Digital Dermatitis in Cattle: Current Bacterial and Immunological Findings
Abstract
:Simple Summary
Abstract
1. Introduction and Digital Dermatitis Lesion Descriptions
1.1. DD Lesion Description
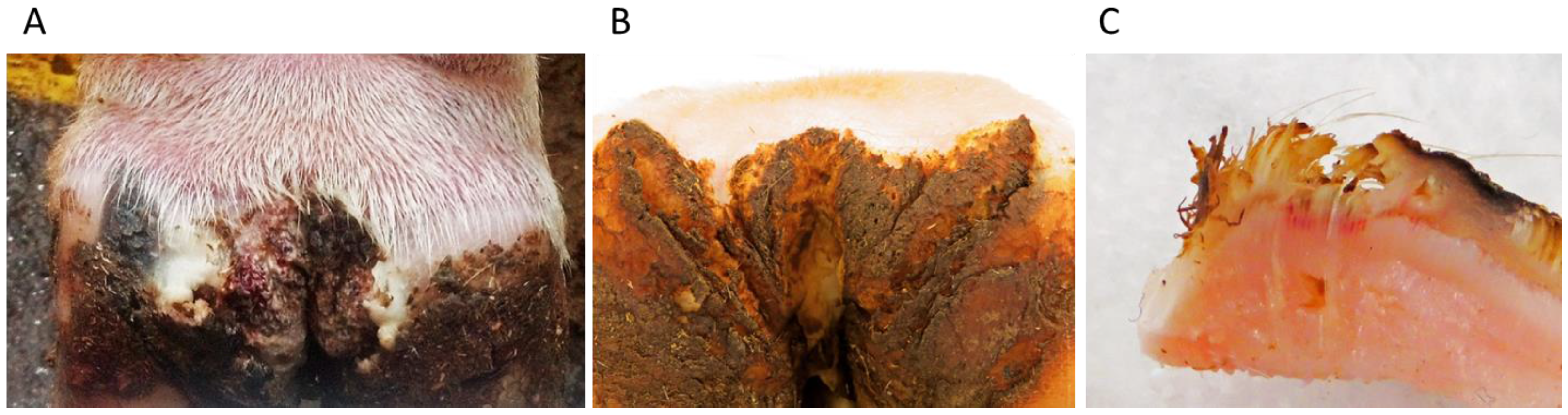
1.2. Multiple Treponema Associated with DD
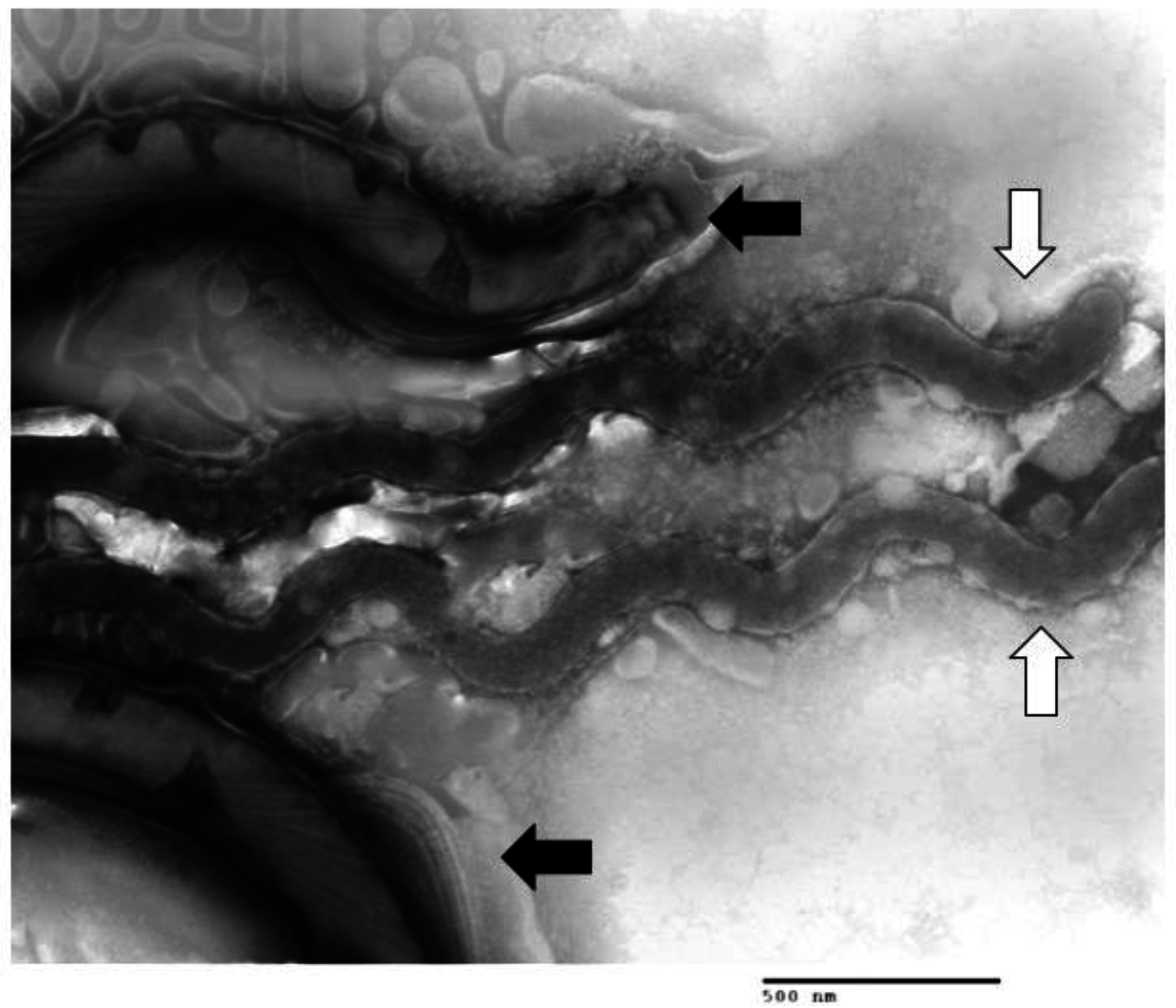
1.3. Other Bacteria Associated with DD

2. Bovine Immune Response to DD
3. Disease Model and Further Research Needs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA. Dairy 2007—Part V: Changes in Dairy Cattle Health and Management Practices in the United States, 1996–2007. USDA–APHIS Veterinary Services: Fort Collins, CO, USA, 2009. [Google Scholar]
- Harvey, D.; Hubbard, C. The Supply chain’s role in improving animal welfare. Animals 2013, 3, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Steiner, A.; Kohler, S.; Koller-Bahler, A.; Wuthrich, M.; Reist, M. Lameness and foot lesions in Swiss dairy cows: I. Prevalence. Schweiz. Arch. Tierheilkd. 2014, 156, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Doherty, N.; More, S.J.; Somers, J. Risk factors for lameness on 10 dairy farms in Ireland. Vet. Rec. 2014. [Google Scholar] [CrossRef] [PubMed]
- Fabian, J.; Laven, R.A.; Whay, H.R. The prevalence of lameness on New Zealand dairy farms: A comparison of farmer estimate and locomotion scoring. Vet. J. 2014, 201, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Refaai, W.; van Aert, M.; Abd El-Aal, A.M.; Behery, A.E.; Opsomer, G. Infectious diseases causing lameness in cattle with a main emphasis on digital dermatitis (Mortellaro disease). Livest. Sci. 2013, 156, 53–63. [Google Scholar] [CrossRef]
- Blowey, R.W.; Sharp, M.W. Digital dermatitis in dairy cattle. Vet. Rec. 1988, 122, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.; Downham, D.Y.; Clarkson, M.J.; Faull, W.B.; Hughes, J.W.; Manson, F.J.; Merritt, J.B.; Russell, W.B.; Sutherst, J.E.; Ward, W.R. Epidemiology of lameness in dairy cattle: description and analysis of foot lesions. Vet. Rec. 1996, 138, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, W.C.; Payne, R.M.; King, J.M.; Wolfe, M.; Begg, S.N. Interdigital papillomatosis in dairy cattle. J. Am. Vet. Med. Assoc. 1980, 177, 437–440. [Google Scholar] [PubMed]
- Rodriguez-Lainz, A.; Melendez-Retamal, P.; Hird, D.W.; Read, D.H. Papillomatous digital dermatitis in Chilean dairies and evaluation of a screening method. Prev. Vet. Med. 1998, 37, 197–207. [Google Scholar] [CrossRef]
- Nally, J.; Wilson-Welder, J.; Alt, D. The etiology of digital dermatitis in ruminants: Recent perspectives. Vet. Med. Res. Rep. 2015. [Google Scholar] [CrossRef]
- Palmer, M.; O’Connell, N. Digital Dermatitis in Dairy Cows: A review of risk factors and potential sources of between-animal variation in susceptibility. Animals 2015, 5, 512–535. [Google Scholar] [CrossRef] [PubMed]
