New 19-Residue Peptaibols from Trichoderma Clade Viride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Peptaibol Extraction
2.3. Analytical Procedures
2.4. Nomenclature of the Identified Peptaibols
2.5. Sequence Selection and Force Field Library Generation for Non-Standard Residues
2.6. Molecular Dynamics Simulations of Trikoningin KA V
2.7. Testing the Inhibitory Effects of Peptaibol Extracts to Strains of Bacteria, Yeasts, and Filamentous Fungi
3. Results and Discussion
3.1. Identification, Sequencing, and Quantitation of Peptaibol Compounds Produced by T. gamsii and T. koningiopsis
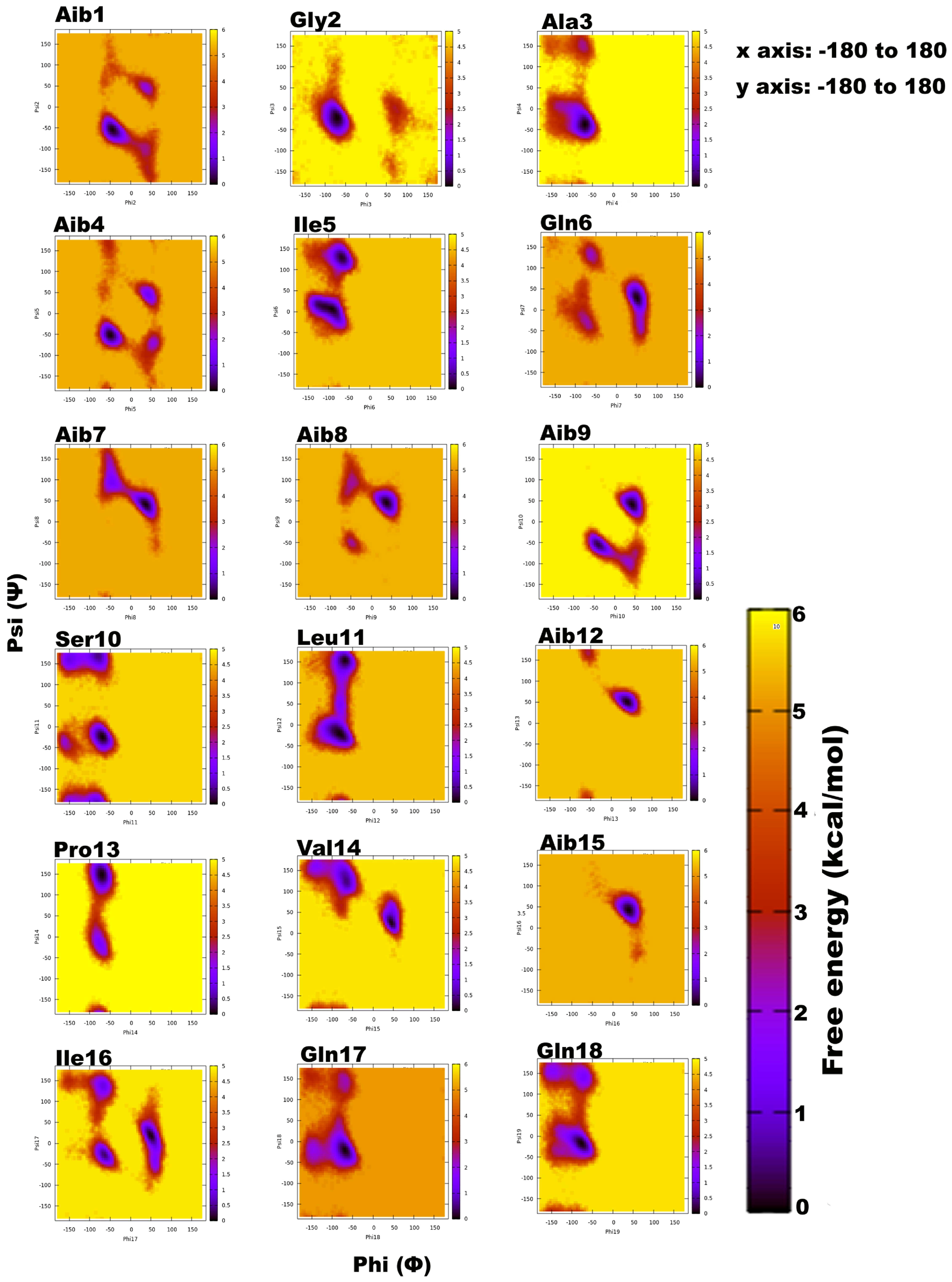
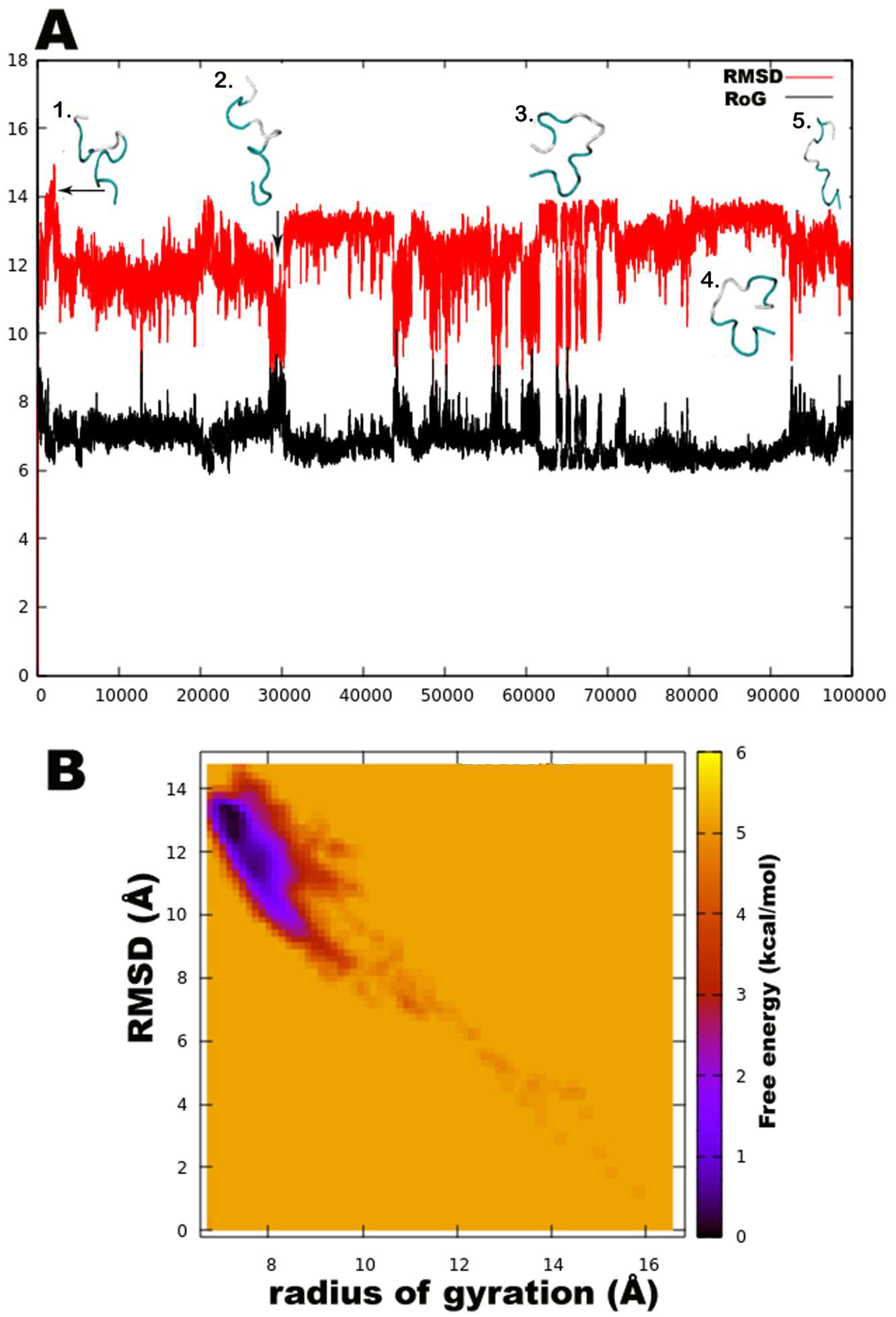
3.2. Structural Elucidation of Trikoningin KA V Based on Short Molecular Dynamics
3.3. Inhibitory Effects of Peptaibol Extracts Towards Bacteria, Yeasts, and Filamentous Fungi
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma names in the year 2015. IMA Fungus 2015, 6, 263–295. [Google Scholar] [CrossRef] [PubMed]
- Bisby, G.R. Trichoderma viride Pers. ex Fries, and notes on Hypocrea. Mycol. Res. 1939, 23, 149–168. [Google Scholar] [CrossRef]
- Rifai, M.A. A revision of the genus Trichoderma. Mycol. Pap. 1969, 116, 1–56. [Google Scholar]
- Bissett, J. A revision of the genus Trichoderma. II. Infrageneric classification. Can. J. Bot. 1991, 69, 2357–2372. [Google Scholar] [CrossRef]
- Kullnig-Gradinger, C.M.; Szakacs, G.; Kubicek, C.P. Phylogeny and evolution of the genus Trichoderma: A multigene approach. Mycol. Res. 2002, 106, 757–767. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Lu, B.S.; Petrini, O.; Schroers, H.J.; Druzhinina, I.S. The Trichoderma koningii aggregate species. Stud. Mycol. 2006, 56, 67–133. [Google Scholar] [CrossRef] [PubMed]
- Lieckfeldt, E.; Samuels, G.J.; Nirenberg, H.I.; Petrini, O. A morphological and molecular perspective of Trichoderma viride: Is it one or two species? Appl. Environ. Microbiol. 1999, 65, 2418–2428. [Google Scholar] [PubMed]
- Jaklitsch, W.M.; Samuels, G.J.; Dodd, S.L.; Lu, B.S.; Druzhinina, I.S. Hypocrea rufa/Trichoderma viride: A reassessment, and description of five closely related species with and without warted conidia. Stud. Mycol. 2006, 56, 135–177. [Google Scholar] [CrossRef] [PubMed]
- Kredics, L.; Hatvani, L.; Naeimi, S.; Körmöczi, P.; Manczinger, L.; Vágvölgyi, C.; Druzhinina, I. Biodiversity of the genus Hypocrea/Trichoderma in different habitats. In Biotechnology and biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: New York, NY, USA, 2014; pp. 3–24. ISBN 978-0-444-59576-8. [Google Scholar]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Degenkolb, T.; Brückner, H. Peptaibiomics: Towards a myriad of bioactive peptides containing C(alpha)-dialkylamino acids? Chem. Biodivers. 2008, 5, 1817–1843. [Google Scholar] [CrossRef] [PubMed]
- Stoppacher, N.; Neumann, N.K.N.; Burgstaller, L.; Zeilinger, S.; Degenkolb, T.; Brückner, H.; Schuhmacher, R. The comprehensive peptaibiotics database. Chem. Biodivers. 2013, 10, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Marahiel, M.A. Protein templates for the biosynthesis of peptide antibiotics. Chem. Biol. 1997, 4, 561–567. [Google Scholar] [CrossRef]
- Bushley, K.E.; Turgeon, B.G. Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol. Biol. 2010, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Duclohier, H. Helical kink and channel behaviour: A comparative study with the peptaibols alamethicin, trichotoxin and antiamoebin. Eur. Biophys. J. 2004, 33, 169–174. [Google Scholar] [CrossRef] [PubMed]
