Early Onset of Laying and Bumblefoot Favor Keel Bone Fractures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Animals and Housing
2.2. Data Collection
2.3. Data Analysis
3. Results and Discussion
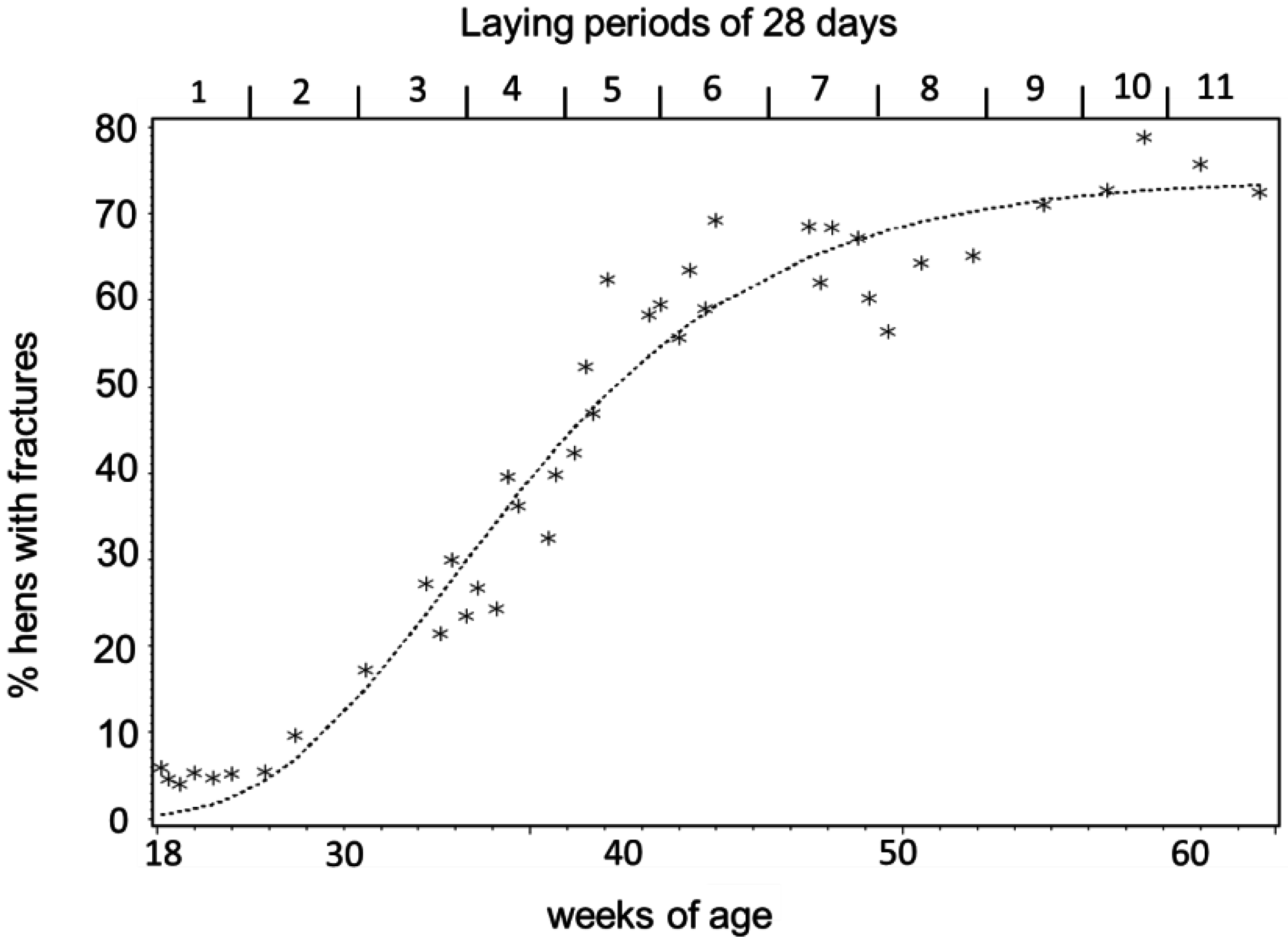
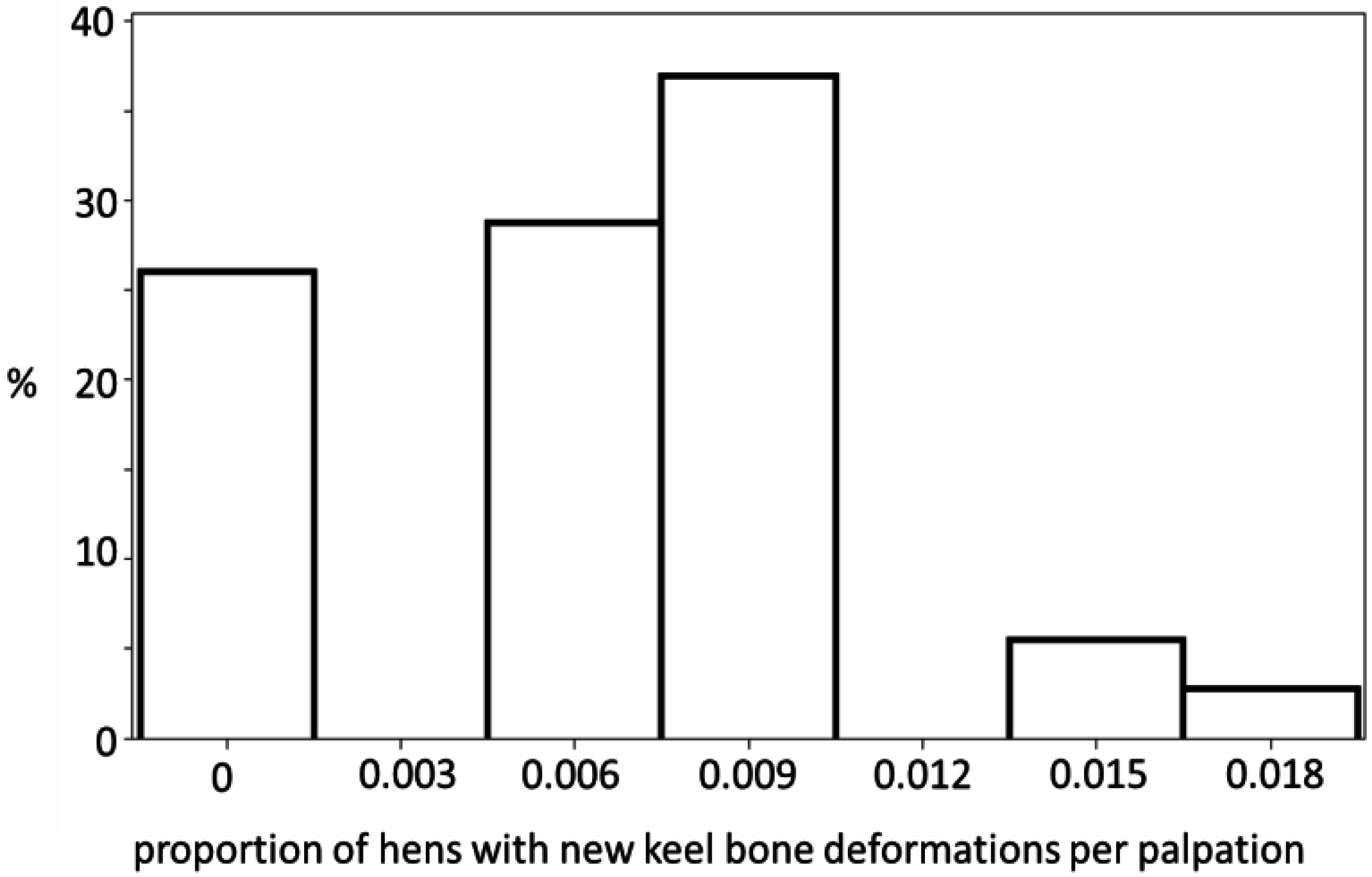
3.1. Keel Bone Deviations
| Time | No New Deviations | <0.0105 | >0.0105 |
|---|---|---|---|
| Laying period 1 | 4 | 2 | 0 |
| Periods 2–6 | 5 | 17 | 2 |
| Periods 7–11.5 | 10 | 3 | 0 |
3.2. Palpation
| Change | Number | % of Total | % of Changes | Median Age (Weeks) |
|---|---|---|---|---|
| Period change | 2532 | 80.03 | - | |
| 4 to 3 | 161 | 5.1 | 26.0 | 36 |
| 4 to 2 | 34 | 1.08 | 5.48 | 38.3 |
