Diversity of Myxobacteria—We Only See the Tip of the Iceberg
Abstract
:1. Introduction
2. Biology and Phylogeny of Myxobacteria
3. Current Status of Antibiotics and Myxobacterial Secondary Metabolites
4. The Great Plate Count Anomaly and Microbial Biogeography
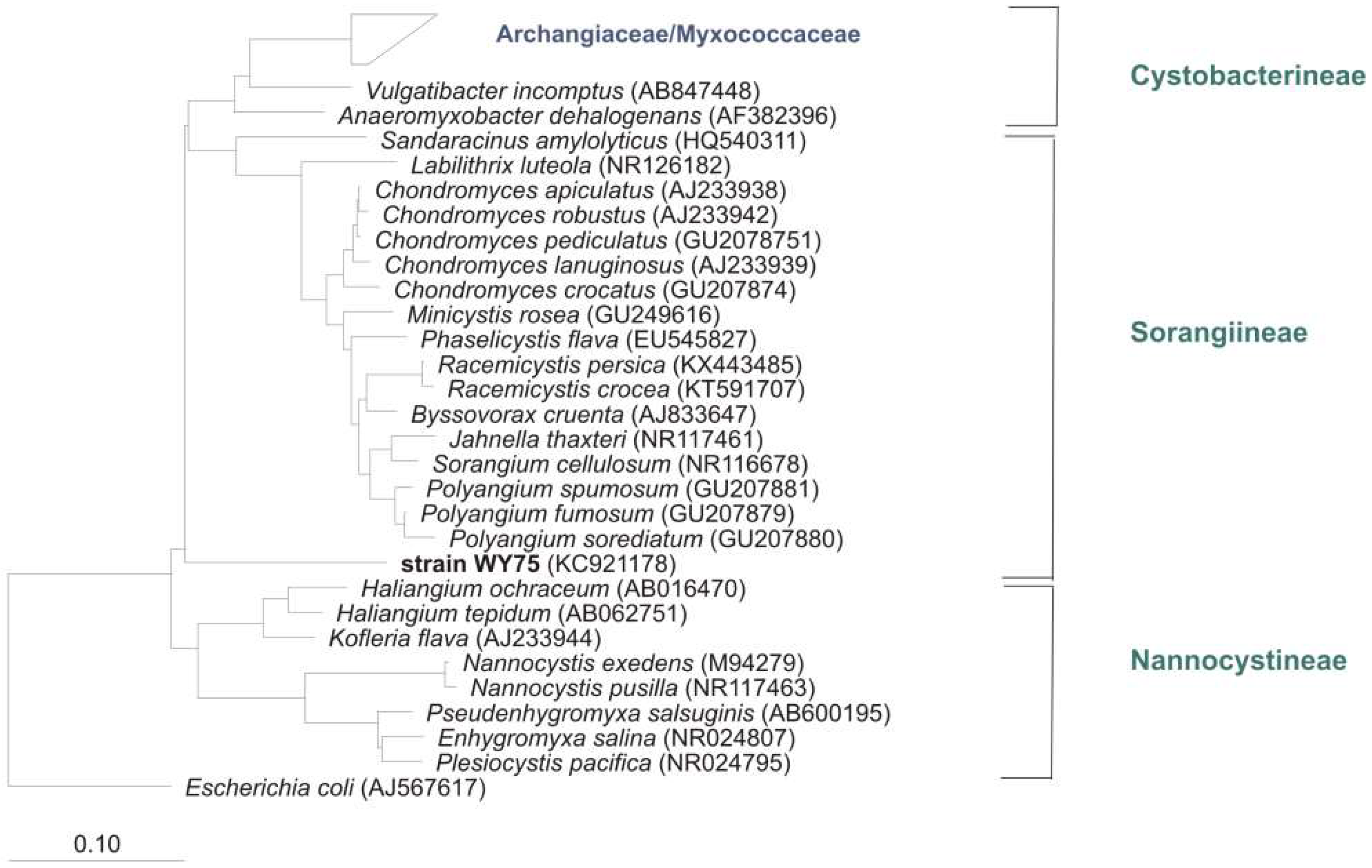
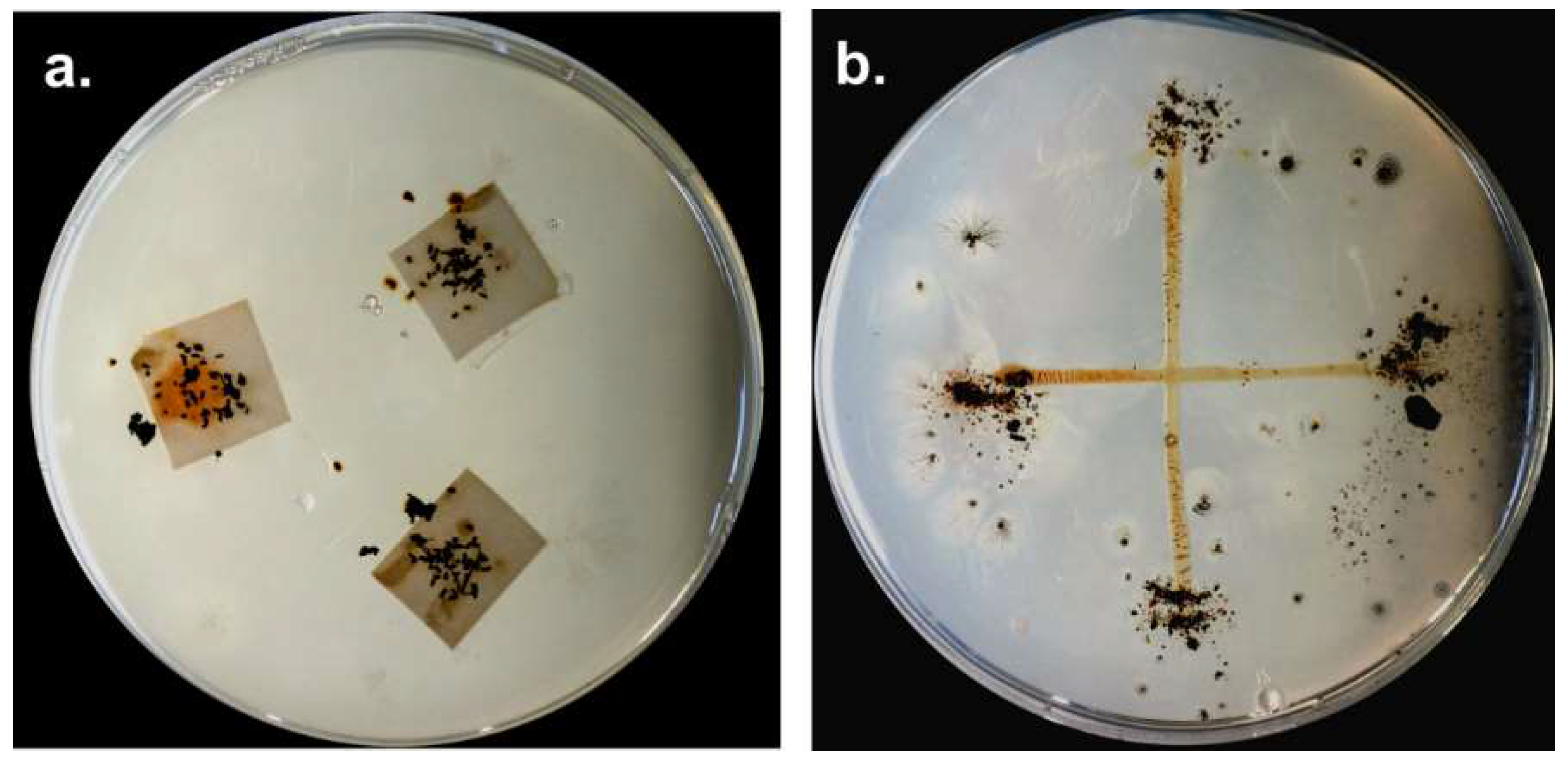
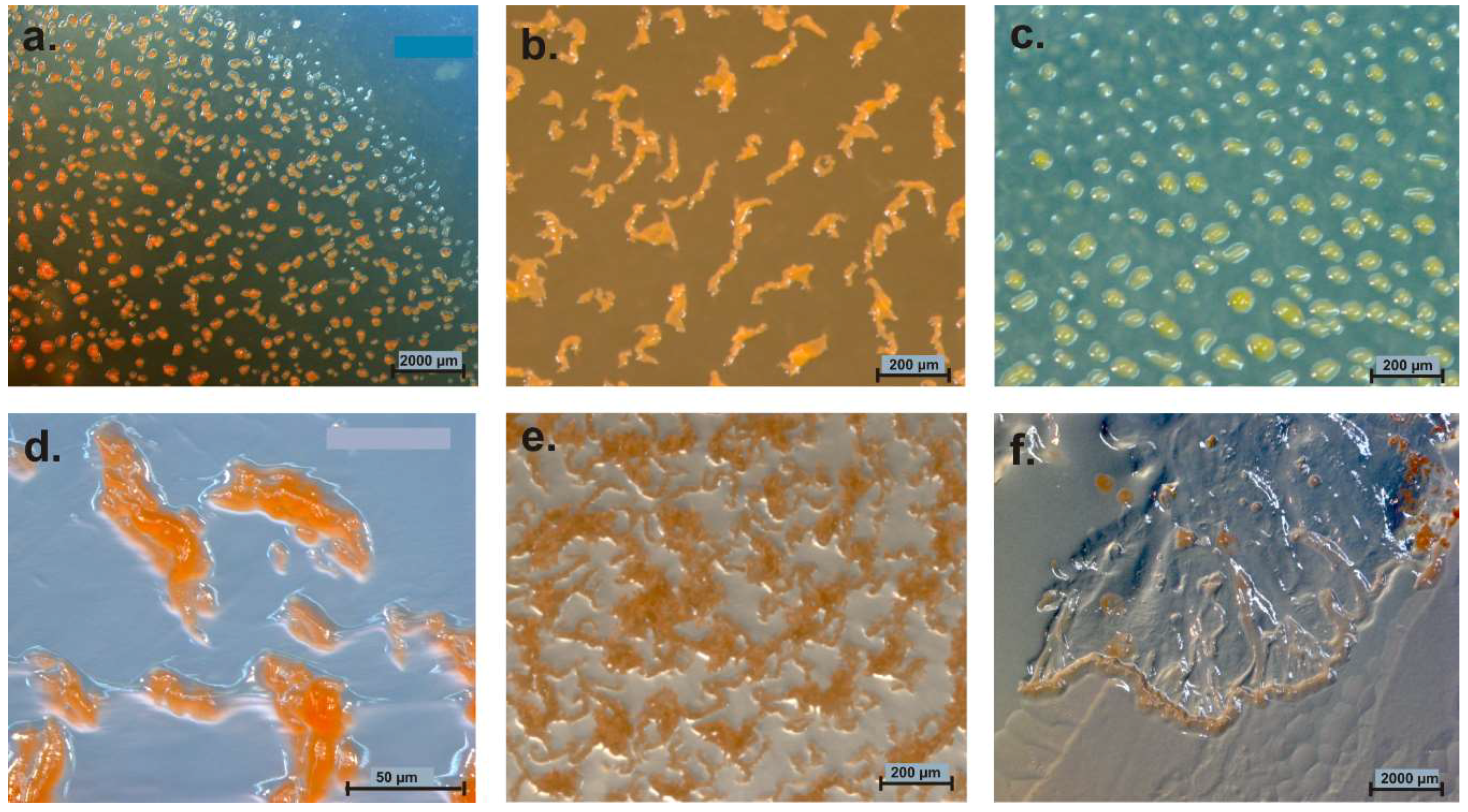
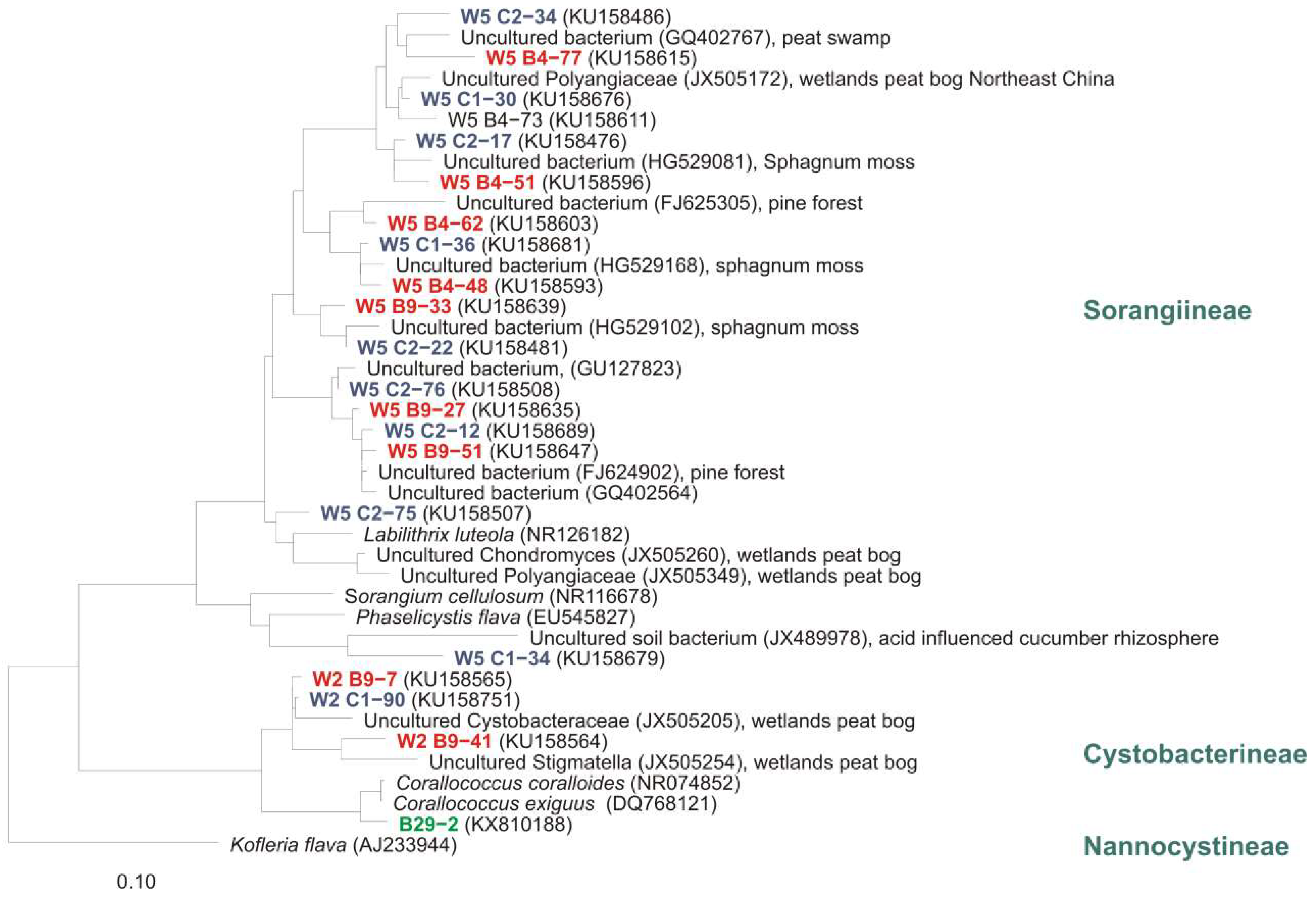
5. Distribution of Myxobacteria in Different Habitats
5.1. Terrestrial Habitats
5.2. Acidic and Alkaline Habitats
5.3. Freshwater Habitats
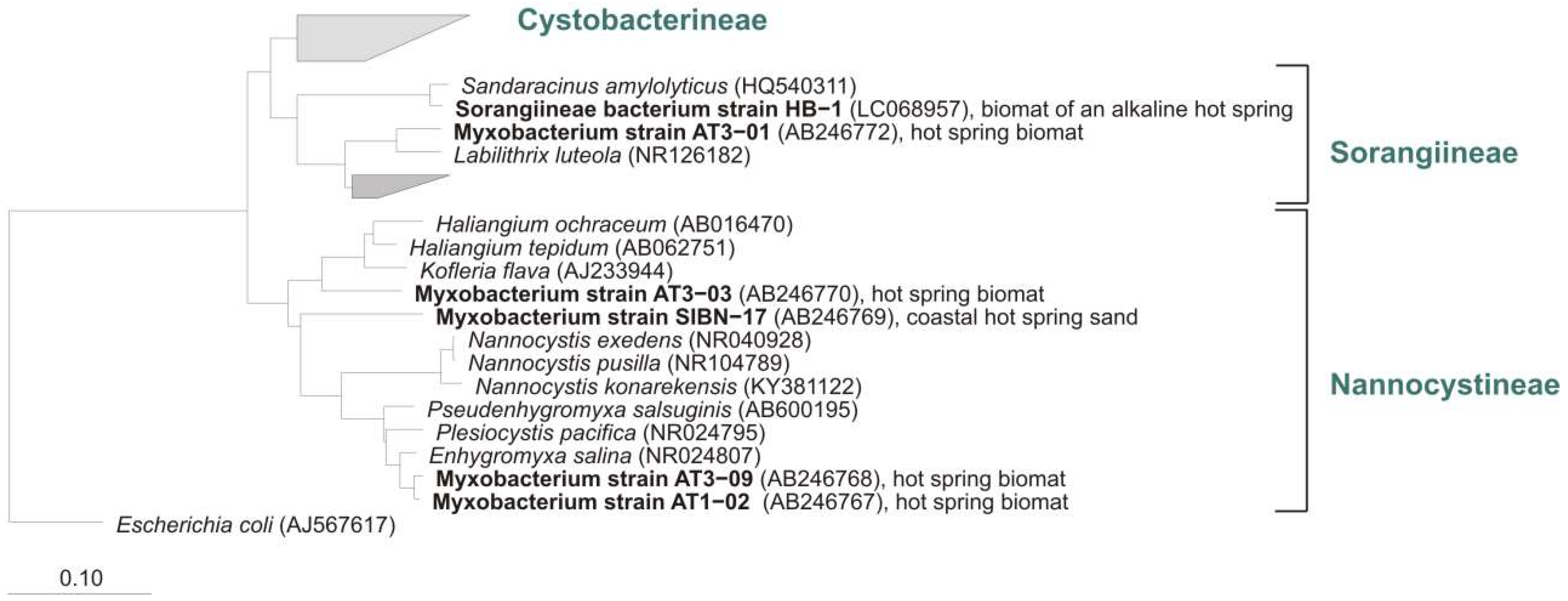
5.4. Marine/Saline Environments
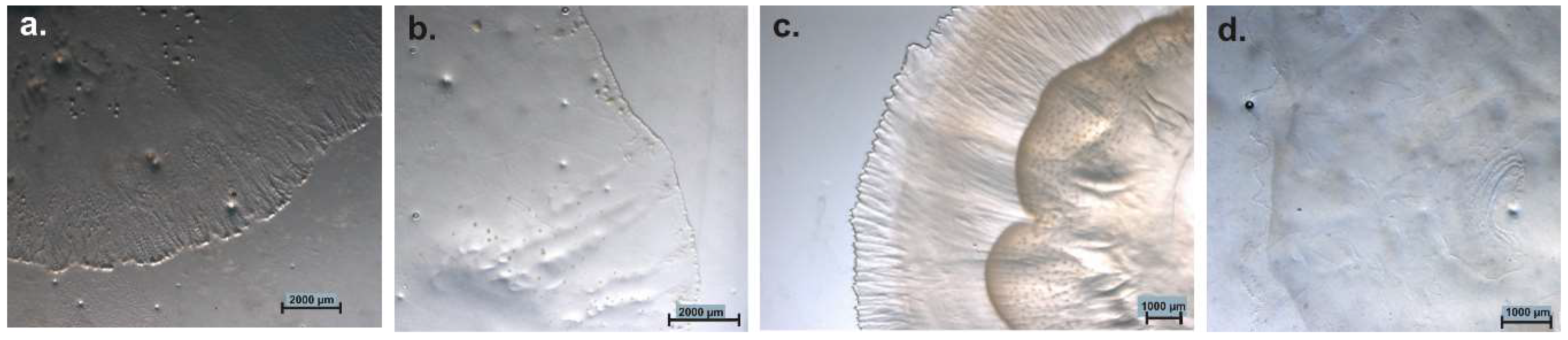
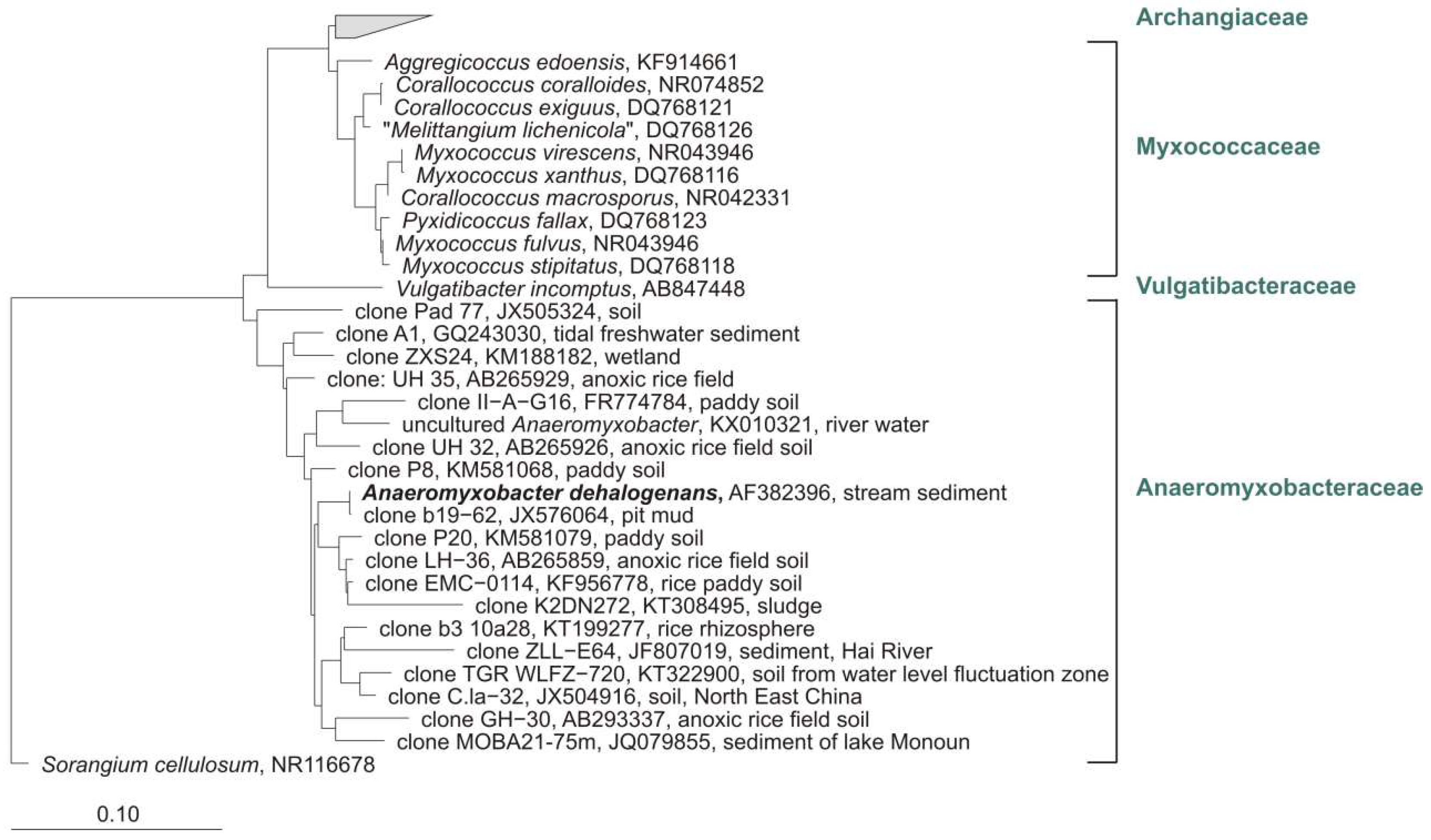
5.5. Facultative or Strictly Anaerobic Myxobacteria
5.6. Moderate to Extreme Hot or Cold Environments
6. Conclusion
Acknowledgments
Conflicts of Interest
References
- Dawid, W. Biology and global distribution of myxobacteria in soils. FEMS Microbiol. Rev. 2000, 24, 403–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, K.I.; Zindler, T.; Wink, J.; Wilharm, E.; Stadler, M. Myxobacteria in high moor and fen: An astonishing diversity in a neglected extreme habitat. MicrobiologyOpen 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Dawid, W. Myxobakterien in ungestörten Hochmooren des Hohen Venn (Hautes Fagnes, Belgien). Syst. Appl. Microbiol. 1984, 5, 555–563. [Google Scholar] [CrossRef]
- Fudou, R.; Jojima, Y.; Iizuka, T.; Yamanaka, S. Haliangium ochraceum gen. nov., sp. nov. and Haliangium tepidum sp. nov.: Novel moderately halophilic myxobacteria isolated from coastal saline environments. J. Gen. Appl. Microbiol. 2002, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Jojima, Y.; Fudou, R.; Hiraishi, A.; Ahn, J.W.; Yamanaka, S. Plesiocystis pacifica gen. nov., sp. nov., a marine myxobacterium that contains dihydro-genated menaquinone, isolated from the Pacific coasts of Japan. IJSEM 2003, 53, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Jojima, Y.; Fudou, R.; Tokura, M.; Hiraishi, A.; Yamanaka, S. Enhygromyxa salina gen. nov., sp. nov., a slightly halophilic myxobacterium isolated from the coastal areas of Japan. Syst. Appl. Microbiol. 2003, 26, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Jojima, Y.; Hayakawa, A.; Fujii, T.; Yamanaka, S.; Fudou, R. Pseudenhygromyxa salsuginis gen. nov., sp. nov., a myxobacterium isolated from an estuarine marsh. IJSEM 2013, 63, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Menne, B.; Rückert, G. Myxobakterien (Myxobacterales) in Höhlensedimenten des Hagengebirges (Nördliche Kalkalpen). Die Höhle. Z Karst Höhlenkd 1988, 39, 120–131. [Google Scholar]
- Mohr, K.I.; Stechling, M.; Wink, J.; Wilharm, E.; Stadler, M. Comparison of Myxobacterial Diversity and Evaluation of Isolation Success in two niches: Kiritimati Island and German Compost. MicrobiologyOpen 2016, 5, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Shimkets, L.J.; Dworkin, M.; Reichenbach, H. The Myxobacteria. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 31–115. [Google Scholar]
- Sanford, R.A.; Cole, J.R.; Tiedje, J.M. Characterization and Description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an Aryl-Halorespiring Facultative Anaerobic Myxobacterium. AEM 2002, 68, 893–900. [Google Scholar] [CrossRef]
