Preliminary Validation and Reliability Testing of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians, in a Colony of Laboratory Cats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Part I: Scale Development, and Content and Face Validity
2.3. Part II: Reliability Assessment and Construct Validity
2.4. Statistical Analyses
3. Results
3.1. Part I: Scale Content and Face Validity
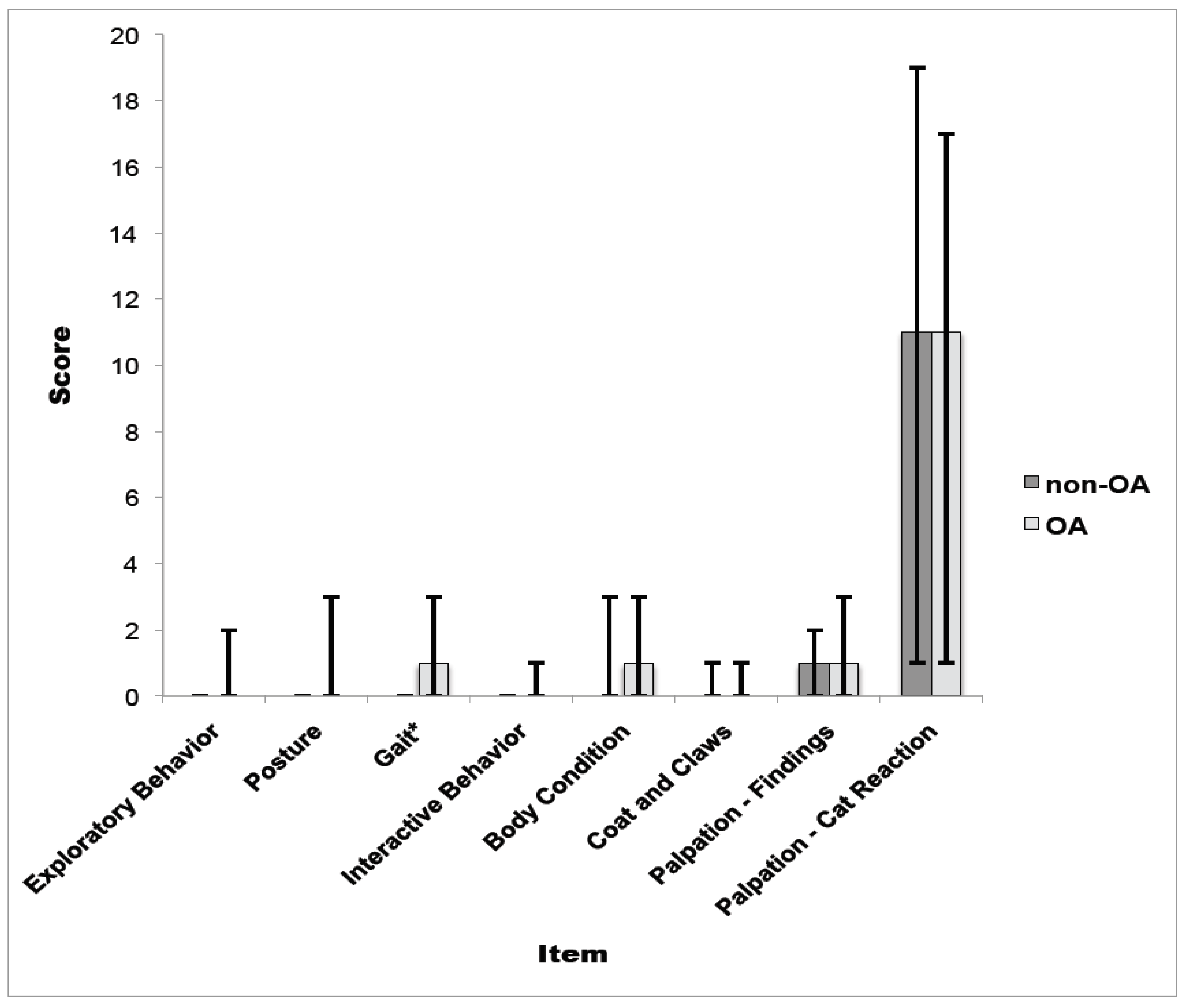
3.2. Part II: Reliability Assessment and Construct Validity
| Scale Item | Days 0 and 7 | Days 32 and 35 | ||
|---|---|---|---|---|
| Kappa | Rhos | Kappa | Rhos | |
| Exploratory Behavior | 0.83 | 0.82 (p < 0.0001) | 0.33 | 0.64 (p = 0.03) |
| Body Posture | 0.64 | 0.60 (p = 0.05) | 0.64 | 0.71 (p = 0.01) |
| Gait/Locomotion | −0.11 | NS | −0.11 | NE |
| Interactive Behavior | 1.00 | 1.00 (p < 0.0001) | 1.00 | 1.00 (p < 0.0001) |
| Body Condition | 0.37 | 0.65 (p = 0.03) | 0.37 | 1.00 (p < 0.0001) |
| Coat and Claws | 0.80 | 0.82 (p < 0.0001) | 0.80 | NS |
| Palpation—Findings | 0.18 | NS | 0.18 | 0.62 (p = 0.04) |
| Palpation—Cat Reaction | 0.32 | 0.64 (p = 0.04) | 0.32 | 0.64 (p = 0.04) |
| Scale Item | Day 0 | Day 35 | ||
|---|---|---|---|---|
| Κappa | Rhos | Kappa | Rhos | |
| Exploratory Behavior | 0.37 | NS | 0.84 | 0.85 (p < 0.01) |
| Body Posture | −0.41 | NS | 0.32 | 0.67 (p = 0.02) |
| Gait/Locomotion | 0.10 | NS | 0.33 | 0.81 (p < 0.01) |
| Interactive Behavior | 0.33 | 0.64 (p = 0.03) | 0.62 | 0.67 (p = 0.02) |
| Body Condition | 0.38 | NS | 0.52 | 0.59 (p = 0.05) |
| Coat and Claws | NE | NE | 1.00 | 1.00 (p < 0.01) |
| Palpation—Cat Reaction | 0.55 | 0.94 (p < 0.01) | 0.68 | 0.86 (p < 0.01) |
| Scale Item | Day 0–Day 7 | Day 7–Day 14 | Day 0–Day 14 | |||
|---|---|---|---|---|---|---|
| Kappa | Rhos | Kappa | Rhos | Kappa | Rhos | |
| Exploratory Behavior | 0.41 | 0.60 (p = 0.0002) | 0.43 | 0.60 (p = 0.0002) | 0.60 | 0.69 (p < 0.0001) |
| Body Posture | ||||||
| Axial | 0.58 | 0.72 (p < 0.0001) | 0.69 | 0.86 (p < 0.0001) | 0.84 | 0.85 (p < 0.0001) |
| Forelimbs | 0.36 | 0.48 (p = 0.0041) | 0.44 | 0.54 (p = 0.001) | 0.40 | 0.40 (p = 0.0176) |
| Hind limbs | 0.51 | 0.62 (p < 0.0001) | 0.18 | NS | 0.57 | 0.74 (p < 0.0001) |
| Posture Total | 0.40 | 0.69 (p < 0.0001) | 0.40 | 0.57 (p = 0.0004) | 0.54 | 0.78 (p < 0.0001) |
| Gait | 0.33 | 0.68 (p < 0.0001) | 0.38 | 0.45 (p = 0.0072) | 0.28 | 0.54 (p = 0.0011) |
| Interactive Behavior | 0.57 | 0.66 (p < 0.0001) | 0.78 | 0.86 (p < 0.0001) | 0.48 | 0.55 (p = 0.0008) |
| Reaction to Palpation | ||||||
| Axial | NE | 0.62 (p < 0.0001) | NE | 0.62 (p < 0.0001) | NE | 0.65 (p < 0.0001) |
| Forelimbs | 0.26 | 0.72 (p < 0.0001) | 0.36 | 0.52 (p = 0.0018) | 0.38 | 0.56 (p = 0.0006) |