| 4 to 1 | 3 | 0.09 | 0.48 | 47.9 |
| From 3 to 2 | 118 | 3.74 | 19.03 | 41 |
| From 3 to 1 | 0 | 0 | 0 | - |
| From 2 to 1 | 32 | 3.74 | 5.16 | 42.9 |
| From 1 to 2 | 27 | 0.86 | 4.36 | 46.4 |
| From 1 to 3 | 0 | 0 | 0 | - |
| From 1 to 4 | 0 | 0 | 0 | - |
| From 2 to 3 | 100 | 3.17 | 16.13 | 41.1 |
| From 2 to 4 | 11 | 0.35 | 1.77 | 41 |
| From 3 to 4 | 42 | 1.08 | 6.77 | 37 |
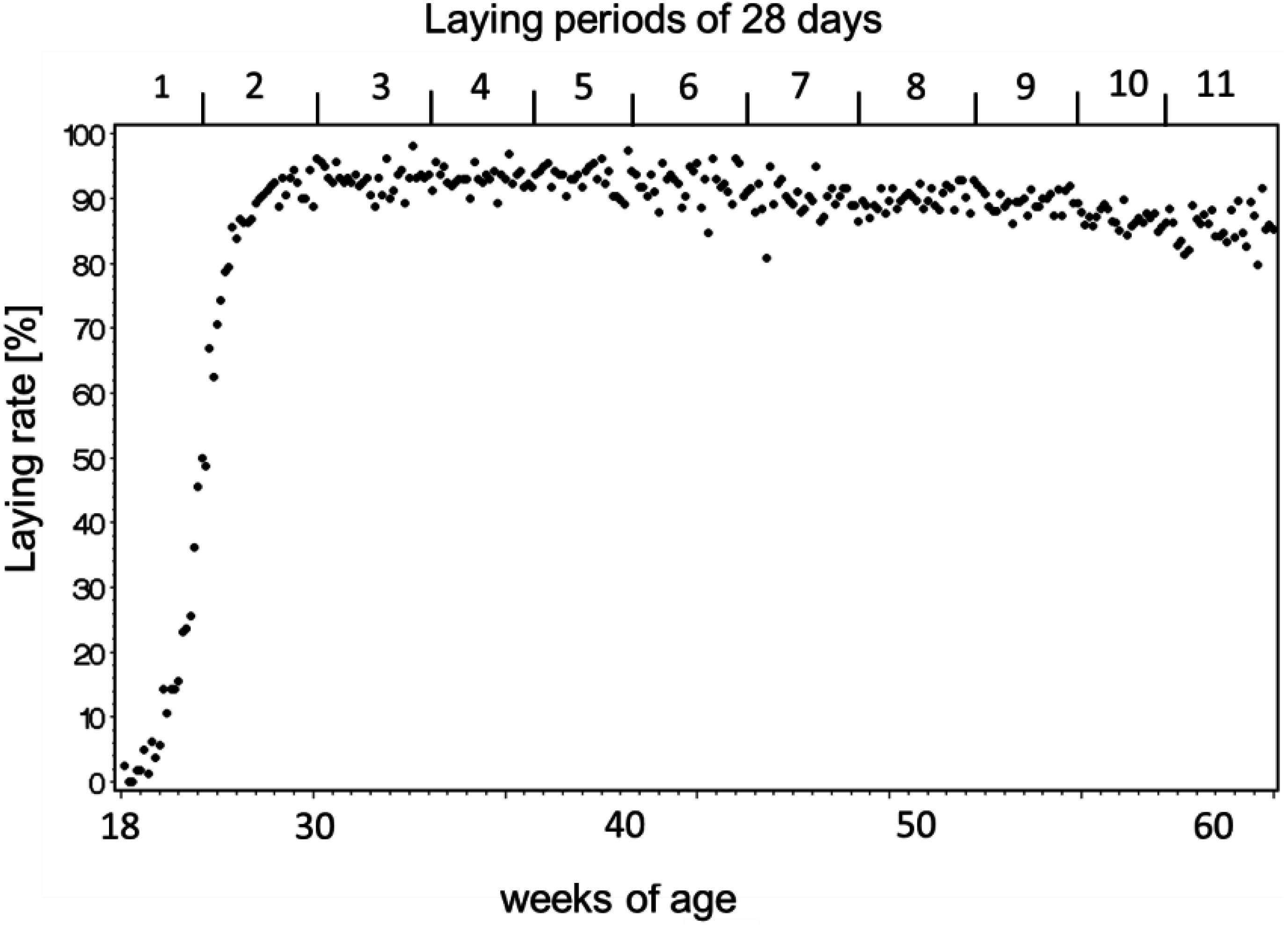
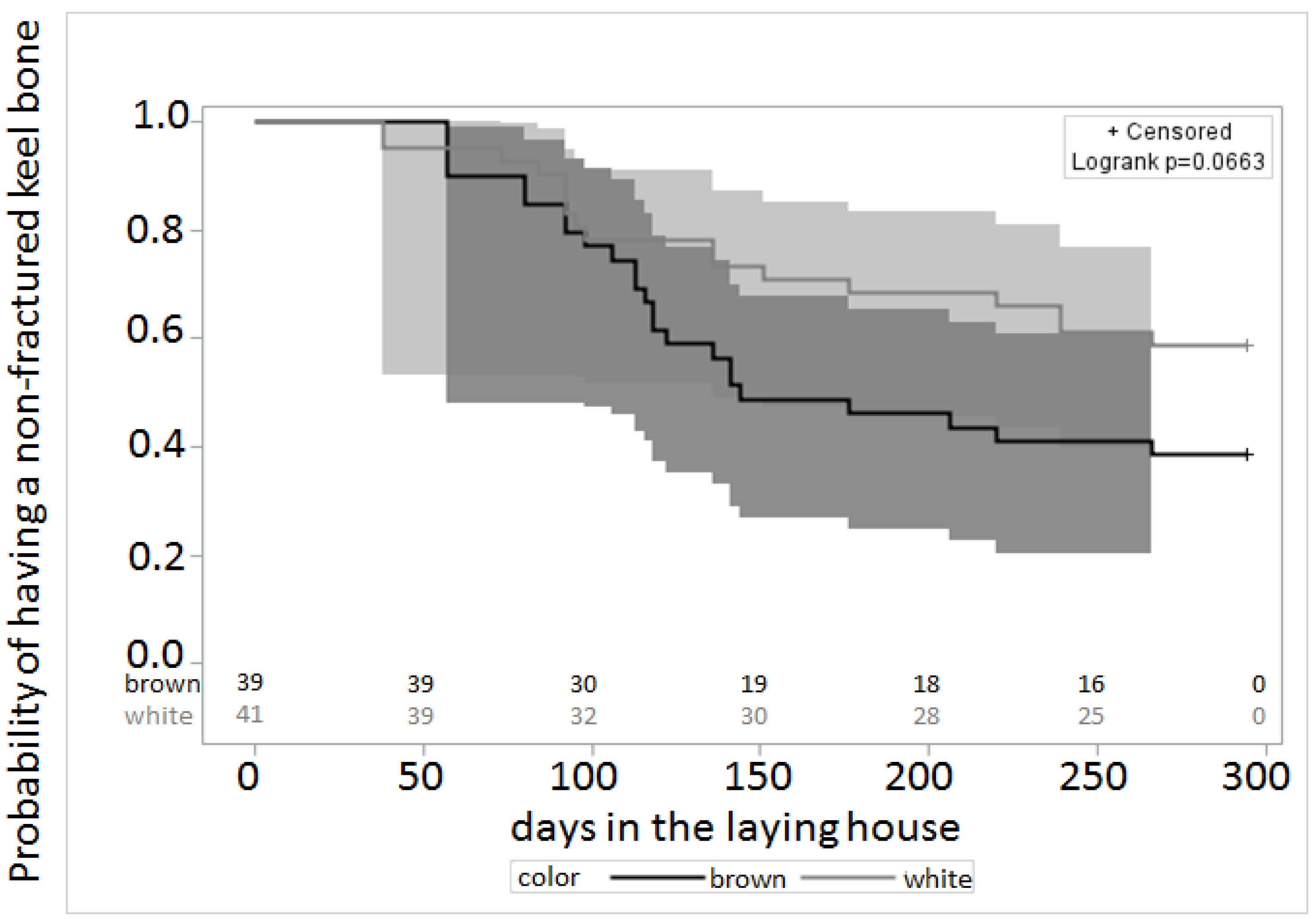
3.3. Egg-Laying and Keel Bone Score
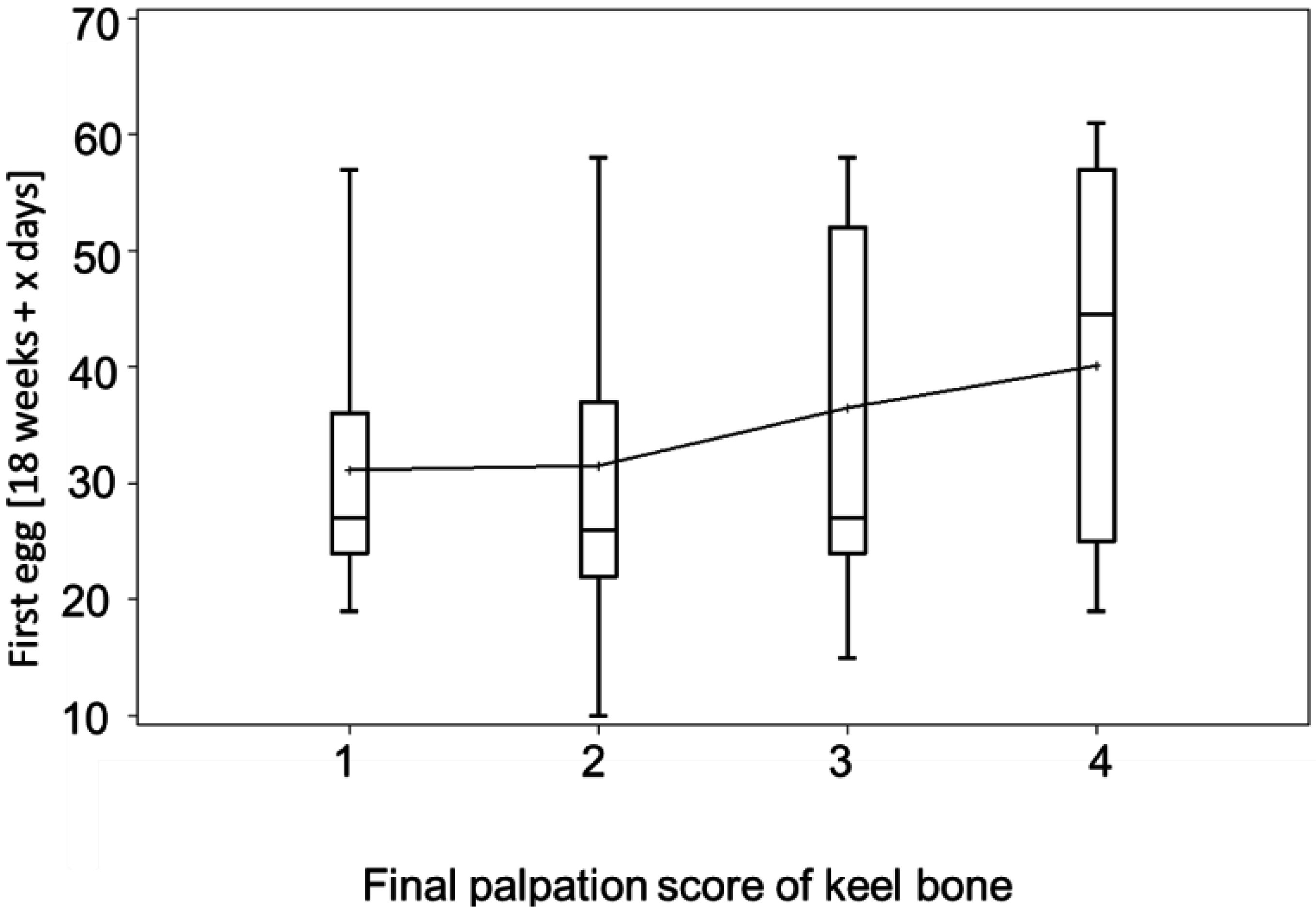
3.4. Bumblefoot
| Bumble Foot | Keel Bone | |||
|---|---|---|---|---|
| Score 1 | Score 2 | Score 3 | Score 4 | |
| No bumble foot | 2 | 23 | 18 | 21 |