- Reichenbach, H. The ecology of the myxobacteria. Environ. Microbiol. 1999, 1, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, B.; Chen, J.; Neu, J.C.; Berry, R.M.; Oster, G.; Zusman, D.R. Myxobacteria gliding motility requires cytoskeleton rotation powered by proton motive force. Proc. Natl. Acad. Sci. USA 2011, 108, 2498–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, D.; Robinson, M.; Kroos, L. Myxobacteria, Polarity, and Multicellular Morphogenesis. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, E.M.F.; Mignot, T.; Yang, Z.; Zusman, D.R. Gliding Motility Revisited: How Do the Myxobacteria move without Flagella? Microbiol. Mol. Biol. Rev. 2010, 74, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Faure, L.M.; Fiche, J.B.; Espinosa, L.; Ducret, A.; Anantharaman, V.; Luciano, J.; Lhospice, S.; Islam, S.T.; Tréguier, J.; Sotes, M.; et al. The mechanism of force transmission at bacterial focal adhesion complexes. Nature 2016, 539, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Burchard, R.P.; Dworkin, M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J. Bacteriol. 1966, 91, 535–545. [Google Scholar] [PubMed]
- Zusman, D.R.; Scott, A.E.; Yang, Z.; Kirby, J.R. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 2007, 5, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Thaxter, R. On the Myxobacteriaceae, a new order of the Schizomycetes. Bot. Gaz. 1892, 17, 389. [Google Scholar] [CrossRef]
- Kofler, L. Die Myxobakterien der Umgebung von Wien. Sitzungsberichte der Akademie der Wissenschaften mathematisch-naturwissenschaftliche Klasse 1913, 122, 845–876. [Google Scholar]
- Baur, E. Myxobakterienstudien. Arch. Protistenkunde 1904, 5, 42. [Google Scholar]
- Jahn, E. Beiträge zur botanischen Protistologie; Gebrüder Borntraeger: Leipzig, Germany, 1924. [Google Scholar]
- Jahn, E. Kulturmethoden und Stoffwechseluntersuchungen bei Myxobakterien (Polyangiden); Urban and Schwarzenberg: Berlin, Germany, 1936. [Google Scholar]
- Kühlwein, H. Beiträge zur Biologie und Entwicklungsgeschichte der Myxobakterien. Arch. Mikrobiol. 1950, 14, 678–704. [Google Scholar] [CrossRef]
- Reichenbach, H.; Höfle, G. Biologically active secondary metabolites from myxobacteria. Biotechnol. Adv. 1993, 11, 219–277. [Google Scholar] [CrossRef]
- Mohr, K.I. History of antibiotics research. In How to Overcome the Antibiotic Crisis—Facts, Challenges, Technologies & Future Perspective; Stadler, M., Dersch, P., Eds.; Springer: Berlin, Germany, 2017; Volume 398, pp. 237–272. [Google Scholar]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2011, 1, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karwehl, S.; Stadler, M. Exploitation of fungal biodiversity for discovery of novel antibiotics. In How to Overcome the Antibiotic Crisis—Facts, Challenges, Technologies & Future Perspective; Stadler, M., Dersch, P., Eds.; Springer: Berlin, Germany, 2017; Volume 398, pp. 303–338. [Google Scholar]
- Waksman, S.A.; Woodruff, H.B. Bacteriostatic and bacteriocidal substances produced by soil actinomycetes. Proc. Soc. Exp. Biol. 1940, 45, 609–614. [Google Scholar] [CrossRef]
