An Investigation of a Cluster of Parapoxvirus Cases in Missouri, Feb–May 2006: Epidemiologic, Clinical and Molecular Aspects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Investigation
2.2. Herder Survey and Investigation
2.3. Veterinarian Survey
2.4. County/State Fair Investigation
2.5. Laboratory Investigation
2.6. Parapoxvirus-Generic and Orf-Specific Real Time-Polymerase Chain Reaction Assay
- (1)
- Parapoxvirus-generic assay:
- Parapox-generic forward primer (5'-GTA_CCG_CGC_CAT_GTC_CA-3')
- Parapox-generic reverse primer (5'-CCT_TCG_GCA_AGG_ACG_ACG-3')
- Parapox-genericprobe (5'-CC_AGC_GAG_TAG_TTC_GCG_TAC_ATG_TCC-3')
- (2)
- Orf-specific assay:
- Orf forward primer (5'-GCT_CGC_TGG_CCA_CCA_G-3')
- Orf reverse primer (5'-CGG_GCC_CAT_GTC_CGT-3')
- Orf probe (5'-CT_CTA_TCT_CCG_AGC_GCT_GGC_GC-3')
2.7. Parapoxvirus Serologic Assay
2.8. Sequencing and Phylogenetic Analysis
3. Results
3.1. Case Investigation
| Case | Sex | Age | Animal source | Animal overtly ill? | Risk factor/ animal contact | Virus identified | Closed operation? * | Used orf vaccine? | Glove use? |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 34 | dairy calf; | yes | tube feeding | pseudocowpox virus | no | n/a † | no |
| family farm | |||||||||
| 2 ‡ | M | 41 | sheep; | yes | bottle feeding | orf virus § | yes | no | no |
| family farm | |||||||||
| 3 ‡ | M | 10 | sheep; | yes | bottle feeding | orf virus § | yes | no | no |
| family farm | |||||||||
| 4 | F | 18 | sheep; | no | shearing | orf virus | yes | no | no |
| family farm |
3.2. Herder Survey and Investigation

| Characteristic | Ever Infected | No History of Infection | ||||
|---|---|---|---|---|---|---|
| n (N = 22) | % | n (N = 81) | % | OR | 95% CI | |
| Sex | ||||||
| Male | 16 | 72.7 | 30 | 37.0 | 4.5† | 1.6–12.8 |
| Female | 6 | 27.3 | 51 | 63.0 | reference | |
| Occupation | ||||||
| Herder (>50% livestock) | 6 | 27.3 | 16 | 19.8 | reference | |
| Others | 16 | 72.7 | 65 | 80.3 | 1.5 | 0.5–4.5 |
| Occupation | ||||||
| Herder (any livestock) | 11 | 50.0 | 23 | 28.4 | reference | |
| Farm worker (non-owner) | 2 | 9.1 | 2 | 2.5 | 2.1 | 0.36–16.9 |
| Homemaker | 2 | 9.1 | 5 | 6.2 | 0.8 | 0.4–5.0 |
| Student | 3 | 13.6 | 13 | 16.1 | 0.5 | 1.0–2.0 |
| Others | 4 | 18.2 | 38 | 46.9 | 0.2* | 0.1–0.8 |