| One bumble foot | 2 | 2 | 1 | 5 |
| Two bumble feet | 0 | 6 | 0 | 0 |
| Total | 4 | 31 | 19 | 26 |
3.5. Behavior
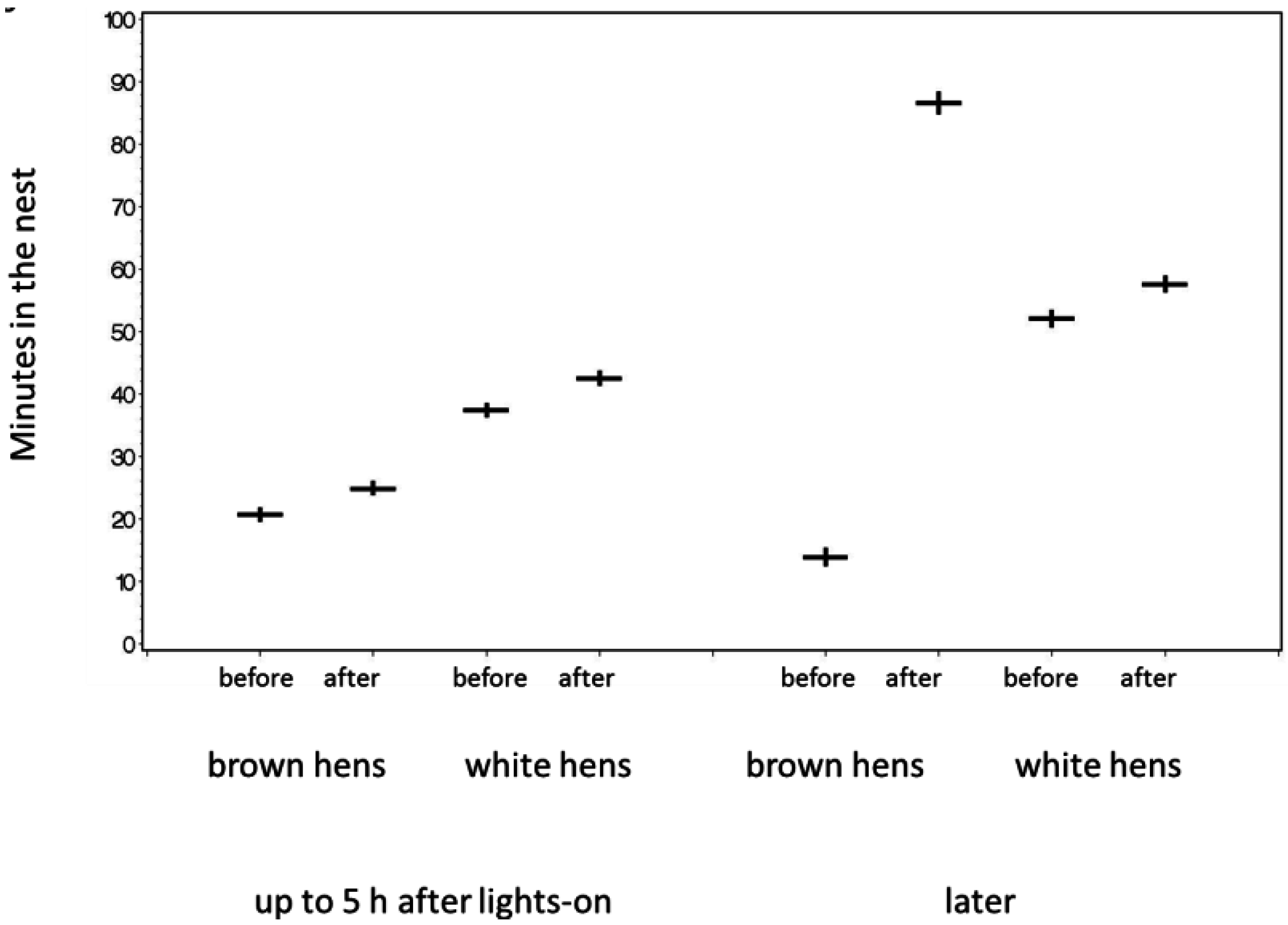
3.6. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Käppeli, S.; Gebhardt-Henrich, S.G.; Fröhlich, E.; Pfulg, A.; Stoffel, M.H. Prevalence of keel bone deformities in Swiss laying hens. Brit. Poult. Sci. 2011, 52, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.; Wilkins, L.; Eleperuma, S.; Ballantyne, A.; Overfield, N. Broken bones in domestic fowls: Effect of husbandry system and stunning method in end-of-lay hens. Brit. Poult. Sci. 1990, 31, 59–69. [Google Scholar] [CrossRef]