- Schatz, A.; Bugie, E.; Waksman, S. Streptomycin: A substance exhibiting antibiotic activity against gram positive and gram negative bacteria. Proc. Exp. Biol. Med. 1944, 55, 66–69. [Google Scholar] [CrossRef]
- Duggar, B.M. Aureomycin: A product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 1948, 30, 177–181. [Google Scholar] [CrossRef]
- McGuire, J.M.; Bunch, R.L.; Anderson, R.C.; Boaz, H.E.; Flynn, E.H.; Powell, H.M.; Smith, J.W. Ilotycin, a new antibiotic. Antibiot. Chemother. 1952, 2, 281–283. [Google Scholar]
- Drews, J. Drug discovery: A historical perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, J.G.; Gilbert, D.N.; Spellberg, B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 2013, 56, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Hesterkamp, T. Antibiotics Clinical Development and Pipeline. In How to Overcome the Antibiotic Crisis—Facts, Challenges, Technologies & Future Perspective; Stadler, M., Dersch, P., Eds.; Springer: Berlin, Germany, 2016; Volume 398, pp. 447–474. [Google Scholar]
- Hoffmann, T.; Krug, D.; Bozkurt, N.; Duddela, S.; Jansen, R.; Garcia, R.; Gerth, K.; Steinmetz, H.; Müller, R. Correlating chemical diversity with taxonomic distance for discovery of natural products in myxobacteria. Nat. Commun. 2018, 9, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landwehr, W.; Wolf, C.; Wink, J. Actinobacteria and Myxobacteria—Two of the Most Important Bacterial Resources for Novel Antibiotics. In How to Overcome the Antibiotic Crisis—Facts, Challenges, Technologies & Future Perspective; Stadler, M., Dersch, P., Eds.; Spinger: Berlin, Germany, 2016. [Google Scholar]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N. Myxobacteria in Soils and Composts; their Distribution, Number and Lytic Action on Bacteria. Microbiology 1947, 1, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, S.; Dudani, A. Lysis of human pathogenic bacteria by myxobacteria. Nature 1955, 15, 125. [Google Scholar] [CrossRef]
- Noren, B.; Raper, K.B. Antibiotic activity of myxobacteria in relation to their bacteriolytic capacity. J. Bacteriol. 1962, 84, 157–162. [Google Scholar] [PubMed]
- Ringel, S.M.; Greenough, R.C.; Roemer, S.; Connor, D.; Gutt, A.L.; Blair, B.; Kanter, G.; von Strandtmann, M. Ambruticin (W7783), a new antifungal antibiotic. J. Antibiot. 1977, 30, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; König, G.M. The chemistry of gliding bacteria. Nat. Prod. Rep. 2007, 24, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Herrmann, J.; Raju, R.; Steinmetz, H.; Mohr, K.I.; Hüttel, S.; Harmrolfs, K.; Stadler, M.; Müller, R. Cystobactamids: Myxobacterial Topoisomerase Inhibitors Exhibiting Potent Antibacterial Activity. Angew. Chem. Int. Ed. 2014, 53, 14605–14609. [Google Scholar] [CrossRef] [PubMed]
- Surup, F.; Viehrig, K.; Mohr, K.I.; Jansen, R.; Herrmann, J.; Müller, R. Disciformycins A and B, unprecedented 12-membered Macrolide-Glycoside Antibiotics from the Myxobacterium Pyxidicoccus fallax active against multiresistant Staphylococci. Angew. Chem. Int. Ed. 2014, 53, 13588–13591. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Garcia, R.; Bifulco, G.; Martinez, J.P.; Hüttel, S.; Sasse, F.; Meyerhans, A.; Stadler, M.; Müller, R. Aetheramides A and B, Potent HIV-Inhibitory Depsipeptides from a Myxobacterium of the New Genus “Aetherobacter”. Org. Lett. 2012, 14, 2854–2857. [Google Scholar] [CrossRef] [PubMed]
