensity to feather peck. Br. Poult. Sci. 1995, 36, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, T.B.; Buitenhuis, A.J.; Ask, B.; Uitdehaag, K.A.; Koene, P.; van der Poel, J.J.; van Arendonk, J.A.M.; Bovenhuis, H. Genetic and phenotypic correlations between feather pecking and open-field response in laying hens at two different ages. Behav. Genet. 2004, 34, 407–415. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.A.; Parker, M.O.; Davey, E.L.; Grist, H.; Owen, R.C.; Szladovits, B.; Demmers, T.G.M.; Wathes, C.M.; Abeyesinghe, S.M. Effect of low light and high noise on behavioural activity, physiological indicators of stress and production in laying hens. Br. Poult. Sci. 2011, 52, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Bertin, A.; Richard-Yris, M.-A. Mothers’fear of human affects the emotional reactivity of young in domestic japanese quail. Appl. Anim. Behav. Sci. 2004, 89, 215–231. [Google Scholar] [CrossRef]
- Houdelier, C.; Lumineau, S.; Bertin, A.; Guibert, F.; de Margerie, E.; Augery, M.; Richard-Yris, M.A. Development of fearfulness in birds: Genetic factors modulate non-genetic maternal influences. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Pittet, F.; Coignard, M.; Houdelier, C.; Richard-Yris, M.A.; Lumineau, S. Effects of maternal experience on fearfulness and maternal behaviour in a precocial bird. Anim. Behav. 2013, 85, 797–805. [Google Scholar] [CrossRef]
- Angevaare, M.J.; Prins, S.; van der Staay, F.J.; Nordquist, R.E. The effect of maternal care and infrared beak trimming on development, performance and behavior of silver nick hens. Appl. Anim. Behav. Sci. 2012, 140, 70–84. [Google Scholar] [CrossRef]
- Rodenburg, T.B.; de Haas, E.N.; Nielsen, B.L.; Buitenhuis, A.J. Fearfulness and feather damage in laying hens divergently selected for high and low feather pecking. Appl. Anim. Behav. Sci. 2010, 128, 91–96. [Google Scholar] [CrossRef]
- Uitdehaag, K.A.; Komen, H.; Rodenburg, T.B.; Kemp, B.; von Arendonk, J. The novel object test as predictor of feather damage in cage-housed Rhode Island Red and White Leghorn laying hens. Appl. Anim. Behav. Sci. 2008, 109, 292–305. [Google Scholar] [CrossRef]
- Hughes, B.O.; Duncan, I.J.H. Influence of strain and environmental factors upon feather pecking and cannibalism in fowls. Br. Poult. Sci. 1972, 13, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Ouart, M.D.; Adams, A.W. Effects of cage design and bird density on layers. 1: Productivity, feathering, and nervousness. Poult. Sci. 1982, 61, 1606–1613. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riber, A.B.; Guzman, D.A. Effects of Dark Brooders on Behavior and Fearfulness in Layers. Animals 2016, 6, 3. https://doi.org/10.3390/ani6010003
Riber AB, Guzman DA. Effects of Dark Brooders on Behavior and Fearfulness in Layers. Animals. 2016; 6(1):3. https://doi.org/10.3390/ani6010003
Chicago/Turabian StyleRiber, Anja B., and Diego A. Guzman. 2016. "Effects of Dark Brooders on Behavior and Fearfulness in Layers" Animals 6, no. 1: 3. https://doi.org/10.3390/ani6010003