- Sandilands, V.; Moinard, C.; Sparks, N.H.C. Providing laying hens with perches: Fulfilling behavioural needs but causing injury? Brit. Poult. Sci. 2009, 50, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Harlander-Matauschek, A.; Rodenburg, T.B.; Sandilands, V.; Tobalske, B.W.; Toscano, M.J. Causes of keel bone damage and their solutions in laying hens. Worlds Poult. Sci. J. 2015, 71, 461–472. [Google Scholar] [CrossRef]
- Wilkins, L.J.; McKinstry, J.L.; Avery, N.C.; Knowles, T.G.; Brown, S.N.; Tarlton, J.; Nicol, C.J. Influence of housing system and design on bone strength and keel bone fractures in laying hens. Vet. Rec. 2011, 169. [Google Scholar] [CrossRef] [PubMed]
- Pickel, T.; Scholz, B.; Schrader, L. Roosting behaviour in laying hens on perches of different temperatures: Trade-offs between thermoregulation, energy budget, vigilance and resting. Appl. Anim. Behav. Sci. 2011, 134, 164–169. [Google Scholar] [CrossRef]
- Scholz, B.; Pickel, T.; Schrader, L. Pressure load on keel bone and foot pads in perching laying hens. In Proceedings of the XIII European Poultry Conference, Tours, France, 23–27 August 2010.
- Pickel, T.; Schrader, L.; Scholz, B. Pressure load on keel bone and foot pads in perching laying hens in relation to perch design. Poult. Sci. 2011, 90, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.H.; McCormack, H.A.; McTeir, L.; Whitehead, C.C. Relationships between