| n (N = 21) | % | n (N = 80) | % | OR | 95% CI | |
| Has heard of parapoxvirus infections before survey | ||||||
| Yes | 20 | 95.2 | 54 | 67.5 | 9.6 * | 1.2–75.7 |
| No | 1 | 4.8 | 26 | 32.5 | reference | |
| n (N = 21) | % | n (N = 79) | % | OR | 95% CI | |
| Know of others who have had parapoxvirus infection | ||||||
| Yes | 5 | 23.8 | 9 | 11.4 | 1.7 | 0.5–6.4 |
| No | 16 | 76.2 | 70 | 88.6 | reference | |
| Ever Infected | No History of Infection | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | |
| Number of animals in contact with | ||||||
| 25–99 | 5/22 | 22.7 | 32/81 | 39.5 | reference | |
| <25 | 3/22 | 13.6 | 17/81 | 21.0 | 1.1 | 0.2–5.3 |
| >99 | 14/22 | 63.6 | 32/81 | 39.5 | 2.8 | 0.9–8.7 |
| Smallpox vaccination | ||||||
| Yes | 12/20 | 60.0 | 56/69 | 81.2 | ||
| No | 8/20 | 40.0 | 13/69 | 18.8 | 0.3 | 0.1–1.0 |
| Daily contact | ||||||
| Yes | 9/22 | 40.9 | 44/81 | 54.3 | 0.6 | 0.2–1.5 |
| No | 13/22 | 59.1 | 37/81 | 45.7 | ||
| Grooming | ||||||
| Yes | 19/22 | 13.6 | 65/81 | 80.3 | 1.6 | 0.4–5.9 |
| No | 3/22 | 86.4 | 16/81 | 19.8 | ||
| Bottle/Tube Feed | ||||||
| Yes | 19/22 | 86.4 | 66/81 | 81.5 | 1.4 | 0.4–5.5 |
| No | 3/22 | 13.6 | 15/81 | 18.5 | ||
| Birthing | ||||||
| Yes | 20/22 | 90.9 | 66/81 | 81.5 | 2.3 | 0.5–10.8 |
| No | 2/22 | 9.1 | 15/81 | 18.5 | ||
| Currently raise sheep | ||||||
| Yes | 15/22 | 68.2 | 55/81 | 67.9 | 1.0 | 0.4–2.8 |
| No | 7/22 | 31.8 | 26/81 | 32.1 | ||
| Currently raise goats | ||||||
| Yes | 3/22 | 13.6 | 9/81 | 11.1 | 1.3 | 0.3–5.1 |
| No | 19/22 | 86.4 | 72/81 | 88.9 | ||
| Currently raise cattle | ||||||
| Yes | 9/22 | 40.8 | 47/81 | 58.0 | 0.5 | 0.2–1.3 |
| No | 13/22 | 59.1 | 34/81 | 42.0 | ||
| Has seen infection in their | ||||||
| Yes | 19/22 | 86.4 | 51/77 | 66.2 | 3.2 | 0.9–11.9 |
| No | 3/22 | 13.6 | 26/77 | 33.8 | ||
| Uses gloves * | ||||||
| Yes | 17/22 | 77.3 | 31/80 | 38.8 | 2.2 | 0.7–6.4 |
| No | 5/22 | 22.7 | 49/80 | 61.3 | ||
| Has “facilitated” infection ** | ||||||
| Yes | 6/16 | 37.5 | 33/61 | 54.1 | 0.5 | 0.2–1.6 |
| No | 10/16 | 62.5 | 28/61 | 45.9 | ||
| Has used orf vaccine on sheep/goat | ||||||
| Yes | 8/19 | 42.1 | 22/61 | 36.1 | 1.3 | 0.5–3.7 |
| No | 11/19 | 57.9 | 39/61 | 63.9 | ||
| Has seen infection in animal within last year | ||||||