- Read, D.H.; Walker, R.L. Papillomatous digital dermatitis (footwarts) in California dairy cattle: Clinical and gross pathologic findings. J. Vet. Diagn. Invest. 1998, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.L.; Read, D.H.; Loretz, K.J.; Nordhausen, R.W. Spirochetes isolated from dairy cattle with papillomatous digital dermatitis and interdigital dermatitis. Vet. Microbiol. 1995, 47, 343–355. [Google Scholar] [CrossRef]
- Holzhauer, M.; Bartels, C.J.; Dopfer, D.; van Schaik, G. Clinical course of digital dermatitis lesions in an endemically infected herd without preventive herd strategies. Vet. J. 2008, 177, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.C.; Kilgo, P.D.; Jacobsen, K.L. Prevalence of papillomatous digital dermatitis among culled adult cattle in the southeastern United States. Am. J. Vet. Res. 2000, 61, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.E.; Carter, S.D.; Blowey, R.; Duncan, J.S.; Grove-White, D.; Evans, N.J. Digital dermatitis in beef cattle. Vet. Rec. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.E.; Evans, N.J.; Blowey, R.W.; Grove-White, D.H.; Clegg, S.R.; Duncan, J.S.; Carter, S.D. A molecular epidemiology of treponemes in beef cattle digital dermatitis lesions and comparative analyses with sheep contagious ovine digital dermatitis and dairy cattle digital dermatitis lesions. Vet. Microbiol. 2015, 178, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Clegg, S.R.; Mansfield, K.G.; Newbrook, K.; Sullivan, L.E.; Blowey, R.W.; Carter, S.D.; Evans, N.J. Isolation of digital dermatitis treponemes from hoof lesions in wild North American Elk (Cervus elaphus) in Washington State, USA. J. Clin. Microbiol. 2014, 53, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Angell, J.W.; Carter, S.D.; Evans, N.J.; Sullivan, L.E.; Grove-White, D.H. Contagious ovine digital dermatitis: An emerging disease. Vet. J. 2014, 201, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mansfield, K.G. Severe hoof disease in free-ranging Roosevelt Elk (Cervus elaphus roosevelti) in southwestern Washington, USA. J. Wildl. Dis. 2014, 50, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.; Evans, N.; Clegg, S.; Carter, S.; Horsfield, J.; Grove-White, D.; Duncan, J. Digital dermatitis treponemes associated with a severe foot disease in dairy goats. Vet. Rec. 2014, 176, 283. [Google Scholar] [CrossRef] [PubMed]
- Knappe-Poindecker, M.; Gilhuus, M.; Jensen, T.K.; Klitgaard, K.; Larssen, R.B.; Fjeldaas, T. Interdigital dermatitis, heel horn erosion, and digital dermatitis in 14 Norwegian dairy herds. J. Dairy Sci. 2013, 96, 7617–7629. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Blowey, R.W.; Timofte, D.; Isherwood, D.R.; Brown, J.M.; Murray, R.; Paton, R.J.; Carter, S.D. Association between bovine digital dermatitis treponemes and a range of “non-healing” bovine hoof disorders. Vet. Rec. 2011. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.L.; Read, D.H.; Famula, T.R.; Mongini, A.; Dopfer, D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 2012, 193, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Zinicola, M.; Lima, F.; Lima, S.; Machado, V.; Gomez, M.; Dopfer, D.; Guard, C.; Bicalho, R. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.J.; Bell, M.J.; Knowles, T.G.; Whay, H.R.; Main, D.J.; Webster, A.J. The development, implementation and testing of a lameness control programme based on HACCP principles and designed for heifers on dairy farms. Vet. J. 2009, 180, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Bassett, H.F.; Monaghan, M.L.; Lenhan, P.; Doherty, M.L.; Carter, M.E. Bovine digital dermatitis. Vet. Rec. 1990, 126, 164–165. [Google Scholar] [PubMed]
- Krull, A.C.; Shearer, J.K.; Gorden, P.J.; Cooper, V.L.; Phillips, G.J.; Plummer, P.J. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 2014, 82, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.L.; Read, D.H.; Walker, R.L.; Famula, T.R. Clinical, histologic, and bacteriologic findings in dairy cows with digital dermatitis (footwarts) one month after topical treatment with lincomycin hydrochloride or oxytetracycline hydrochloride. J. Am. Vet. Med. Assoc. 2010, 237, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Apprich, V.; Hackl, V.; Tober, R.; Danzer, M.; Kainzbauer, C.; Gabriel, C.; Stanek, C.; Kofler, J. Prevalence of bovine papillomavirus and Treponema DNA in bovine digital dermatitis lesions. Vet. Microbiol. 2011, 148, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Collighan, R.J.; Woodward, M.J. Spirochaetes and other bacterial species associated with bovine digital dermatitis. FEMS Microbiol. Lett. 1997, 156, 37–41. [Google Scholar] [CrossRef]
- Cruz, C.; Driemeier, D.; Cerva, C.; Corbellini, L.G. Bovine digital dermatitis in southern Brazil. Vet. Rec. 2001, 148, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Dopfer, D.; Koopmans, A.; Meijer, F.A.; Szakall, I.; Schukken, Y.H.; Klee, W.; Bosma, R.B.; Cornelisse, J.L.; van Asten, A.J.; ter Huurne, A.A. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec 1997, 140, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, K.; Boye, M.; Capion, N.; Jensen, T.K. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 2008, 46, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, K.; Foix Breto, A.; Boye, M.; Jensen, T.K. Targeting the treponemal microbiome of digital dermatitis infections by high-resolution phylogenetic analyses and comparison with fluorescent in situ hybridization. J. Clin. Microbiol. 2013, 51, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Krull, A.; Rabenold, J.; Elliot, M.; Gorden, J.; Shearer, J.K.; Leuschen, B. The Potential Symbiotic Relationship of Anaerobic Bacteria along with Treponema spp. in the Development of Papillomatous Digital dermatitis. In Proceedings of the Conference of Research Workers in Animal Disease, Chicago, IL, USA, 4–6 December 2011.