genetic, environmental and nutritional factors influencing osteoporosis in laying hens. Brit. Poult. Sci. 2006, 47, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Gentle, M.J. Pain issues in poultry. Appl. Anim. Behav. Sci. 2011, 135, 252–258. [Google Scholar] [CrossRef]
- Nasr, M.; Murrell, J.; Wilkins, L.J.; Nicol, C.J. The effect of keel fractures on egg-production parameters, mobility and behaviour in individual laying hens. Anim. Welf. 2012, 21, 127–135. [Google Scholar] [CrossRef]
- Nasr, M.; Nicol, C.; Murrell, J. Do laying hens with keel bone fractures experience pain? PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Browne, W.J.; Caplen, G.; Hothersall, B.; Murrell, J.C.; Nicol, C.J. Positive affective state induced by opioid analgesia in laying hens with bone fractures. Appl. Anim. Behav. Sci. 2013, 147, 127–131. [Google Scholar] [CrossRef]
- Richards, G.J.; Wilkins, L.J.; Knowles, T.G.; Booth, F.; Toscano, M.J.; Nicol, C.J.; Brown, S.N. Pop hole use by hens with different keel fracture status monitored throughout the laying period. Vet. Rec. 2012, 170, 494–498. [Google Scholar] [CrossRef] [PubMed]
- H&N International. Breeders and Distributors of the World Finest Layers. Available online: www.hn-int.com (accessed on 20 February 2015).
- Raidex. Available online: http://www.raidex.de/produkte/viehzeichenspray.html (accessed on 8 January 2015).