- McMullin, D.R.; Renaud, J.B.; Barasubiye, T.; Sumarah, M.W.; Miller, J.D. Metabolites of Trichoderma species isolated from damp building materials. Can. J. Microbiol. 2017, 63, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, H.L.; Chen, L.; Chen, A.J.; Lan, J.; Chen, X.D.; Zhang, H.W.; Chen, H.; Liu, X.Z.; Zou, Z.M. Cytochalasans with different amino-acid origin from the plant endophytic fungus Trichoderma gamsii. J. Antibiot. 2012, 65, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Chen, L.; Chen, A.; Tian, X.; Chen, X.; Zhang, H.; Chen, H.; Liu, X.Z.; Zhang, Y.; Zou, Z.M. Trichalasins C and D from the plant endophytic fungus Trichoderma gamsii. Fitoterapia 2012, 83, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, H.; Li, L.; Song, B.; Chen, H.; Zhang, H.; Liu, X.; Zou, Z. Trichodermone, a spiro-cytochalasan with a tetracyclic nucleus (7/5/6/5) skeleton from the plant endophytic fungus Trichoderma gamsii. J. Nat. Prod. 2014, 77, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.T.; Song, B.; Zhang, H.W.; Ding, G.; Liu, X.Z.; Gu, Y.C.; Zou, Z.M. Stereochemical determination of new cytochalasans from the plant endophytic fungus Trichoderma gamsii. Fitoterapia 2014, 96, 115–122. [Google Scholar] [CrossRef]
- Ding, G.; Chen, L.; Zhou, C.; Jia, H.M.; Liu, Y.T.; Chang, X.; Song, B.; Liu, X.Z.; Gu, Y.C.; Zou, Z.M. Trichoderamides A and B, a pair of stereoisomers from the plant endophytic fungus Trichoderma gamsii. J. Antibiot. 2015, 68, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Niu, S.B.; Li, L.; Ding, G.; Yu, M.; Zhang, G.S.; Wang, M.H.; Li, L.Y.; Zhang, T.; Jia, H.M.; et al. Trichoderpyrone, a unique polyketide hybrid with a cyclopentenone-pyrone skeleton from the plant endophytic fungus Trichoderma gamsii. J. Nat. Prod. 2017, 80, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Sun, S.Z.; Miao, C.P.; Wu, K.; Chen, Y.W.; Xu, L.H.; Guan, H.L.; Zhao, L.X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2016, 40, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, E.; Marik, T.; Mikkola, L.; Kredics, L.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Indoor Trichoderma strains emitting peptaibols in guttation droplets. J. Appl. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vanquelef, E.; Simon, S.; Marquant, G.; Garcia, E.; Klimerak, G.; Delepine, J.C.; Cieplak, P.; Dupradeau, F.Y. RED Server: A web service for deriving RESP and ESP charges and building force field libraries for new molecules and molecular fragments. Nucleic Acids Res. 2011, 39, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Dupradeau, F.Y.; Pigache, A.; Zaffran, T.; Savineau, C.; Lelong, R.; Grivel, N.; Lelong, D.; Rosanski, W.; Cieplak, P. The REd. Tools: Advances in RESP and ESP charge derivation and force field library building. Phys. Chem. Chem. Phys. 2010, 12, 7821–7839. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, P.; Cornell, W.D.; Bayly, C.; Kollman, P.A. Application of the multimolecule and multiconformational RESP methodology to biopolymers: Charge derivation for DNA, RNA, and proteins. J. Comput. Chem. 1995, 16, 1357–1377. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. Amber 2018 Reference Manual; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham III, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory. Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Marik, T.; Szekeres, A.; Várszegi, C.; Czifra, D.; Vágvölgyi, C.; Kredics, L. Rapid bioactivity-based pre-screening method for the detection of peptaibiotic-producing Trichoderma strains. Acta Biol. Szeged. 2013, 57, 1–7. [Google Scholar]