- Gerth, K.; Bedorf, N.; Irschik, H.; Höfle, G.; Reichenbach, H. The soraphens: A family of novel antifungal compounds from Sorangium cellulosum (Myxobacteria). I. Soraphen A1 alpha: Fermentation, isolation, biological properties. J. Antibiot. 1994, 47, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gerth, K.; Bedorf, N.; Höfle, G.; Irschik, H.; Reichenbach, H. Epothilons A and B: Antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria). Production, physico-chemical and biological properties. J. Antibiot. 1996, 49, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Sasse, F.; Steinmetz, H.; Schupp, T.; Petersen, F.; Memmert, K.; Hofmann, H.; Heusser, C.; Brinkmann, V.; von Matt, P.; Höfle, G.; et al. Argyrins, immunosuppressive cyclic peptides from myxobacteria. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. 2002, 55, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Held, J.; Gebru, T.; Kalesse, M.; Jansen, R.; Gerth, K.; Müller, R.; Mordmüller, B. Antimalarial activity of the myxobacterial macrolide chlorotonil a. Antimicrob. Agents Chemother. 2014, 58, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.C.; Müller, R. The impact of genomics on the exploitation of the myxobacterial secondary metabolome. Nat. Prod. Rep. 2009, 26, 1385–1407. [Google Scholar] [CrossRef] [PubMed]
- Schneiker, S.; Perlova, O.; Kaiser, O.; Gerth, K.; Alici, A.; Altmeyer, M.O.; Bartels, D.; Bekel, T.; Beyer, S.; Bode, E.; et al. Complete genome sequence of the myxobacterium Sorangium cellulosum. Nat. Biotechnol. 2007, 25, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Goldman, B.S.; Nierman, W.C.; Kaiser, D.; Slater, S.C.; Durkin, A.S.; Eisen, J.A.; Ronning, C.M.; Barbazuk, W.B.; Blanchard, M.; Field, C.; et al. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl. Acad. Sci. USA 2006, 10, 15200–15205. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, H.; Mohr, K.I.; Zander, W.; Jansen, R.; Gerth, K.; Müller, R. Indiacens A and B: Prenyl Indoles from the Myxobacterium Sandaracinus amylolyticus. J. Nat. Prod. 2012, 75, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Garcia, R.O.; Gerth, K.; Irschik, H.; Müller, R. Sandaracinus amylolyticus gen. nov., sp. nov., a starch-degrading soil myxobacterium, and description of Sandaracinaceae fam. nov. IJSEM 2012, 62, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Stadler, M.; Gemperlein, K.; Müller, R. Aetherobacter fasciculatus gen. nov., sp. nov. and Aetherobacter rufus gen. nov., sp. nov., two novel myxobacteria with promising biotechnological applications. IJSEM 2015, 66, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Mohr, K.I.; Bernecker, S.; Stadler, M.; Müller, R. Indothiazinone, an indolyl-thiazolyl-ketone from a novel myxobacterium belonging to the Sorangiineae. J. Nat. Prod. 2014, 25, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Awal, R.P.; Wink, J.; Mohr, K.I.; Rohde, M.; Stadler, M.; Kämpfer, P.; Glaeser, S.; Schumann, P.; Garcia, R.; et al. Aggregicoccus edonensis gen. nov., sp. nov., an unusually aggregating myxobacterium isolated from a soil sample. IJSEM 2014, 65, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Karwehl, S.; Mohr, K.I.; Jansen, R.; Sood, S.; Bernecker, S.; Stadler, M. Edonamides, the first secondary metabolites from the recently described Myxobacterium Aggregicoccus edonensis. Tetrahedron Lett. 2015, 56, 6402–6404. [Google Scholar] [CrossRef]