| Hind limbs | 0.25 | 0.68 (p < 0.0001) | 0.23 | 0.48 (p = 0.004) | 0.44 | 0.75 (p < 0.0001) |
| Palpation Total | −0.02 | 0.72 (p < 0.0001) | 0.00 | 0.63 (p < 0.0001) | NE | 0.72 (p < 0.0001) |
| Scale Item | Kappa | Rhos |
|---|---|---|
| Exploratory Behavior | 0.45 | 0.55 (p < 0.0001) |
| Body Posture | ||
| Axial | 0.31 | NS |
| Forelimbs | −0.02 | NS |
| Hind limbs | 0.44 | 0.42 (p = 0.0014) |
| Posture Total | 0.41 | 0.42 (p = 0.0012) |
| Gait | 0.22 | NS |
| Interactive Behavior | 0.55 | 0.60 (p < 0.0001) |
| Reaction to Palpation | ||
| Axial | 0.24 | 0.29 (p = 0.0306) |
| Forelimbs | 0.03 | NS |
| Hind limbs | 0.35 | 0.38 (p = 0.0035) |
| Palpation Total | 0.21 | NS |
| Scale Item | Day 0 | Day 7 | Day 14 | |||
|---|---|---|---|---|---|---|
| OA | Non-OA | OA | Non-OA | OA | Non-OA | |
| Exploratory Behavior | 1 (1–6) | 1 (0–5) | 1 (0–6) | 1 (0–5) | 1 (0–5) | 1 (0–5) |
| Body Posture | ||||||
| Axial | 0 (0–1) | 0 (0) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0) |
| Forelimbs | 0 (0–1) | 0 (0) | 0 (0–2) | 0 (0) | 0 (0–1) | 0 (0) |
| Hind limbs | 1 (0–2) | 0 (0–1) | 0 (0–2) | 0 (0) | 0 (0–2) | 0 (0–2) |
| Posture Total | 1 (0–3) | 0 (0–1) | 1 (0–3) | 0 (0–1) | 1 (0–2) | 0 (0–2) |
| Gait | 3 (0–5) | 1 (0–3) | 3 (0–5) | 0 (0–3) | 3 (0–5) | 2 (0–4) |
| Interactive Behavior | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–1) |
| Reaction to Palpation | ||||||
| Axial | 1 (0–5) | 1 (0–3) | 1 (0–5) | 1 (0–2) | 1.5 (0–4) | 2 (0–3) |
| Forelimbs | 2 (0–4) | 3 (1–3) | 2 (0–4) | 3 (1–4) | 2 (0–4) | 3 (2–4) |
| Hind limbs | 5 (0–8) | 6 (3–7) | 5 (1–9) | 5 (2–6) | 5 (2–7) | 5 (4–6) |
| Palpation Total | 5 (0–8) | 6 (3–7) | 5 (1–9) | 5 (2–6) | 5 (2–7) | 5 (4–6) |
| Scale Total | 9 (2–17) | 11 (4–13) | 8 (3–18) | 10 (2–14) | 9 (5–13) | 10 (3–16) |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lascelles, B.D.X.; Henry, J.B., III; Brown, J.; Roberston, I.; Thomson Sumrell, A.; Simpson, W.; Wheeler, S.; Hansen, B.D.; Zamprogno, H.; Freire, M.; et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet. Surg. 2010, 39, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, D.; Robertson, S. DJD-associated pain in cats: What can we do to promote patient comfort? J. Feline Med. Surg. 2010, 12, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Zainal Ariffin, S.M.; Johnston, P. Osteoarthritis in the cat: 1. How common is it and how easy to recognize? J. Feline Med. Surg. 2012, 14, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.P.; Bennett, D. Feline osteoarthritis: A prospective study of 28 cases. J. Small Anim. Pract. 2006, 47, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; Hansen, B.D.; Roe, S.; DePuy, V.; Thomson, A.; Pierce, C.C.; Smith, E.S.; Rowinski, E. Evaluation of client-specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J. Vet. Int. Med. 2007, 21, 410–416. [Google Scholar] [CrossRef]