- Marik, T.; Várszegi, C.; Kredics, L.; Vágvölgyi, C.; Szekeres, A. Mass spectrometric investigation of alamethicin. Acta Biol. Szeged. 2013, 57, 109–112. [Google Scholar]
- Jaworski, A.; Kirschbaum, J.; Brückner, H. Structures of trichovirins II, peptaibol antibiotics from the mold Trichoderma viride NRRL 5243. J. Pept. Sci. 1999, 5, 341–351. [Google Scholar] [CrossRef]
- Kirschbaum, J.; Krause, C.; Winzheimer, R.K.; Brückner, H. Sequences of alamethicins F30 and F50 reconsidered and reconciled. J. Pept. Sci. 2003, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Kirschbaum, J.; Brückner, H. Peptaibiomics: An advanced, rapid and selective analysis of peptaibiotics/peptaibols by SPE/LC-ES-MS. Amino Acids 2006, 30, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Röhrich, C.R.; Iversen, A.; Jaklitsch, W.M.; Voglmayr, H.; Vilcinskas, A.; Nielsen, K.F.; Thrane, U.; von Döhren, H.; Brückner, H.; Degenkolb, T. Screening the biosphere: The fungicolous fungus Trichoderma phellinicola, a prolific source of hypophellins, new 17-, 18-, 19-, and 20-residue peptaibiotics. Chem. Biodivers. 2013, 10, 787–812. [Google Scholar] [CrossRef] [PubMed]
- Goulard, C.; Hlimi, S.; Rebuffat, S.; Bodo, B. Trichorzins HA and MA, antibiotic peptides from Trichoderma harzianum. J. Antibiot. 1995, 48, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Auvin-Guette, C.; Rebuffat, S.; Vuidepot, I.; Massias, M.; Bodo, B. Structural elucidation of trikoningins KA and KB, peptaibols from Trichoderma koningii. J. Chem. Soc. Perkin Trans. I 1993, 249–255. [Google Scholar] [CrossRef]
- Degenkolb, T.; Gräfenhan, T.; Berg, A.; Nirenberg, H.I.; Gams, W.; Brückner, H. Peptaibiomics: Screening for polypeptide antibiotics (peptaibiotics) from plant-protective Trichoderma species. Chem. Biodivers. 2006, 3, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Gräfenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Ismaiel, A.; Brückner, H.; von Döhren, H.; et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptaibiotics, and mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef]
- Neuhof, T.; Dieckmann, R.; Druzhinina, I.S.; Kubicek, C.P.; von Döhren, H. Intact-cell MALDI-TOF mass spectrometry analysis of peptaibol formation by the genus Trichoderma/Hypocrea: Can molecular phylogeny of species predict peptaibol structures? Microbiology 2007, 153, 3417–3437. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, S.; Prigent, Y.; Auvin-Guette, C.; Bodo, B. Tricholongins BI and BII, 19-residue peptaibols from Trichoderma longibrachiatum. FEBS J. 1991, 201, 661–674. [Google Scholar] [CrossRef]
- Hajji, M.E.; Rebuffat, S.; Lecommandeur, D.; Bodo, B. Isolation and sequence determination of trichorzianines A antifungal peptides from Trichoderma harzianum. Int. J. Pept. Protein Res. 1987, 29, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Pócsfalvi, G.; Scala, F.; Lorito, M.; Ritieni, A.; Randazzo, G.; Ferranti, P.; Vékey, K.; Malorni, A. Microheterogeneity characterization of a trichorzianine-A mixture from Trichoderma harzianum. J. Mass Spectrom. 1998, 33, 154–163. [Google Scholar] [CrossRef]
- Rebuffat, S.; Hajji, M.; Hennig, P.; Davoust, D.; Bodo, B. Isolation, sequence, and conformation of seven trichorzianines from Trichoderma harzianum. Chem. Biol. Drug Des. 1989, 34, 200–210. [Google Scholar] [CrossRef]