- Muyzer, G. Genetic fingerprinting of microbial communities: Present status and future perspective. In Microbial Biosystems: New Frontiers, Proceedings of the 8th International Symposium, Halifax, Canada, 9–14 August 1998; Bell, C.R., Brylinsky, M., Johnson-Green, P., Eds.; Microbial Ecology Atlantic Canada Society for Microbial Ecology: Halifax, Canada, 2000. [Google Scholar]
- Vaz-Moreira, I.; Silva, M.E.; Manaia, C.M.; Nunes, O.C. Diversity of bacterial isolates from commercial and homemade composts. Microbiol. Ecol. 2008, 55, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Winterberg, H. Zur Methodik der Bakterienzahlung. Z. Hyg. 1898, 29, 75–93. [Google Scholar] [CrossRef]
- Ward, D.M.; Weller, R.; Bateson, M.M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature 1990, 345, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Disc. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.; Brown, J.H. Biogeography; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Ramette, A.; Tiedje, J.M. Biogeography: An Emerging Cornerstone for Understanding Prokaryotic Diversity, Ecology, and Evolution. Microb. Ecol. 2005, 53, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.-C.; Martiny, J.B.H. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.E.; Pace, N.R. Environmental diversity of Bacteria and Archaea. Syst. Biol. 2001, 50, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Bano, N.; Ruffin, S.; Ransom, B.; Hollibaugh, J.T. Phylogenetic composition of Arctic Ocean archaeal assemblages and comparison with Antarctic assemblages. Appl. Environ. Microbiol. 2004, 70, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Glöckner, F.O.; Zaichikov, E.; Belkova, N.; Denissova, L.; Pernthaler, J.; Pernthaler, A.; Amann, R. Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters including an abundant group of actinobacteria. Appl. Environ. Microbiol. 2000, 66, 5053–5065. [Google Scholar] [CrossRef] [PubMed]
- Rejmánková, E.; Komárek, J.; Komárková, J. Cyanobacteria—A neglected component of biodiversity: Patterns of species diversity in inland marshes of northern Belize (Central America). Divers. Distrib. 2004, 10, 189–199. [Google Scholar] [CrossRef]
- Hedlund, B.P.; Staley, J.T. Microbial endemism and biogeography. In Microbial Diversity and Bioprospecting; Bull, A.T., Ed.; ASM Press: Washington, DC, USA, 2003; pp. 225–231. [Google Scholar]
- Nemergut, D.R.; Costello, E.K.; Hamady, M.; Lozupone, C.; Jiang, L.; Schmidt, S.K.; Fierer, N.; Townsend, A.R.; Cleveland, C.C.; Stanish, L.; et al. Global patterns in the biogeography of bacterial taxa. Environ. Microbiol. 