- Bennett, D.; Morton, C. A study of owner observed behavioural and lifestyle changes in cats with musculoskeletal disease before and after analgesic therapy. J. Feline Med. Surg. 2009, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X.; DePuy, V.; Thomson, A.; Hansen, B.; Marcellin-Little, D.J.; Biourge, V.; Bauer, J.E. Evaluation of a therapeutic diet for feline degenerative joint disease. J. Vet. Intern. Med. 2010, 24, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Sul, R.M.; Chase, D.; Parkin, T.; Bennett, D. Comparison of meloxicam and a glucosamine-chondroitin supplement in management of feline osteoarthritis. Vet. Comp. Orthop. Traumatol. 2014, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Giraudel, J.M.; Gruet, P.; Alexander, D.G.; Seewald, W.; King, J.N. Evaluation of orally administered robenacoxib versus ketoprofen for treatment of acute pain and inflammation associated with musculoskeletal disorders in cats. Am. J. Vet. Res. 2010, 71, 710–719. [Google Scholar] [CrossRef] [PubMed]
- King, J.N.; King, S.; Budsberg, S.C.; Lascelles, B.D.; Bienhoff, S.E.; Roycroft, L.M.; Roberts, E.S. Clinical safety of robenacoxib in feline osteoarthritis: Results of a randomized, blinded, placebo-controlled clinical trial. J. Feline Med. Surg. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gunew, M.N.; Menrath, V.H.; Marshall, R.D. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J. Feline Med. Surg. 2008, 10, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Corbee, R.J.; Barnier, M.M.C.; Van De Lest, C.H.A.; Hazelwinkel, H.A.W. The effect of dietary long-chain omega-3 fatty acid supplementation on owner’s perception of behaviour and locomotion in cats with naturally occurring osteoarthritis. J. Anim. Physiol. Anim. Nutr. 2013, 97, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.X. Feline degenerative joint disease. Vet. Surg. 2010, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.D.; Dong, Y.-H.; Marcellin-Little, D.J.; Thomson, A.; Wheeler, S.; Correa, M. Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. BMC Vet. Res. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, M.H.; Raekallio, M.R.; Junnila, J.J.T.; Hiem-Björkman, A.K.; Snellman, M.P.M.; Vainio, O.M. A comparison of thermographic imaging, physical examination and modified questionnaire as an instrument to assess painful conditions in cats. J. Feline Med. Surg. 2013, 15, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Klinck, M.P.; Frank, D.; Guillot, M.; Troncy, E. Owner-perceived signs and veterinary diagnosis in 50 cases of feline osteoarthritis. Can. Vet. J. 2012, 53, 1181–1186. [Google Scholar] [PubMed]