- Röhrich, C.R.; Jaklitsch, W.M.; Voglmayr, H.; Iversen, A.; Vilcinskas, A.; Nielsen, K.F.; Thrane, U.; von Döhren, H.; Brückner, H.; Degenkolb, T. Front line defenders of the ecological niche! Screening the structural diversity of peptaibiotics from saprotrophic and fungicolous Trichoderma/Hypocrea species. Fungal Divers. 2014, 69, 117–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röhrich, C.R.; Iversen, A.; Jaklitsch, W.M.; Voglmayr, H.; Berg, A.; Dörfelt, H.; Thrane, U.; Vilcinskas, A.; Nielsen, K.F.; Von Döhren, H.; et al. Hypopulvins, novel peptaibiotics from the polyporicolous fungus Hypocrea pulvinata, are produced during infection of its natural hosts. Fungal Boil. 2012, 116, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Koide, N.; Asami, K.; Fujita, T. Ion-channels formed by hypelcins, antibiotic peptides, in planar bilayer lipid membranes. Biochim. Biophys. Acta 1997, 1326, 47–53. [Google Scholar] [CrossRef]
- Duclohier, H.; Molle, G.; Spach, G. The influence of the trichorzianin C-terminal residues on the ion channel conductance in lipid bilayers. Biochim. Biophys. Acta 1989, 987, 133–136. [Google Scholar] [CrossRef]
- Molle, G.; Duclohier, H.; Julien, S.; Spach, G. Synthetic analogues of alamethicin: Effect of C-terminal residue substitutions and chain length on the ion channel lifetimes. Biochim. Biophys. Acta 1991, 1064, 365–369. [Google Scholar] [CrossRef]
- Condamine, E.; Rebuffat, S.; Prigent, Y.; Ségalas, I.; Bodo, B.; Davoust, D. Three-dimensional structure of the ion-chanel forming peptide trichorzianin TA VII bound to sodium dodecyl sulfate micelles. Biopolymers 1998, 46, 75–88. [Google Scholar] [CrossRef]
- Van Bohemen, A.-I.; Zalouk-Vergnoux, A.; Poirier, L.; Phuong, N.N.; Inguimbert, N.; Salah, K.B.H.; Ruiz, N.; Pouchus, Y.F. Development and validation of LC–MS methods for peptaibol quantification in fungal extracts according to their lengths. J. Chromatogr. B Biomed. Sci. Appl. 2016, 1009–1010, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, S.; Goulard, C.; Bodo, B.; Roquebert, M.-F. The peptaibol antibiotics from Trichoderma soil fungi; structural diversity and membrane properties. Recent Res. Devel. Org. Biorg. Chem. 1999, 3, 65–91. [Google Scholar]
- Aravinda, S.; Shamala, N.; Balaram, P. Aib residues in peptaibiotics and synthetic sequences: Analysis of nonhelical conformations. Chem. Biodivers. 2008, 5, 1238–1262. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, R.; Balaram, P. The use of D-amino acids in peptide design. In D-Amino Acids: A New Frontier in Amino Acid and Protein Research—Practical Methods and Protocols; Konno, R., Brückner, H., D’Aniello, A., Fisher, G.H., Fujii, N., Homma, H., Eds.; Nova Science Publishers: New York, NY, USA, 2007; pp. 415–430. [Google Scholar]
- Wałęsa, R.; Broda, M.A. The influence of solvent on conformational properties of peptides with Aib residue—a DFT study. J. Mol. Model. 2017, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer-Stenner, R.; Gonzales, W.; Bourne, G.T.; Feng, J.A.; Marshall, G.R. Conformational manifold of α-aminoisobutyric acid (Aib) containing alanine-based tripeptides in aqueous solution explored by vibrational spectroscopy, electronic circular dichroism spectroscopy, and molecular dynamics simulations. J. Am. Chem. Soc. 2007, 129, 13095–13109. [Google Scholar] [CrossRef] [PubMed]
- Brückner, H.; Graf, H. Paracelsin, a peptide antibiotic containing α-aminoisobutyric acid, isolated from Trichoderma ressei Simmons Part A. Experientia 1983, 39, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Jen, W.C.; Jones, G.A.; Brewer, D.; Parkinson, V.O.; Taylor, A. The antibacterial activity of alamethicins and zervamicins. J. Appl. Microbiol. 1987, 63, 293–298. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; D’Ambrosio, M.; Harman, G.E.; Hayes, C.K.; Kubicek, C.P.; Scala, F. Synergistic interaction between cell wall degrading enzymes and membrane affecting compounds. Mol. Plant Microbe Interact. 1996, 9, 206–213. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Lorito, M.; Farkas, V.; Rebuffat, S.; Bodo, B.; Kubicek, C.P. Cell wall synthesis is a major target of mycoparasitic antagonism by Trichoderma harzianum. J. Bacteriol. 1996, 178, 6382–6385. [Google Scholar] [CrossRef] [PubMed]