2011, 13, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.M.; Kato, C.; Zhou, X.W.; Wu, Z.H.; Sato, T.; Li, Y.Z. Phylogeographic separation of marine and soil myxobacteria at high levels of classification. ISME J. 2010, 4, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Brinkhoff, T.; Fischer, D.; Vollmers, J.; Voget, S.; Beardsley, C.; Thole, S.; Mussmann, M.; Kunze, B.; Wagner-Döbler, I.; Daniel, R.; et al. Biogeography and phylogenetic diversity of a cluster of exclusively marine myxobacteria. IJSEM 2012, 6, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Wielgoss, S.; Didelot, X.; Chaudhuri, R.R.; Liu, X.; Weedall, G.D.; Velicer, G.J.; Vos, M. A barrier to homologous recombination between sympatric strains of the cooperative soil bacterium Myxococcus xanthus. ISME J. 2016, 10, 2468–2477. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Wielgoss, S.; Fiegna, F.; Velicer, G.J. The biogeography of kin discrimination across microbial neighbourhoods. Mol. Ecol. 2016, 25, 4875–4888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerth, K.; Pradella, S.; Perlova, O.; Beyer, S.; Müller, R. Myxobacteria: Proficient producers of novel natural products with various biological activities—Past and future biotechnological aspects with the focus on the genus Sorangium. J. Biotechnol. 2003, 106, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Velicer, G.J.; Mendes-Soares, H.; Wielgoss, S. Whence Comes Social Diversity? Ecological and Evolutionary Analysis of the Myxobacteria. In Myxobacteria: Genomics, Cellular and Molecular Biology; Yang, Z., Higgs, P.I., Eds.; Caister Academic Press: Poole, UK, 2014. [Google Scholar]
- Tian, F.; Yong, Y.; Chen, B.; Li, H.; Yao, Y.-F.; Guo, X.-K. Bacterial, archaeal and eukaryotic diversity in Arctic sediment as revealed by 16S rRNA and 18S rRNA gene clone libraries analysis. Polar Biol. 2009, 32, 93–103. [Google Scholar] [CrossRef]
- Wu, Z.H.; Jiang, D.M.; Li, P.; Li, Y.Z. Exploring the diversity of myxobacteria in a soil niche by myxobacteria-specific primers and probes. Environ. Microbiol. 2005, 7, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Wu, Z.H.; Zhao, J.Y.; Li, Y.Z. Fruiting and non-fruiting myxobacteria: A phylogenetic perspective of cultured and uncultured members of this group. Mol. Phylogenet. Evol. 2007, 44, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Russell, J.A.; Moreau, C.S.; Kautz, S.; Sullam, K.E.; Hu, Y.; Basinger, U.; Mott, B.M.; Buck, N.; Wheeler, D.E. Highly similar microbial communities are shared among related and trophically similar ant species. Mol. Ecol. 2012, 21, 2282–2296. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N.; Pankratov, T.A.; Belova, S.E.; Kulichevskaya, I.S.; Liesack, W. Phylogenetic Analysis and In Situ Identification of Bacteria Community Composition in an Acidic Sphagnum Peat Bog. Appl. Environ. Microbiol. 2005, 72, 2110–2117. [Google Scholar] [CrossRef] [PubMed]
- Hook, L.A. Distribution of Myxobacters in Aquatic Habitats of an Alkaline Bog. AEM 1977, 34, 333–335. [Google Scholar]
- Rückert, G. Myxobakterien-Artenspektren von Boden in Abhängigkeit von bodenbildenden Faktoren unter besonderer Berücksichtigung der Bodenreaktion. Z. Pflanzenernaehr. Bodenkd. 1979, 142, 330–343. [Google Scholar] [CrossRef]
- Pacha, R.E.; Porter, S. Characteristics of Myxobacteria Isolated from the Surface of Freshwater Fish. Appl. Microbiol. 1968, 16, 1901–1906. [Google Scholar] [PubMed]