| Yes | 12/21 | 42.9 | 34/59 | 57.6 | 1.0 | 0.4–2.7 |
| No | 9/21 | 57.1 | 25/59 | 42.4 | ||
| Questions | Number (%) of Respondents Reporting “Yes” * |
|---|---|
| Recall more than one parapoxvirus infection? | 2/21 † (9.5) |
| Seek healthcare during the infection? | 13/21 † (61.9) |
| If no, why? | |
| Not severe enough | – |
| Already aware of diagnosis | 7/8 (87.5) |
| No access to healthcare | – |
| What type of physician(s) did you see? ‡ | |
| Urgent care | 2/13 (15.4) |
| Family practice | 10/13 (76.9) |
| Internal medicine | – |
| Sub-specialist (dermatology, infectious diseases) | 3/13 (23.1) |
| Repeat visits before diagnosis was made? | 4/13(30.8) |
| Received antibiotics | 11/13 (84.6) |
| If topical therapy were available, would this be worthwhile? | 19/22 (86.4) |
3.3. Veterinary Survey
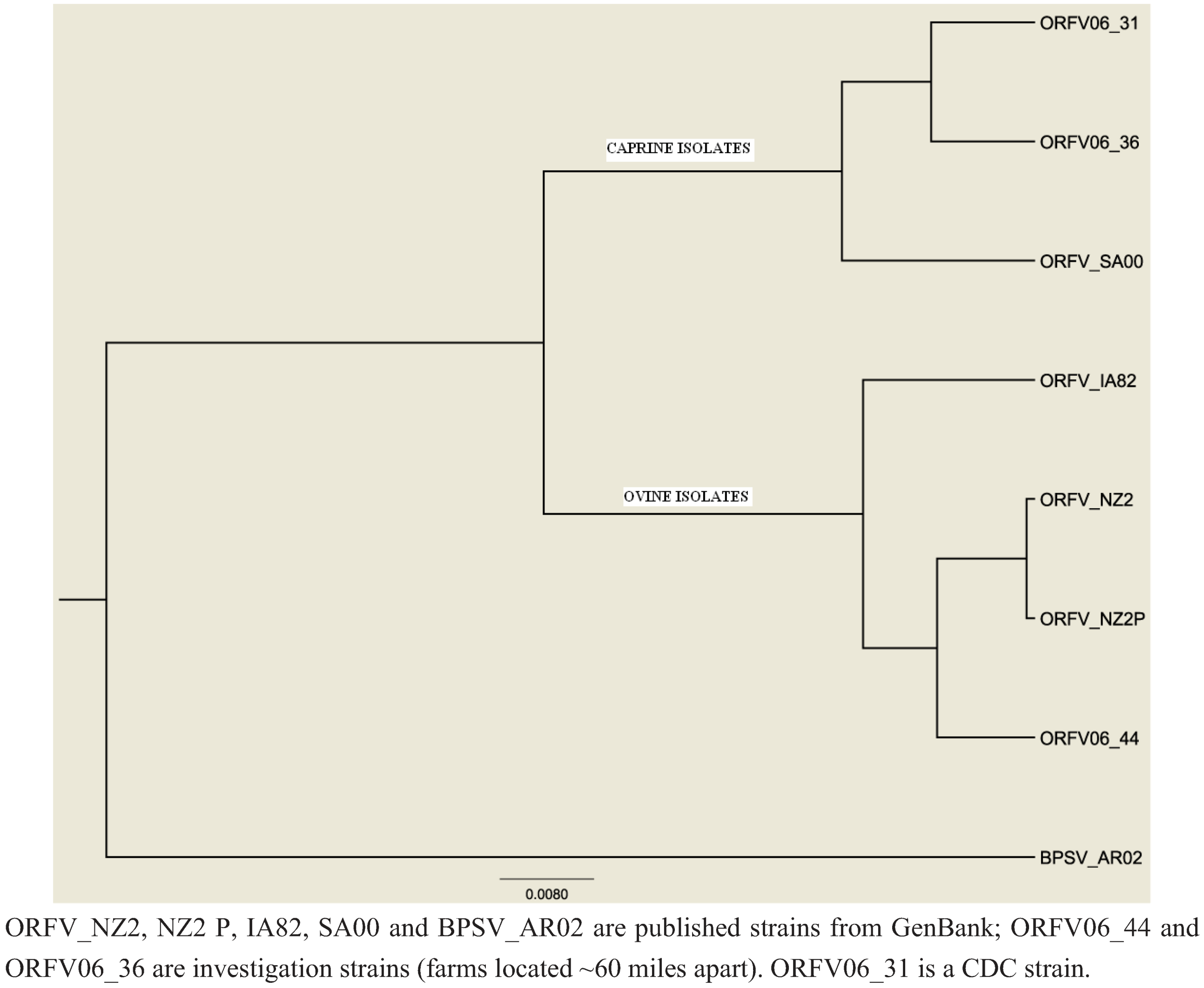
3.4. Laboratory Investigation (Case, Herder and County/State Fair Investigation)
4. Discussion
5. Conclusion
Conflict of Interest
Acknowledgements
References
- Mercer, A.; Fleming, S.; Robinson, A.; Nettleton, P.; Reid, H. Molecular genetic analyses of parapoxviruses pathogenic for humans. Arch. Virol. Suppl. 1997, 13, 25–34. [Google Scholar]
- Leavell, U.W., Jr.; McNamara, M.J.; Muelling, R.; Talbert, W.M.; Rucker, R.C.; Dalton, A.J. Orf: Report of 19 human cases with clinical and pathological observations. J. Am. Med. Assoc. 1968, 203, 657–664. [Google Scholar] [CrossRef]
- Rucker, R.C. Clinical picture of orf in Northern California. Cutis 1977, 20, 109–111. [Google Scholar]
- Carmignani, S.; Hamory, B.H. Orf occurring in Missouri. South. Med. J. 1983, 76, 499–501. [Google Scholar] [CrossRef]
- de Wet, C.; Murie, J. Lamb pays lip service: Two cases of ecthyma contagiosum (orf). Scott. Med. J. 2011, 56. [Google Scholar] [CrossRef]
- Lederman, E.R.; Green, G.M.; DeGroot, H.E.; Dahl, P.; Goldman, E.; Greer, P.W.; Li, Y.; Zhao, H.; Paddock, C.D.; Damon, I.K. Progressive ORF virus infection in a patient with lymphoma: successful treatment using imiquimod. Clin. Infect. Dis. 2007, 44, e100–e103. [Google Scholar] [CrossRef]
- Geernick, K.; Lukito, G.; Snoeck, R.; DeVos, R.; DeClerq, E.; Vanrenterghem, Y.; Degreef, H.; Maes, B. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J. Med. Virol. 2001, 64, 543–549. [Google Scholar] [CrossRef]