- Cutler, H.G.; Himmelsbach, D.S.; Yagen, B.; Arrendale, R.F.; Jacyno, J.M.; Cole, P.D.; Cox, R.H. Koninginin B: A biologically active congener of koninginin A from Trichoderma koningii. J. Agric. Food Chem. 1991, 39, 977–980. [Google Scholar] [CrossRef]
- Song, X.Y.; Shen, Q.T.; Xie, S.T.; Chen, X.L.; Sun, C.Y.; Zhang, Y.Z. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiol. Lett. 2006, 260, 119–125. [Google Scholar] [CrossRef]
- Marik, T.; Urbán, P.; Tyagi, C.; Szekeres, A.; Leitgeb, B.; Vágvölgyi, M.; Manczinger, L.; Druzhinina, I.S.; Kredics, L. Diversity profile and dynamics of peptaibols produced by green mould Trichoderma species in interactions with their hosts Agaricus bisporus and Pleurotus ostreatus. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
| Peptide | M | [M+Na]+ | [M+2Na]2+ | b12 | y7 | rt-GK (min) | Area size | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | R13 | R14 | R15 | R16 | R17 | R18 | R19 | Compound identical or positionally isomeric with | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pept-Ia | 1861.1 | 1884.1 | 953.55 | 1108.5 | 754.4 | 50.504 | 0.28% | AcAib | Gly | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: Trikoningin KA V: [Ile]5→[Vxx]5 and [Ile]16→[Vxx]16 | [39,40] |
| New: Tricholongin LBII, LBIV: [Phe]3→[Ala]3 and [Aib]5→[Vxx]5 | [41] | |||||||||||||||||||||||||||
| New: Tricholongin BII (LBII): [Phe]3→[Ala]3 and [Aib]5→[Vxx]5 | [44] | |||||||||||||||||||||||||||
| Pept-Ib | 1876.2 | 1899.2 | 961.1 | 1108.5 | 768.4 | 51.073 | 1.51% | AcAib | Gly | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Trikoningin KA V: [Ile]5→[Vxx]5 (Positional isomer of Pept-IIb; | [39,40] |
| Pept-IIIb; Pept-IVb) | ||||||||||||||||||||||||||||
| Pept-IIa | 1876.1 | 1899.1 | 961.05 | 1122.5 | 754.4 | 51.175 | 1.66% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: Trikoningin KA V: [Ile]16→[Vxx]16 (Positional isomer of Pept-IIIa; | [39,40] |
| Pept-IVa; Pept-Va; Pept-VIa; Pept-VIIIa) | ||||||||||||||||||||||||||||
| New: Tricholongin LBII, LBIV: [Phe]3→[Ala]3 and [Aib]5→[Lxx]5 | [41] | |||||||||||||||||||||||||||
| New: Tricholongin BII: [Phe]3→[Ala]3 and [Aib]5→[Lxx]5 | [44] | |||||||||||||||||||||||||||
| New: Trichostrigocin TSG-A, TSG-B: [Ala]2→[Gly]2 and [Aib]3→[Ala]3 | [41] | |||||||||||||||||||||||||||
| Pept-IIb | 1875.2 | 1898.2 | 960.6 | 1108.5 | 768.4 | 52.316 | 1.99% | AcAib | Gly | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-Ib; Pept-IIIb; Pept-IVb) | → Pept-Ib |
| Pept-IIIa | 1876.2 | 1899.2 | 961.1 | 1122.5 | 754.4 | 53.005 | 0.56% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-IIa; Pept-IVa; Pept-Va; Pept-VIa; Pept-VIIIa) | → Pept-IIa |
| Pept-IIIb | 1875.3 | 1898.3 | 960.65 | 1108.5 | 768.4 | 53.292 | 2.96% | AcAib | Gly | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-Ib; Pept-IIb; Pept-IVb) | → Pept-Ib |
| Pept-IVa | 1875.2 | 1898.2 | 960.6 | 1122.5 | 754.4 | 53.555 | 3.70% | AcAib | Gly | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-IIa; Pept-IIIa; Pept-Va; Pept-VIa; Pept-VIIIa) | → Pept-IIa |