- Moe, K.K.; Yano, T.; Misumi, K.; Kubota, C.; Nibe, K.; Yamazaki, W.; Muguruma, M.; Misawa, N. Detection of antibodies against Fusobacterium necrophorum and Porphyromonas levii-like species in dairy cattle with papillomatous digital dermatitis. Microbiol. Immunol. 2010, 54, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Ohya, T.; Yamaguchi, H.; Nii, Y.; Ito, H. Isolation of Campylobacter sputorum from lesions of papillomatous digital dermatitis in dairy cattle. Vet. Rec. 1999, 145, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, S.; Nordhoff, M.; Wyss, C.; Strub, S.; Hubner, J.; Gescher, D.M.; Petrich, A.; Gobel, U.B.; Moter, A. Involvement of Guggenheimella bovis in digital dermatitis lesions of dairy cows. Vet. Microbiol. 2008, 128, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, C.M.; Parlor, K.W.; Marsh, T.L.; Ames, N.K.; Goeman, A.K.; Walker, R.D. Characterization of the predominant anaerobic bacterium recovered from digital dermatitis lesions in three Michigan dairy cows. Anaerobe 2003, 9, 151–155. [Google Scholar] [CrossRef]
- Strub, S.; van der Ploeg, J.R.; Nuss, K.; Wyss, C.; Luginbuhl, A.; Steiner, A. Quantitation of Guggenheimella bovis and treponemes in bovine tissues related to digital dermatitis. FEMS Microbiol. Lett. 2007, 269, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyss, C.; Dewhirst, F.E.; Paster, B.J.; Thurnheer, T.; Luginbuhl, A. Guggenheimella bovis gen. nov., sp. nov., isolated from lesions of bovine dermatitis digitalis. Int. J. Syst. Evol. Microbiol. 2005, 55, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Apley, M.D. Clinical evidence for individual animal therapy for papillomatous digital dermatitis (hairy heel wart) and infectious bovine pododermatitis (foot rot). Vet. Clin. North. Am. Food Anim. Pract. 2015, 31, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Britt, J.S.; Gaska, J.; Garrett, E.F.; Konkle, D.; Mealy, M. Comparison of topical application of three products for treatment of papillomatous digital dermatitis in dairy cattle. J. Am. Vet. Med. Assoc. 1996, 209, 1134–1136. [Google Scholar] [PubMed]
- Cutler, J.H.; Cramer, G.; Walter, J.J.; Millman, S.T.; Kelton, D.F. Randomized clinical trial of tetracycline hydrochloride bandage and paste treatments for resolution of lesions and pain associated with digital dermatitis in dairy cattle. J. Dairy Sci. 2013, 96, 7550–7557. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Shearer, J.K. Efficacy of oxytetracycline for treatment of papillomatous digital dermatitis lesions on various anatomic locations in dairy cows. J. Am. Vet. Med. Assoc. 2000, 216, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Laven, R.A. Efficacy of systemic cefquinome and erythromycin against digital dermatitis in cattle. Vet. Rec. 2006, 159, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Laven, R.A.; Hunt, H. Comparison of valnemulin and lincomycin in the treatment of digital dermatitis by individually applied topical spray. Vet. Rec. 2001, 149, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Laven, R.A.; Logue, D.N. Treatment strategies for digital dermatitis for the UK. Vet. J. 2006, 171, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, A.; Taguchi, K. Healing of digital dermatitis after a single treatment with topical oxytetracycline in 89 dairy cows. Vet. Rec. 2008, 163, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Relun, A.; Lehebel, A.; Bruggink, M.; Bareille, N.; Guatteo, R. Estimation of the relative impact of treatment and herd management practices on prevention of digital dermatitis in French dairy herds. Prev. Vet. Med. 2013, 110, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Silva, C.A.; Borges, J.R.; Fioravanti, M.C.; Borges, G.T.; Atayde, I.B. A clinical trial to assess the use of sodium hypochlorite and oxytetracycline on the healing of digital dermatitis lesions in cattle. Can. Vet. J. 2005, 46, 345–348. [Google Scholar] [PubMed]
- Yano, T.; Moe, K.K.; Chuma, T.; Misawa, N. Antimicrobial susceptibility of Treponema phagedenis-like spirochetes isolated from dairy cattle with papillomatous digital dermatitis lesions in Japan. J. Vet. Med. Sci. 2010, 72, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Demirkan, I.; Murray, R.D.; Vink, W.D.; Blowey, R.W.; Hart, C.A.; Carter, S.D. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 2008, 130, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Demirkan, I.; Singh, P.; Getty, B.; Timofte, D.; Vink, W.D.; Murray, R.D.; Blowey, R.W.; Birtles, R.J.; et al. Association of unique, isolated treponemes with bovine digital dermatitis lesions. J. Clin. Microbiol. 