- Li, S.G.; Zhou, X.W.; Li, P.F.; Han, K.; Li, W.; Li, Z.F.; Wu, Z.H.; Li, Y.Z. The existence and diversity of myxobacteria in lake mud—A previously unexplored myxobacteria habitat. Environ. Microbiol. Rep. 2012, 4, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Kou, W.; Zhang, J.; Lu, X.; Ma, Y.; Mou, X.; Wu, L. Identification of bacterial communities in sediments of Poyang Lake, the largest freshwater lake in China. SpringerPlus 2016, 1, 401. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Angel, R.; Klose, M.; Claus, P.; Marotta, H.; Pinho, L.; Enrich-Prast, A.; Conrad, R. Structure and function of methanogenic microbial communities in sediments of Amazonian lakes with different water types. Environ. Microbiol. 2016, 18, 5082–5100. [Google Scholar] [CrossRef] [PubMed]
- Kandel, P.P.; Pasternak, Z.; van Rijn, J.; Nahum, O.; Jurkevitch, E. Abundance, diversity and seasonal dynamics of predatory bacteria in aquaculture zero discharge systems. FEMS Microbiol. Ecol. 2014, 89, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yao, Q.; Cai, Z.; Xie, X.; Zhu, H. Isolation and Identification of Myxobacteria from Saline-Alkaline Soils in Xinjiang, China. PLoS ONE 2013, 8, e70466. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Gresea, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Brockman, E.R. Fruiting myxobacteria from the South Carolina coast. J. Bacteriol. 1963, 94, 1253–1254. [Google Scholar]
- Li, B.; Yao, Q.; Zhu, H. Approach to analyze the diversity of myxobacteria in soil by semi-nested PCR-denaturing gradient gel electrophoresis (DGGE) based on taxon-specific gene. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Albataineh, H.D.; Stevens, D.C. Marine Myxobacteria: A Few Good Halophiles. Mar. Drugs 2018, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Schäberle, T.F.; Goralski, E.; Neu, E.; Erol, O.; Hölzl, G.; Dörmann, P.; Bierbaum, G.; König, G.M. Marine myxobacteria as a source of antibiotics--comparison of physiology, polyketide-type genes and antibiotic production of three new isolates of Enhygromyxa salina. Mar. Drugs 2010, 8, 2466–2479. [Google Scholar] [CrossRef] [PubMed]
- Fudou, R.; Iizuka, T.; Sato, S.; Ando, T.; Shimba, N.; Yamanaka, S. Haliangicin, a novel antifungal metabolite produced by a marine myxobacterium. 2. Isolation and structural elucidation. J. Antibiot. 2001, 54, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Dreisigacker, S.; Kehraus, S.; Neu, E.; Bierbaum, G.; Wright, P.R.; Menche, D.; Schäberle, T.F.; König, G.M. Salimabromide: Unexpected chemistry from the obligate marine myxobacterium Enhygromxya salina. Chemistry 2013, 19, 9319–9324. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Kehraus, S.; Neu, E.; Bierbaum, G.; Schäberle, T.F.; König, G.M. Salimyxins and enhygrolides: Antibiotic, sponge-related metabolites from the obligate marine myxobacterium Enhygromyxa salina. ChemBioChem 2013, 14, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tomura, T.; Sato, J.; Iizuka, T.; Fudou, R.; Ojika, M. Isolation and Biosynthetic Analysis of Haliamide, a New PKS-NRPS Hybrid Metabolite from the Marine Myxobacterium Haliangium ochraceum. Molecules 2016, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Treude, N.; Rosencrantz, D.; Liesack, W.; Schnell, S. Strain FAc12, a dissimilatory iron-reducing member of the Anaeromyxobacter subgroup of Myxococcales. FEMS Microbiol. Ecol. 2003, 44, 261–269. [Google Scholar] [CrossRef]