| Pept-IVb | 1875.2 | 1898.2 | 960.6 | 1108.5 | 768.4 | 53.996 | 9.21% | AcAib | Gly | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-Ib; Pept-IIb; Pept-IIIb) | → Pept-Ib |
| Pept-Va | 1876.2 | 1899.2 | 961.1 | 1122.5 | 754.4 | 54.776 | 4.49% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-IIa; Pept-IIIa; Pept-IVa; Pept-VIa; Pept-VIIIa) | → Pept-IIa |
| Pept-Vb | 1890.2 | 1913.2 | 968.1 | 1122.5 | 768.4 | 55.313 | 5.41% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | Trikoningin KA V (Positional isomer of Pept-VIb; Pept-VII; Pept-VIIIb) | [39,40] |
| Pept-VIa | 1876.2 | 1899.2 | 961.1 | 1122.5 | 754.4 | 55.617 | 0.84% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-IIa; Pept-IIIa; Pept-IVa; Pept-Va; Pept-VIIIa) | → Pept-IIa |
| Pept-VIb | 1890.2 | 1913.2 | 968.1 | 1122.5 | 768.4 | 55.798 | 23.32% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | (Positional isomer of Pept-Vb; Pept-VII; Pept-VIIIb) | → Pept-Vb |
| Pept-VII | 1889.3 | 1912.3 | 967.65 | 1122.5 | 768.4 | 56.403 | 4.16% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | (Positional isomer of Pept-Vb; Pept-VIb; Pept-VIIIb) | → Pept-Vb |
| Pept-VIIIa | 1874.2 | 1897.2 | 960.1 | 1122.5 | 754.4 | 56.806 | 0.49% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-IIa; Pept-IIIa; Pept-IVa; Pept-Va; Pept-VIa) | → Pept-IIa |
| Pept-VIIIb | 1890.2 | 1913.2 | 968.1 | 1122.5 | 768.4 | 56.826 | 27.99% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | (Positional isomer of Pept-Vb; Pept-VIb; Pept-VII) | → Pept-Vb |
| Pept-IX | 1874.5 | 1897.5 | 960.25 | 1106.5 | 768.4 | 57.554 | 5.92% | AcAib | Gly | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ala | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Tricholongin LBIII: [Phe]3→[Ala]3, [Aib]5→[Lxx]5 and [Lxx]16→[Vxx]16 | [41] |
| Pept-X | 1904.2 | 1927.2 | 975.1 | 1136.4 | AcAib | Ala | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Trikoningin KA V: Gly]2→[Ala]2 (Positional isomer of Pept-XI; Pept-XII) | [39,40] | |||
| New: Trichostrigocin TSG-A, TSG-B: [Aib]3→[Ala]3 and [Vxx]16→[Lxx]16 | [41] | |||||||||||||||||||||||||||
| New: Trichorzianin TA IIIb, TA IIIb: [Aib]5→[Lxx]5 and [Trpol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TA VIb: [Aib]5→[Lxx]5 and [Pheol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TA IVb: [Iva]5→[Lxx]5 and [Trpol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TA VII: [Iva]5→[Lxx]5 and [Pheol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TAP 14a: [Aib]5→[Lxx]5 and [Trpol]19→[Lxxol]19 | [46] | |||||||||||||||||||||||||||
| New: Trichorzianin TB IIIc: [Aib]5→[Lxx]5 and [Trpol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| New: Trichorzianin TB IVb: [Iva]5→[Lxx]5 and [Trpol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| New: Trichorzianin TB VIb: [Aib]5→[Lxx]5 and [Pheol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| New: Trichorzianin TB VII: [Iva]5→[Lxx]5 and [Pheol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| Pept-XI | 1903.3 | 1926.3 | 974.65 | 1136.5 | 768.4 | 58.602 | 4.82% | AcAib | Ala | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-X; Pept-XII) | → Pept-X |
| Pept-XII | 1904.2 | 1927.2 | 975.1 | 1136.6 | 768.4 | 59.264 | 0.69% | AcAib | Ala | Ala | Aib | Lxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: (Positional isomer of Pept-X; Pept-X) | → Pept-X |
| Peptide | M | [M+Na]+ | [M+2Na]2+ | b12 | y7 | rt-GK (min) | Area size | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | R13 | R14 | R15 | R16 | R17 | R18 | R19 | Compound identical or positionally isomeric with | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Koningiopsin Ia | 1875.5 | 1898.5 | 960.75 | 1121.9 | 754.5 | 53.121 | 0.91% | AcAib | Ala | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: Trichorzianin TAP-14b: [Pheol]19→[Lxxol]19 | [46] |