2009, 47, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Capion, N.; Klitgaard, K.; Rogdo, T.; Fjeldaas, T.; Boye, M.; Jensen, T.K. Bovine digital dermatitis: Possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Microbiol. 2012, 160, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Stamm, L.V.; Bergen, H.L.; Walker, R.L. Molecular typing of papillomatous digital dermatitis-associated Treponema isolates based on analysis of 16S-23S ribosomal DNA intergenic spacer regions. J. Clin. Microbiol. 2002, 40, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Nordhoff, M.; Moter, A.; Schrank, K.; Wieler, L.H. High prevalence of treponemes in bovine digital dermatitis-a molecular epidemiology. Vet. Microbiol. 2008, 131, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Moe, K.K.; Yamazaki, K.; Ooka, T.; Hayashi, T.; Misawa, N. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 2010, 143, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Capion, N.; Boye, M.; Ekstrom, C.T.; Jensen, T.K. Infection dynamics of digital dermatitis in first-lactation Holstein cows in an infected herd. J. Dairy Sci. 2012, 95, 6457–6464. [Google Scholar] [CrossRef] [PubMed]
- Pringle, M.; Bergsten, C.; Fernstrom, L.L.; Hook, H.; Johansson, K.E. Isolation and characterization of Treponema phagedenis-like spirochetes from digital dermatitis lesions in Swedish dairy cattle. Acta. Vet. Scand. 2008. [Google Scholar] [CrossRef] [PubMed]
- Trott, D.J.; Moeller, M.R.; Zuerner, R.L.; Goff, J.P.; Waters, W.R.; Alt, D.P.; Walker, R.L.; Wannemuehler, M.J. Characterization of Treponema phagedenis-like spirochetes isolated from papillomatous digital dermatitis lesions in dairy cattle. J. Clin. Microbiol. 2003, 41, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Yamagami, R.; Misumi, K.; Kubota, C.; Moe, K.K.; Hayashi, T.; Yoshitani, K.; Ohtake, O.; Misawa, N. Genetic heterogeneity among strains of Treponema phagedenis-like spirochetes isolated from dairy cattle with papillomatous digital dermatitis in Japan. J. Clin. Microbiol. 2009, 47, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, I.; Carter, S.D.; Hart, C.A.; Woodward, M.J. Isolation and cultivation of a spirochaete from bovine digital dermatitis. Vet. Rec. 1999, 145, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, I.; Williams, H.F.; Dhawi, A.; Carter, S.D.; Winstanley, C.; Bruce, K.D.; Hart, C.A. Characterization of a spirochaete isolated from a case of bovine digital dermatitis. J. Appl. Microbiol. 2006, 101, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Dopfer, D.; Anklam, K.; Mikheil, D.; Ladell, P. Growth curves and morphology of three Treponema subtypes isolated from digital dermatitis in cattle. Vet. J. 2012, 193, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Demirkan, I.; Murray, R.D.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Treponema pedis sp. nov., a spirochaete isolated from bovine digital dermatitis lesions. Int. J. Syst. Evol. Microbiol. 2009, 59, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Schrank, K.; Choi, B.K.; Grund, S.; Moter, A.; Heuner, K.; Nattermann, H.; Gobel, U.B. Treponema brennaborense sp. nov., a novel spirochaete isolated from a dairy cow suffering from digital dermatitis. Int. J. Syst. Bacteriol. 1999, 49 Pt 1, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Welder, J.H.; Elliott, M.K.; Zuerner, R.L.; Bayles, D.O.; Alt, D.P.; Stanton, T.B. Biochemical and molecular characterization of Treponema phagedenis-like spirochetes isolated from a bovine digital dermatitis lesion. BMC Microbiol. 2013, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Dymock, D.; Jenkinson, H.F. From tooth to hoof: Treponemes in tissue-destructive diseases. J. Appl. Microbiol. 2003, 94, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.; Klitgaard, K.; Jensen, T.K. Identification of Treponema pedis as the predominant Treponema species in porcine skin ulcers by fluorescence in situ hybridization and high-throughput sequencing. Vet. Microbiol. 2014, 171, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pringle, M.; Fellström, C. Treponema pedis isolated from a sow shoulder ulcer. Vet. Microbiol. 2010, 142, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Svartstrom, O.; Mushtaq, M.; Pringle, M.; Segerman, B. Genome-wide relatedness of Treponema pedis, from gingiva and necrotic skin lesions of pigs, with the human oral pathogen Treponema denticola. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.K.; Yano, T.; Kuwano, A.; Sasaki, S.; Misawa, N. Detection of treponemes in canker lesions of horses by 16S rRNA clonal sequencing analysis. J. Vet. Med. Sci. 2010, 72, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, C.M.; Castro, F.; Buchanan, B.; Schumacher, J.; Craig, L.E. Proliferative pododermatitis (canker) with intralesional spirochetes in three horses. J. Vet. Diagn. Invest. 