- Gebhardt-Henrich, S.G.; Fröhlich, E.K.; Burose, F.; Fleurent, J.; Gantner, M.; Zähner, M. Individual tracking of laying hens with an RFID-System. Landtechnik 2014, 69, 301–306. [Google Scholar]
- Casey-Trott, T.; Heerkens, J.L.T.; Petrik, M.; Regmi, P.; Schrader, L.; Toscano, M.J.; Widowski, T. Methods for assessment of keel bone damage in poultry. Poult. Sci. 2015, 94, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Käppeli, S.; Gebhardt-Henrich, S.G.; Fröhlich, E.K.; Pfulg, A.; Schäublin, H.; Stoffel, M.H. Effects of housing, perches, genetics, and 25-hydroxycholecalciferol on keel bone deformities in laying hens. Poult. Sci. 2011, 90, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Rönchen, S.; Harmann, H.; Hewicker-Trautwein, M.; Distl, O. Keel bone condition in laying hens: A histological evaluation of macroscopically assessed keel bones. Berl. Münch. Tierärztl. Wochenschr. 2008, 121, 89–94. [Google Scholar] [PubMed]
- Tauson, R.; Abrahamsson, P. Foot and keel bone disorders in laying hens: Effects of artificial perch material and hybrid. Acta Agric. Scand. Sect. A Anim. Sci. 1996, 46, 239–246. [Google Scholar] [CrossRef]
- Savegnago, R.P.; Cruz, V.A.R.; Ramos, S.B.; Caetano, S.L.; Schmidt, G.S.; Ledur, M.C.; El Faro, L.; Munari, D.P. Egg production curve fitting using nonlinear models for selected and nonselected lines of White Leghorn hens. Poult. Sci. 2012, 91, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; GraphPad Software Inc.: San Diego, CA, USA, 2003. [Google Scholar]
- Stratmann, A.; Fröhlich, E.K.; Harlander-Matauschek, A.; Würbel, H.; Gebhardt-Henrich, S. Bewegungen und Abstürze in einem Volierensystem: Auswirkungen von Sitzstangenpolstern auf Brustbeindeformationen bei Legehennen. In Current Research in Applied Ethology; Erhard, M., Pollmann, U., Puppe, B., Reiter, K., Waiblinger, S., Eds.; KTBL: Darmstadt, Germany, 2012; pp. 61–70. [Google Scholar]
- Rodenburg, T.B.; Tuyttens, F.A.M.; de Reu, K.; Herman, L.; Zoons, J.; Sonck, B. Welfare assessment of laying hens in furnished cages and non-cage systems: Assimilating expert opinion. Anim. Welf. 2008, 17, 355–361. [Google Scholar]
- Stratmann, A.; Fröhlich, E.K.F.; Harlander-Matauschek, A.; Schrader, L.; Toscano, M.J.; Würbel, H.; Gebhardt-Henrich, S.G. Soft perches in an aviary system reduce incidence of keel bone damage in laying hens. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturini, G.C.; Savegnago, R.P.; Nunes, B.N.; Ledur, M.C.; Schmidt, G.S.; el Faro, L.; Munari, D.P. Genetic parameters and principal component analysis for egg production from White Leghorn hens. Poult. Sci. 2013, 92, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in cage layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Murrell, J.; Nicol, C. The effect of keel fractures on egg production, feed and water consumption in individual laying hens. Brit. Poult. Sci. 2013, 54, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Daigle, C.; Siegford, J. Welfare Quality® parameters do not always reflect hen behaviour across the lay cycle in non-cage laying hens. Anim. Welf. 2014, 23, 423–434. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Murrell, J.; Wilkins, L.; Nicol, C. The effect of two classes of NSAIDs on the landing ability of laying hens with and without keel fractures. In Quality of Life in Designed Environments; Waiblinger, S., Winckler, C., Gutmann, A., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; p. 53. [Google Scholar]
- Icken, W.; Thurner, S.; Heinrich, A.; Kaiser, A.; Cavero, D.; Wendl, G.; Fries, R.; Schmutz, M.; Preisinger, R. Higher precision level at individual laying performance tests in noncage housing systems. Poult. Sci. 2013, 92, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebhardt-Henrich, S.G.; Fröhlich, E.K.F. Early Onset of Laying and Bumblefoot Favor Keel Bone Fractures. Animals 2015, 5, 1192-1206. https://doi.org/10.3390/ani5040406
Gebhardt-Henrich SG, Fröhlich EKF. Early Onset of Laying and Bumblefoot Favor Keel Bone Fractures. Animals. 2015; 5(4):1192-1206. https://doi.org/10.3390/ani5040406
Chicago/Turabian StyleGebhardt-Henrich, Sabine G., and Ernst K. F. Fröhlich. 2015. "Early Onset of Laying and Bumblefoot Favor Keel Bone Fractures" Animals 5, no. 4: 1192-1206. https://doi.org/10.3390/ani5040406