| Koningiopsin Ib | 1889.5 | 1912.5 | 967.75 | 1121.5 | 768.5 | 54.267 | 5.96% | AcAib | Ala | Ala | Aib | Vxx | Gln | Aib | Aib | Aib | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Trichorzianin TA IVb: [Trpol]19→[Lxxol]19 | [45] |
| New: Trichorzianin TA VII: [Pheol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| Koningiopsin IIa | 1890.5 | 1913.5 | 968.25 | 1136.7 | 754.5 | 54.69 | 8.13% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Lxx | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: Trichorzianin TA IVb: [Aib]9→[Lxx]9 and [Trpol]19→[Lxxol]19 | [45] |
| New: Trichostrigocin TSG-A, TSG-B:[Aib]3→[Ala]3 and [Aib]9→[Lxx]9 | [41] | |||||||||||||||||||||||||||
| Koningiopsin IIb | 1889.6 | 1912.6 | 967.8 | 1120.8 | 768.6 | 55.513 | 2.88% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Aib | Vxx | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Trichorzianin TA IIIb, TA IIIc: [Ser]10→[Vxx]10 and | [45] |
| [Trpol]19→[Lxxol]19 | ||||||||||||||||||||||||||||
| New: Trichorzianin TA VIb: [Ser]10→[Vxx]10 and [Pheol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TAP-14a: [Ser]10→[Vxx]10 and [Pheol]19→[Lxxol]19 | [46] | |||||||||||||||||||||||||||
| Koningiopsin IIIa | 1891.6 | 1914.6 | 968.8 | 1122.7 | 755.6 | 55.593 | 0.97% | AcAib | Ala | Ala | Aib | Ala | Gln | Aib | Aib | Lxx | Ser | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Glu | Lxxol | New: Trichorzianin TAP-14b: [Aib]5→[Ala]5, [Aib]9→[Lxx]9, | [46] |
| [Gln]18→[Glu]18 and [Pheol]19→[Lxxol]19 | ||||||||||||||||||||||||||||
| New: Trichorzianin TB IIIc, TB IVb: [Aib]5→[Ala]5, [Aib]9→[Lxx]9, [Ile]16→[Vxx]16 and [Trpol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| New: Trichorzianin TB VIb, TB VII: [Aib]5→[Ala]5, [Aib]9→[Lxx]9, [Ile]16→[Vxx]16 and [Trpol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| New: Trichostrigocin TSG-A, TSG-B: [Aib]3→[Ala]3, [Ala]5→[Lxx]5, [Aib]9→[Lxx]9 and [Gln]18→[Glu]18 | [41] | |||||||||||||||||||||||||||
| Koningiopsin IIIb | 1873.6 | 1896.6 | 959.8 | 1120.8 | 754.5 | 55.936 | 2.03% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Aib | Vxx | Lxx | Aib | Pro | Vxx | Aib | Vxx | Gln | Gln | Lxxol | New: Trichorzianin TAP-14b: [Ser]10→[Vxx]10 and [Pheol]19→[Lxxol]19 | [46] |
| Koningiopsin IV | 1903.6 | 1926.6 | 974.8 | 1136.7 | 768.6 | 56.317 | 12.27% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Lxx | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Trichorzianin TA IIIb, TA IIIc: [Aib]9→[Lxx]9 and | [45] |
| [Trpol]19→[Lxxol]19 (Positional isomer of Koningiopsin Va) | ||||||||||||||||||||||||||||
| New: Trichorzianin TA VIb: [Aib]9→[Lxx]9 and [Pheol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TAP-14a: [Aib]9→[Lxx]9 and [Pheol]19→[Lxxol]19 | [46] | |||||||||||||||||||||||||||
| Koningiopsin Va | 1903.6 | 1926.6 | 974.8 | 1136.8 | 768.6 | 56.739 | 47.94% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Lxx | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: (Positional isomer of Koningiopsin IV) | →Koningiopsin IV |
| Koningiopsin Vb | 1905.6 | 1928.6 | 975.8 | 1136.8 | 769.6 | 57.463 | 5.95% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Lxx | Ser | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Glu | Lxxol | New: Trichorzianin TB IIIc: [Aib]9→[Lxx]9, and [Trpol]19→[Lxxol]19 | [47] |
| New: Trichorzianin TBVIb: [Aib]9→[Lxx]9, and [Pheol]19→[Lxxol]19 | [47] | |||||||||||||||||||||||||||