2005, 17, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Rashmir-Raven, A.M.; Black, S.S.; Rickard, L.G.; Akin, M. Papillomatous pastern dermatitis with spirochetes and Pelodera strongyloides in a Tennessee walking horse. J. Vet. Diagn. Invest. 2000, 12, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Sykora, S.; Brandt, S. Occurrence of Treponema DNA in equine hoof canker and normal hoof tissue. Equine Vet. J. 2014, 47, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Lumeij, J.T.; de Koning, J.; Bosma, R.B.; van der Sluis, J.J.; Schellekens, J.F. Treponemal infections in hares in The Netherlands. J. Clin. Microbiol. 1994, 32, 543–546. [Google Scholar] [PubMed]
- Evans, N.J.; Timofte, D.; Carter, S.D.; Brown, J.M.; Scholey, R.; Read, D.H.; Blowey, R.W. Association of treponemes with bovine ulcerative mammary dermatitis. Vet. Rec. 2010, 166, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Stamm, L.V.; Walker, R.L.; Read, D.H. Genetic diversity of bovine ulcerative mammary dermatitis-associated Treponema. Vet. Microbiol. 2009, 136, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.E.; Pescador, C.A.; Nakajima, Y.; Driemeier, D. Immunopathological investigations on bovine digital epidermitis. Vet. Rec. 2005, 157, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.J.; Woodward, M.J.; Grogono-Thomas, R. The occurrence of treponemes in contagious ovine digital dermatitis and the characterisation of associated Dichelobacter nodosus. Vet. Microbiol. 2005, 111, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Svartström, O.; Karlsson, F.; Fellström, C.; Pringle, M. Characterization of Treponema spp. isolates from pigs with ear necrosis and shoulder ulcers. Veterinary microbiology 2013, 166, 617–623. [Google Scholar]
- Moter, A.; Riep, B.; Haban, V.; Heuner, K.; Siebert, G.; Berning, M.; Wyss, C.; Ehmke, B.; Flemmig, T.F.; Gobel, U.B. Molecular epidemiology of oral treponemes in patients with periodontitis and in periodontitis-resistant subjects. J. Clin Microbiol 2006, 44, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.B.; Ellen, R.P. New insights into the emerging role of oral spirochaetes in periodontal disease. Clin Microbiol Infect. 2011, 17, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, O.; Karlsson, F.; Fellström, C.; Pringle, M. Characterization of Treponema spp. isolates from pigs with ear necrosis and shoulder ulcers. Vet. Microbiol. 2013, 166, 617–623. [Google Scholar]
- Mikx, F.H. Comparison of peptidase, glycosidase and esterase activities of oral and non-oral Treponema species. J. Gen. Microbiol. 1991, 137, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Dymock, D.; Woodward, M.J.; Jenkinson, H.F. Genetic relatedness and phenotypic characteristics of Treponema associated with human periodontal tissues and ruminant foot disease. Microbiology 2003, 149, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Nooris, S.J.; Paster, B.J.; Smibert, R.M.; Genus, I.V. Treponema . In Bergey’s Manual of Systematic Bacteriology; Krieg, N.R., Staley, J.T., Brown, D.R., Eds.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Elliott, M.K.; Alt, D.P.; Zuerner, R.L. Lesion formation and antibody response induced by papillomatous digital dermatitis-associated spirochetes in a murine abscess model. Infect. Immun. 2007, 75, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Zuerner, R.L.; Heidari, M.; Elliott, M.K.; Alt, D.P.; Neill, J.D. Papillomatous digital dermatitis spirochetes suppress the bovine macrophage innate immune response. Vet. Microbiol. 2007, 125, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Koniarova, I.; Orsag, A.; Ledecký, V. The role anaerobes in dermatitis digitalis et interdigitalis in cattle. Vet. Med. 1992, 38, 589–596. [Google Scholar]
- Santos, T.M.; Pereira, R.V.; Caixeta, L.S.; Guard, C.L.; Bicalho, R.C. Microbial diversity in bovine papillomatous digital dermatitis in Holstein dairy cows from upstate New York. FEMS Microbiol. Ecol. 2011, 79, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Ohya, T.; Ishii, R.; Ogihara, Y.; Maeda, T.; Ishikawa, Y.; Kadota, K. Concurrent spirochaetal infections of the feet and colon of cattle in Japan. Aust. Vet. J. 2002, 80, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Blowey, R.W.; Done, S.H.; Cooley, W. Observations on the pathogenesis of digital dermatitis in cattle. Vet. Rec. 1994, 135, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Knappe-Poindecker, M.; Gilhuus, M.; Jensen, T.K.; Vatn, S.; Jorgensen, H.J.; Fjeldaas, T. Cross-infection of virulent Dichelobacter nodosus between sheep and co-grazing cattle. Vet. Microbiol. 2014, 170, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.S.; Grove-White, D.; Moks, E.; Carroll, D.; Oultram, J.W.; Phythian, C.J.; Williams, H.W. Impact of footrot vaccination and antibiotic therapy on footrot and contagious ovine digital dermatitis. Vet. Rec. 2012, 170, 462. [Google Scholar] [CrossRef] [PubMed]