- Guillot, M.; Moreau, M.; d’Anjou, M.A.; Martel-Pelletier, J.; Pelletier, J.-P.; Troncy, E. Evaluation of osteoarthritis in cats: Novel information from a pilot study. Vet. Surg. 2012, 41, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Guillot, M.; Pelletier, J.-P.; Martel-Pelletier, J.; Troncy, E. Kinetic peak vertical force measurement in cats afflicted by coxarthritis: Data management and acquisition protocols. Res. Vet. Sci. 2013, 95, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Moreau, M.; Heit, M.; Martel-Pelletier, J.; Pelletier, J.-P.; Troncy, E. Characterization of osteoarthritis in cats and meloxicam efficacy using objective chronic pain evaluation tools. Vet. J. 2013, 196, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, E.; Bockstahler, B. Systematic review of ground reaction force measurements in cats. Vet. J. 2015, 206. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Chartrand, G.; Chav, R.; Rousseau, J.; Beaudoin, J.F.; Martel-Pelletier, J.; Pelletier, J.P.; Lecomte, R.; de Guise, J.A.; Troncy, E. [(18)F]-fluorodeoxyglucose positron emission tomography of the cat brain: A feasibility study to investigate osteoarthritis-associated pain. Vet. J. 2015, 204, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Gravel, P.; Gauthier, M.L.; Leblond, H.; Tremblay, M.; Rossignol, S.; Martel-Pelletier, J.; Pelletier, J.P.; de Guise, J.A.; Troncy, E. Coxofemoral joint kinematics using video fluoroscopic images of treadmill-walking cats: Development of a technique to assess osteoarthritis-associated disability. J. Feline Med. Surg. 2015, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Taylor, P.M.; Rialland, P.; Klinck, M.P.; Martel-Pelletier, J.; Pelletier, J.P.; Troncy, E. Evoked temporal summation in cats to highlight central sensitization related to osteoarthritis-associated chronic pain: A preliminary study. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Managing pain in feline patients. Vet. Clin. North Am. Small Anim. Pract. 2008, 38, 1267–1290. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, H.; Hansen, B.D.; Bondell, H.D.; Thomson Sumrell, A.; Simpson, W.; Robertson, I.D.; Brown, J.; Pease, A.P.; Roe, S.C.; Hardie, E.M.; et al. Item generation and design testing of a questionnaire to assess degenerative joint disease-associated pain in cats. Am. J. Vet. Res. 2010, 71, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; DePuy, V.; Hardie, E.; Zamprogno, H.; Thomson, A.; Simpson, W.; Roe, S.; Hansen, B.; Lascelles, B.D.X. Reliability and discriminatory testing of a client-based metrology instrument, feline