- Thomas, S.H.; Padilla-Crespo, E.; Jardine, P.M.; Sanford, R.A.; Löffler, F.E. Diversity and distribution of anaeromyxobacter strains in a uranium-contaminated subsurface environment with a nonuniform groundwater flow. AEM 2009, 75, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ratering, S.; Schnell, S. Microbial iron cylce in corrosion material of drinking water pipelines. Ann. Agrar. Sci. 2011, 9, 18–25. [Google Scholar]
- Kudo, K.; Yamaguchi, N.; Makino, T.; Ohtsuka, T.; Kimura, K.; Dong, D.T.; Amachi, S. Release of arsenic from soil by a novel dissimilatory arsenatereducing bacterium, Anaeromyxobacter sp. strain PSR-1. AEM 2013, 79, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. IJSEM 2014, 64, 346–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerzghi, H.; Brooks, J.P.; Gerba, C.P. Pepper IL. Influence of long-term land application of Class B biosolids on soil bacterial diversity. J. Appl. Microbiol. 2010, 109, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Moradi, A.; Glaeser, S.P.; Kämpfer, P.; Gemperlein, K.; Nübel, U.; Schumann, P.; Müller, R.; Wink, J. Nannocystis konarekensis sp. nov., a novel myxobacterium from an Iranian desert. IJSEM 2018, 68, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, H.; Li, X.; Chen, J.; Wang, A. Typical methanogenic inhibitors can considerably alter bacterial populations and affect the interaction between fatty acid degraders and homoacetogens. Appl. Microbiol. Biotechnol. 2010, 87, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Brockman, E.R. Myxobacters from Arid Mexican Soil. AEM 1976, 32, 642–644. [Google Scholar]
- Gerth, K.; Müller, R. Moderately thermophilic Myxobacteria: Novel potential for the production of natural products isolation and characterization. Environ. Microbiol. 2005, 7, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Tokura, M.; Jojima, Y.; Hiraishi, A.; Yamanaka, S.; Fudou, R. Enrichment and Phylogenetic Analysis of Moderately Thermophilic Myxobacteria from Hot Springs in Japan. Microbes Environ. 2006, 21, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Dawid, W.; Gallikowski, C.A.; Hirsch, P. Psychrophilic myxobacteria from Antarctic soils. Polarforschung 1988, 58, 271–278. [Google Scholar]
- Brockman, E.R.; Boyd, W.L. Myxobacteria from soils of the Alaskan and Canadian arctic. J. Bacteriol 1963, 86, 605–606. [Google Scholar]
- Müller, R.; Wink, J. Future potential for anti-infectives from bacteria—How to exploit biodiversity and genomic potential. Int. J. Med. Microbiol. 2014, 304, 3–13. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohr, K.I. Diversity of Myxobacteria—We Only See the Tip of the Iceberg. Microorganisms 2018, 6, 84. https://doi.org/10.3390/microorganisms6030084
Mohr KI. Diversity of Myxobacteria—We Only See the Tip of the Iceberg. Microorganisms. 2018; 6(3):84. https://doi.org/10.3390/microorganisms6030084
Chicago/Turabian StyleMohr, Kathrin I. 2018. "Diversity of Myxobacteria—We Only See the Tip of the Iceberg" Microorganisms 6, no. 3: 84. https://doi.org/10.3390/microorganisms6030084