- Blowey, R.W.; Done, S.H. Failure to demonstrate histological changes of digital or interdigital dermatitis in biopsies of slurry heel. Vet. Rec. 1995, 137, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Stauble, A.; Steiner, A.; Frey, J.; Kuhnert, P. Simultaneous detection and discrimination of virulent and benign Dichelobacter nodosus in sheep of flocks affected by foot rot and in clinically healthy flocks by competitive real-time PCR. J. Clin. Microbiol. 2014, 52, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Kirst, M.E.; Li, E.C.; Alfant, B.; Chi, Y.Y.; Walker, C.; Magnusson, I.; Wang, G.P. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl. Environ. Microbiol. 2015, 81, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Chang, M.; Martin, J.; Mitreva, M.; Lux, R.; Klokkevold, P.; Sodergren, E.; Weinstock, G.M.; Haake, S.K.; Li, H. Dynamic changes in the subgingival microbiome and their potential for diagnosis and prognosis of periodontitis. MBio 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P. Defining functional signatures of dysbiosis in periodontitis progression. Genome Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zinicola, M.; Higgins, H.; Lima, S.; Machado, V.; Guard, C.; Bicalho, R. Shotgun metagenomic sequencing reveals functional genes and microbiome associated with bovine digital dermatitis. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, I.; Walker, R.L.; Murray, R.D.; Blowey, R.W.; Carter, S.D. Serological evidence of spirochaetal infections associated with digital dermatitis in dairy cattle. Vet. J. 1999, 157, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.K.; Alt, D.P. Bovine immune response to papillomatous digital dermatitis (PDD)-associated spirochetes is skewed in isolate reactivity and subclass elicitation. Vet. Immunol. Immunopathol. 2009, 130, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.K.; Yano, T.; Misumi, K.; Kubota, C.; Yamazaki, W.; Muguruma, M.; Misawa, N. Analysis of the IgG immune response to Treponema phagedenis-like spirochetes in individual dairy cattle with papillomatous digital dermatitis. Clin. Vaccine Immunol. 2010, 17, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.L.; Read, D.H.; Loretz, K.J.; Hird, D.W.; Berry, S.L. Humoral response of dairy cattle to spirochetes isolated from papillomatous digital dermatitis lesions. Am. J. Vet. Res. 1997, 58, 744–748. [Google Scholar] [PubMed]
- Vink, W.D.; Jones, G.; Johnson, W.O.; Brown, J.; Demirkan, I.; Carter, S.D.; French, N.P. Diagnostic assessment without cut-offs: Application of serology for the modelling of bovine digital dermatitis infection. Prev. Vet. Med. 2009, 92, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Timofte, D.; Isherwood, D.R.; Brown, J.M.; Williams, J.M.; Sherlock, K.; Lehane, M.J.; Murray, R.D.; Birtles, R.J.; Hart, C.A.; et al. Host and environmental reservoirs of infection for bovine digital dermatitis treponemes. Vet. Microbiol. 2012, 156, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Baek, K.J.; Choi, Y.S.; Choi, Y. A periodontal pathogen Treponema denticola hijacks the Fusobacterium nucleatum-driven host response. Immunol. Cell. Biol. 2013, 91, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.C.; Brown, W.C.; Hamilton, M.J.; Wyatt, C.R.; Orden, J.A.; Khalid, A.M.; Naessens, J. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet. Immunol. Immunopathol. 1996, 52, 275–283. [Google Scholar] [CrossRef]
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine gammadelta T cells are a major regulatory T cell subset. J. Immunol. 2014, 193, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Scholey, R.; Evans, N.; Blowey, R.; Massey, J.; Murray, R.; Smith, R.; Ollier, W.; Carter, S. Identifying host pathogenic pathways in bovine digital dermatitis by RNA-Seq analysis. Vet. J. 2013, 197, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Scholey, R.; Murray, R.D.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Differential inflammatory responses of bovine foot skin fibroblasts and keratinocytes to digital dermatitis treponemes. Vet. Immunol. Immunopathol. 2014, 161, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Refaai, W.; Ducatelle, R.; Geldhof, P.; Mihi, B.; El-shair, M.; Opsomer, G. Digital dermatitis in cattle is associated with an excessive innate immune response triggered by the keratinocytes. BMC Vet. Res. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhardt, T.; Carbone, F.R. Unpleasant memories: Tissue-embedded T cell memory drives skin hypersensitivity. Nat. Med. 2015, 21, 551–552. [Google Scholar] [CrossRef] [PubMed]