| Koningiopsin VIa | 1888.6 | 1911.6 | 967.3 | 1120.7 | 768.6 | 57.825 | 11.54% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Lxx | Ala | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Gln | Lxxol | New: Trichorzianin TA IIIb, TA IIIc: [Aib]9→[Lxx]9, [Ser]10→[Ala]10 | [45] |
| and [Trpol]19→[Lxxol]19 | ||||||||||||||||||||||||||||
| New: Trichorzianin TA VIb: [Aib]9→[Lxx]9, [Ser]10→[Ala]10 and [Pheol]19→[Lxxol]19 | [45] | |||||||||||||||||||||||||||
| New: Trichorzianin TAP-14a: [Aib]9→[Lxx]9, [Ser]10→[Ala]10 and [Pheol]19→[Lxxol]19 | [46] | |||||||||||||||||||||||||||
| Koningiopsin VIb | 1888.7 | 1911.7 | 967.35 | 1120.9 | 769.5 | 58.448 | 1.41% | AcAib | Ala | Ala | Aib | Aib | Gln | Aib | Aib | Lxx | Ala | Lxx | Aib | Pro | Vxx | Aib | Lxx | Gln | Glu | Lxxol | New: Trichorzianin TB IIIc: [Aib]9→[Lxx]9, [Ser]10→[Ala]10 and | [47] |
| [Trpol]19→[Lxxol]19 | ||||||||||||||||||||||||||||
| New: Trichorzianin TB VIb: [Aib]9→[Lxx]9, [Ser]10→[Ala]10 and [Pheol]19→[Lxxol]19 | [47] |
| Acceptor | Donor | Fraction | Average Distance |
|---|---|---|---|
| Gly_2 | Aib_7 | 0.2038 | 2.8926 |
| Gln_6 | Aib_9 | 0.1888 | 2.8999 |
| Ala_3 | Gln_6 | 0.175 | 2.8978 |
| Pro_13 | Ile_16 | 0.1729 | 2.8903 |
| Ile_16 | Leu_19 | 0.1611 | 2.8976 |
| Aib_8 | Leu_11 | 0.1475 | 2.8926 |
| Aib_7 | Ser_10 | 0.1211 | 2.8964 |
| Val_14 | Gln_17 | 0.0917 | 2.9024 |
| Ile_5 | Aib_8 | 0.0727 | 2.912 |
| Aib_15 | Gln_17 | 0.0642 | 2.8104 |
| Aib_15 | Gln_18 | 0.0546 | 2.9034 |
| Ile_16 | Gly_2 | 0.0457 | 2.8761 |
| Aib_15 | Gln_6 | 0.0418 | 2.8816 |
| Ser_10 | Aib_12 | 0.0393 | 2.846 |
| Ile_5 | Aib_7 | 0.0352 | 2.8111 |
| Gly_2 | Ile_5 | 0.0344 | 2.9118 |
| Gln_18 | Val_14 | 0.0316 | 2.851 |
| Gln_17 | Gly_2 | 0.0303 | 2.878 |
| AIB_8 | AIB_12 | 0.0303 | 2.9031 |
| Tested Microbial Strain | Sensitivity to T. gamsii SZMC 1656 Extract | Sensitivity to T. koningiopsis SZMC 12500 Extract |
|---|---|---|
| Micrococcus luteus SZMC 0264 | +++++ | ++++ |
| Staphylococcus aureus SZMC 0579 | +++++ | ++++ |
| Escherichia coli SZMC 0582 | + | − |
| Pseudomonas aeruginosa SZMC 0568 | + | ++ |
| Candida boidinii SZMC 0673 | − | − |
| Kluyveromyces lactis SZMC 0683 | − | − |
| Saccharomyces cerevisiae SZMC 0425 | − | − |
| Schizosaccharomyces pombe SZMC 0142 | − | − |
| Tested Filamentous Fungal Strain | Sensitivity to T. gamsii SZMC 1656 Extract | Sensitivity to T. koningiopsis SZMC 12500 Extract |
|---|---|---|
| Alternaria alternata SZMC 16085 | +++++ * | +++ * |
| Fusarium solani species complex SZMC 11467 | ++ | + |
| Rhizoctonia solani SZMC 6252J | ++ * | +++ * |
| Phoma cucurbitacearum SZMC 16088 | ++ * | ++++ * |
| T. aggressivum f. europaeum SZMC 1811 | ++ | + |
| T. pleuroti SZMC 12454 | ++ | + |
| T. gamsii SZMC 1656 | +++ | +++ |
| T. koningiopsis SZMC 12500 | +++ | +++ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marik, T.; Tyagi, C.; Racić, G.; Rakk, D.; Szekeres, A.; Vágvölgyi, C.; Kredics, L. New 19-Residue Peptaibols from Trichoderma Clade Viride. Microorganisms 2018, 6, 85. https://doi.org/10.3390/microorganisms6030085
Marik T, Tyagi C, Racić G, Rakk D, Szekeres A, Vágvölgyi C, Kredics L. New 19-Residue Peptaibols from Trichoderma Clade Viride. Microorganisms. 2018; 6(3):85. https://doi.org/10.3390/microorganisms6030085
Chicago/Turabian StyleMarik, Tamás, Chetna Tyagi, Gordana Racić, Dávid Rakk, András Szekeres, Csaba Vágvölgyi, and László Kredics. 2018. "New 19-Residue Peptaibols from Trichoderma Clade Viride" Microorganisms 6, no. 3: 85. https://doi.org/10.3390/microorganisms6030085