- Tan, S.T.; Blake, G.B.; Chambers, S. Recurrent orf in an immunocompromised host. Br. J. Plast. Surg. 1991, 44, 465–467. [Google Scholar] [CrossRef]
- Rogers, M.; Bale, P.; De Silva, L.M.; Glasson, M.J.; Collins, E. Giant parapox infection in a two year old child. Australas. J. Dermatol. 1989, 30, 87–91. [Google Scholar] [CrossRef]
- Gill, M.J.; Arlette, J.; Buchanan, K.A.; Barber, K. Human Orf. Arch. Dermatol. 1990, 126, 356–358. [Google Scholar] [CrossRef]
- Paiba, G.A.; Thomas, D.R.; Morgan, K.L.; Bennett, M.; Salmon, R.L.; Chalmers, R.; Kench, S.M.; Coleman, T.J.; Meadows, D.; Morgan-Capner, P.; Softley, P.; Sillis, M.; Green, L.E. Orf (contagious pustular dermatitis) in farmworkers: Prevalence and risk factors in three areas of England. Vet. Rec. 1999, 145, 7–11. [Google Scholar] [CrossRef]
- Fritz, C.L.; Kjemtrup, A.M.; Conrad, P.A.; Flores, G.R.; Campbell, G.L.; Schriefer, M.E.; Gallo, D.; Vugia, D.J. Seroepidemiology of emerging tickborne infectious diseases in a northern California community. J. Infect. Dis. 1997, 175, 1432–1439. [Google Scholar]
- BioEdit. Available online: http://www.mbio.ncsu.edu/BioEdit/BioEdit.html (accessed on 23 February 2013).
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl. Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention ORF Virus Infection in Humans—New York, Illinois, California, and Tennessee, 2004–2005. MMWR 2006, 55, 65–68.
- Bayindir, Y.; Bayraktar, M.; Karadag, N.; Ozcan, H.; Kayabas, U.; Otlu, B.; Durmaz, R.; Doganay, M. Investigation and analysis of a human orf outbreak among people living on the same farm. New Microbiol. 2011, 34, 37–43. [Google Scholar]
- Lederman, E.R.; Austin, C.; Trevino, I.; Reynolds, M.G.; Swanson, H.; Cherry, B.; Ragsdale, J.; Dunn, J.; Meidl, S.; Zhao, H.; Li, Y.; Pue, H.; Damon, I.K. Orf virus infection in children: Clinical characteristics, transmission, diagnostic methods and future therapeutics. Pediatr. Infect. Dis. J. 2007, 26, 740–744. [Google Scholar] [CrossRef]
- Stewart, A.C. Epidemiology of orf. NZ Med. J. 1983, 96, 100–101. [Google Scholar]
- Centers for Disease Control and Prevention Human Orf Mimicking Cutaneous Anthrax—California. MMWR 1973, 22, 108.