musculoskeletal pain index (FMPI) for the evaluation of degenerative joint disease-associated pain in cats. Vet. J. 2013, 196, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Hansen, B.; DePuy, V.; Davidson, G.S.; Thomson, A.; Simpson, W.; Roe, S.; Hardie, E.; Lascelles, B.D.X. Feline musculoskeletal pain index: Responsiveness and testing of criterion validity. J. Vet. Intern. Med. 2013, 27, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Streiner, D.L.; Norman, G.R. Validity. In Health Measurement Scales: A Practical Guide to Their Development and Use, 4th ed.; Oxford University Press: Oxford, UK, 2009; Available online: http://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780199231881.001.0001/acprof-9780199231881 (accessed on 30 July 2015).
- Crellin, D.; Sullivan, T.P.; Babl, F.E.; O’Sullivan, R.; Hutchinson, A. Analysis of the validation of existing behavioral pain and distress scales for use in the procedural setting. Paediatr. Anaesth. 2007, 17, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Kundel, H.L.; Polansky, M. Measurement of observer agreement. Radiology 2003, 228, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zaebst, D.; Seel, L. A Macro to Calculate Kappa Statistics for Categorizations by Multiple Raters. In Proceedings of the 30th Annual SAS Users Group International Conference, Cary, NC, USA, 10–13 April 2005.
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Slingerland, L.I.; Hazelwinkel, H.A.; Meij, B.P.; Picavet, P.; Voorhout, G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet. J. 2011, 187, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, J.M.; Donoghue, S. Associations between body condition and disease in cats. J. Am. Vet. Med. Assoc. 1998, 212, 1725–1731. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinck, M.P.; Rialland, P.; Guillot, M.; Moreau, M.; Frank, D.; Troncy, E. Preliminary Validation and Reliability Testing of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians, in a Colony of Laboratory Cats. Animals 2015, 5, 1252-1267. https://doi.org/10.3390/ani5040410
Klinck MP, Rialland P, Guillot M, Moreau M, Frank D, Troncy E. Preliminary Validation and Reliability Testing of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians, in a Colony of Laboratory Cats. Animals. 2015; 5(4):1252-1267. https://doi.org/10.3390/ani5040410
Chicago/Turabian StyleKlinck, Mary P., Pascale Rialland, Martin Guillot, Maxim Moreau, Diane Frank, and Eric Troncy. 2015. "Preliminary Validation and Reliability Testing of the Montreal Instrument for Cat Arthritis Testing, for Use by Veterinarians, in a Colony of Laboratory Cats" Animals 5, no. 4: 1252-1267. https://doi.org/10.3390/ani5040410