- McCully, M.L.; Collins, P.J.; Hughes, T.R.; Thomas, C.P.; Billen, J.; O’Donnell, V.B.; Moser, B. Skin Metabolites Define a New Paradigm in the Localization of Skin Tropic Memory T Cells. J. Immunol. 2015, 195, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, M.; Santema, W.; Van Rhijn, I.; Rutten, V.; Koets, A. gammadelta T cell homing to skin and migration to skin-draining lymph nodes is CCR7 independent. J. Immunol. 2012, 188, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Gehad, A.; Yang, C.; Scott, L.L.; Teague, J.E.; Schlapbach, C.; Elco, C.P.; Huang, V.; Matos, T.R.; Kupper, T.S.; et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Cook, N.B.; Bernardoni, N.D.; Rieman, J.; Dusick, A.F.; Hartshorn, R.; Socha, M.T.; Read, D.H.; Dopfer, D. An experimental infection model to induce digital dermatitis infection in cattle. J. Dairy Sci. 2012, 95, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Fine, D.; Teng, Y.T.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Nutter, W.T.; Moffitt, J.A. Digital dermatitis control. Vet. Rec. 1990, 126, 200–201. [Google Scholar]
- Blowey, R.W. Control of digital dermatitis. Vet. Rec. 2000, 146, 295. [Google Scholar] [PubMed]
- Holzhauer, M.; Bartels, C.J.; van Barneveld, M.; Vulders, C.; Lam, T. Curative effect of topical treatment of digital dermatitis with a gel containing activated copper and zinc chelate. Vet. Rec. 2011. [Google Scholar] [CrossRef] [PubMed]
- Holzhauer, M.; Dopfer, D.; de Boer, J.; van Schaik, G. Effects of different intervention strategies on the incidence of papillomatous digital dermatitis in dairy cows. Vet. Rec. 2008, 162, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Nuss, K. Footbaths: The solution to digital dermatitis? Vet. J. 2006, 171, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.K.; Hernandez, J. Efficacy of two modified nonantibiotic formulations (Victory) for treatment of papillomatous digital dermatitis in dairy cows. J. Dairy Sci. 2000, 83, 741–745. [Google Scholar] [CrossRef]
- Smith, A.C.; Wood, C.L.; McQuerry, K.J.; Bewley, J.M. Effect of a tea tree oil and organic acid footbath solution on digital dermatitis in dairy cows. J. Dairy Sci. 2014, 97, 2498–2501. [Google Scholar] [CrossRef] [PubMed]
- Speijers, M.H.; Baird, L.G.; Finney, G.A.; McBride, J.; Kilpatrick, D.J.; Logue, D.N.; O’Connell, N.E. Effectiveness of different footbath solutions in the treatment of digital dermatitis in dairy cows. J. Dairy Sci. 2010, 93, 5782–5791. [Google Scholar] [CrossRef] [PubMed]
- Tyler, H.D.; Ensminger, M.E. Dairy Cattle Science; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Travis, D.A.; Sriramarao, P.; Cardona, C.; Steer, C.J.; Kennedy, S.; Sreevatsan, S.; Murtaugh, M.P. One Medicine One Science: A framework for exploring challenges at the intersection of animals, humans, and the environment. Ann. N Y Acad. Sci. 2014, 1334, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services (FDA). #209 Guidance for Industry: The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals; Department of Health and Human Services (FDA): Washington, DC, USA, 2012.
- Doane, M.; Sarenbo, S. Exposure of farm laborers and dairy cattle to formaldehyde from footbath use at a dairy farm in New York State. Sci. Total Environ. 2014, 487, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hansi, M.; Weidenhamer, J.D.; Sinkkonen, A. Plant growth responses to inorganic environmental contaminants are density-dependent: Experiments with copper sulfate, barley and lettuce. Environ. Pollut. 2014, 184, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. Rev. Environ. Contam. Toxicol. 2011, 213, 1–26. [Google Scholar] [PubMed]
- Kumar, V.; Kalita, J.; Misra, U.K.; Bora, H.K. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med. Biol. 2015, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson-Welder, J.H.; Alt, D.P.; Nally, J.E. Digital Dermatitis in Cattle: Current Bacterial and Immunological Findings. Animals 2015, 5, 1114-1135. https://doi.org/10.3390/ani5040400
Wilson-Welder JH, Alt DP, Nally JE. Digital Dermatitis in Cattle: Current Bacterial and Immunological Findings. Animals. 2015; 5(4):1114-1135. https://doi.org/10.3390/ani5040400
Chicago/Turabian StyleWilson-Welder, Jennifer H., David P. Alt, and Jarlath E. Nally. 2015. "Digital Dermatitis in Cattle: Current Bacterial and Immunological Findings" Animals 5, no. 4: 1114-1135. https://doi.org/10.3390/ani5040400