- Centers for Disease Control and Prevention Human Orf Virus Infection from Household Exposures—United States, 2009–2011. MMWR 2012, 61, 245–248.
- CDC Online Resources. Orf Frequently Asked Questions. Available online: http://www.cdc.gov/ncidod/dvrd/orf_virus/ (accessed on 23 February 2013).
- Robinson, A.J.; Petersen, G.V. Orf virus infection of workers in the meat industry. NZ Med. J. 1983, 96, 81–85. [Google Scholar]
- de la Concha-Bermejillo, A.; Gui, J.; Zhang, Z.; Waldron, D. Severe persistent orf in young goats. J. Vet. Diagn. Invest. 2003, 15, 423–431. [Google Scholar] [CrossRef]
- Scagliarini, A.; Piovesana, S.; Turrini, F.; Savini, F.; Sithole, F.; McCrindle, C.M. Orf in South Africa: Endemic but neglected. Onderstepoort. J. Vet. Res. 2012, 79, E1–E8. [Google Scholar]
- Marshall, K. Animal Plant Health Inspection Service. Personal Communication, United States Department of Agriculture: Richmond, VA, USA, 2006. [Google Scholar]
- MacNeil, A.; Lederman, E.; Reynolds, M.G.; Ragade, N.J.; Talken, R.; Friedman, D.; Hall, W.; Shwe, T.; Li, Y.; Zhao, H.; Smith, S.; Davidson, W.; Hughes, C.; Damon, I.K. Diagnosis of bovine-associated parapoxvirus infections in humans: Molecular and epidemiological evidence. Zoonoses Public Health 2010, 57, e161–e164. [Google Scholar] [CrossRef]
- Abrahao, J.S.; Silva-Fernandes, A.T.; Assis, F.L.; Guedes, M.I.; Drumond, B.P.; Leite, J.A.; Coelho, L.F.; Turrini, F.; Fonseca, F.G.; Lobato, Z.I.; Madureira, M.; Ferreira, P.C.; Bonjardim, C.A.; Trindade, G.S.; Kroon, E.G. Human Vaccinia virus and Pseudocowpox virus co-infection: Clinical description and phylogenetic characterization. J. Clin. Virol. 2010, 48, 69–72. [Google Scholar] [CrossRef]
- Tondury, B.; Kuhne, A.; Kutzner, H.; Palmedo, G.; Lautenschlager, S.; Borelli, S. Molecular diagnostics of parapox virus infections. J. Dtsch. Dermatol. Ges. 2010, 8, 681–684. [Google Scholar]
- USDA-APHIS. National Animal Health Monitoring Survey—Sheep 2001. Available online: http://www.aphis.usda.gov/animal_health/nahms/sheep/downloads/sheep01/Sheep01_is_PartII_III_Highlights.pdf (accessed on 23 February 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lederman, E.R.; Tao, M.; Reynolds, M.G.; Li, Y.; Zhao, H.; Smith, S.K.; Sitler, L.; Haberling, D.L.; Davidson, W.; Hutson, C.; et al. An Investigation of a Cluster of Parapoxvirus Cases in Missouri, Feb–May 2006: Epidemiologic, Clinical and Molecular Aspects. Animals 2013, 3, 142-157. https://doi.org/10.3390/ani3010142
Lederman ER, Tao M, Reynolds MG, Li Y, Zhao H, Smith SK, Sitler L, Haberling DL, Davidson W, Hutson C, et al. An Investigation of a Cluster of Parapoxvirus Cases in Missouri, Feb–May 2006: Epidemiologic, Clinical and Molecular Aspects. Animals. 2013; 3(1):142-157. https://doi.org/10.3390/ani3010142
Chicago/Turabian StyleLederman, Edith R., Min Tao, Mary G. Reynolds, Yu Li, Hui Zhao, Scott K. Smith, Lisa Sitler, Dana L. Haberling, Whitni Davidson, Christina Hutson, and et al. 2013. "An Investigation of a Cluster of Parapoxvirus Cases in Missouri, Feb–May 2006: Epidemiologic, Clinical and Molecular Aspects" Animals 3, no. 1: 142-157. https://doi.org/10.3390/ani3010142




