Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota
Abstract
:1. Introduction
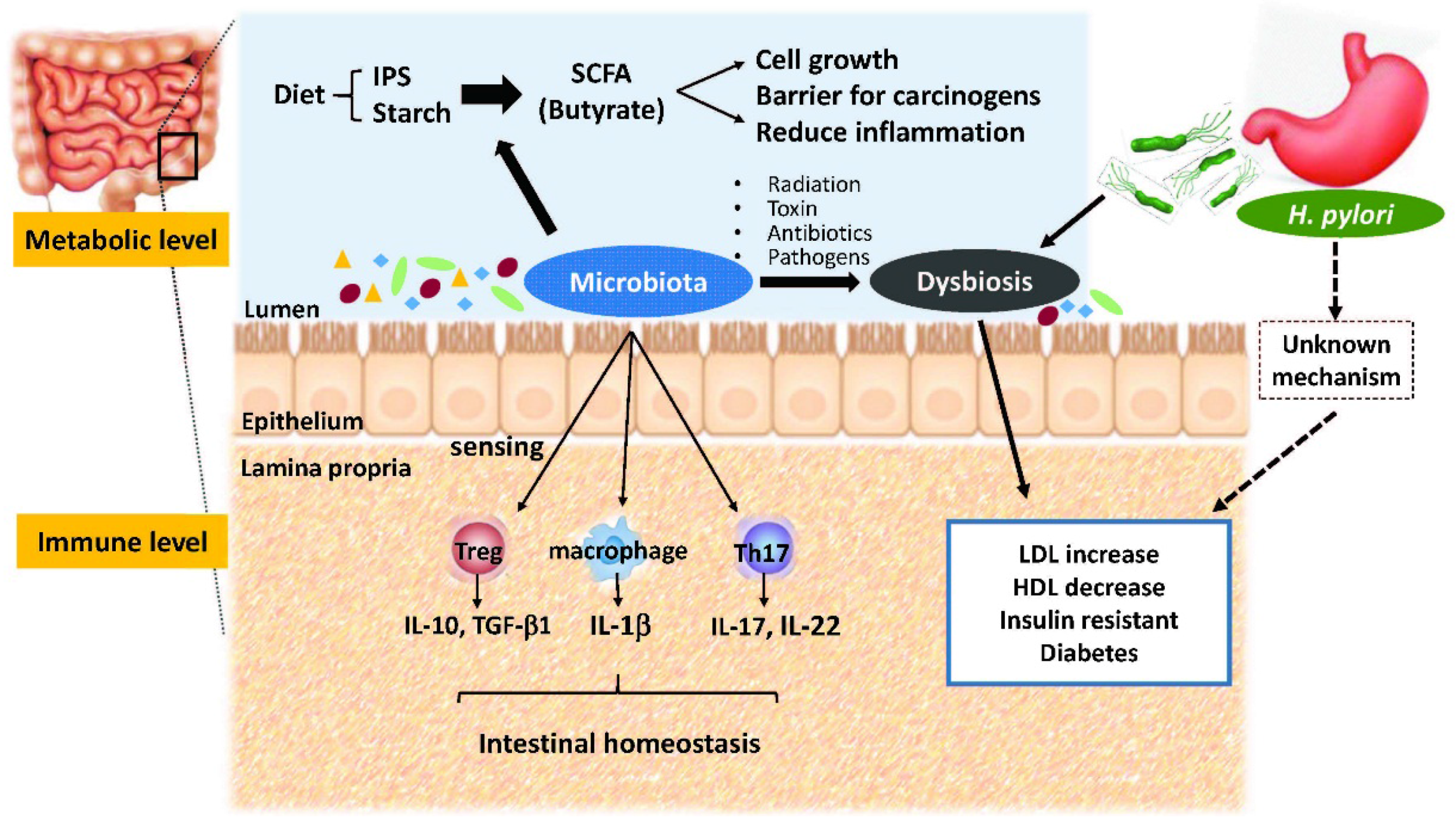
2. Crosstalk between Microbiota and Intestines
3. Metabolic Roles of Gut Microbiota
4. Gut Dysbiosis and Human Metabolic Disorders
5. H. pylori Infection and Metabolic Diseases
6. H. pylori Infection and Gut Microbiota
7. Inflammatory Bowel Diseases and Intestinal Microbiota
8. Inflammatory Bowel Diseases and Helicobacter Infection
9. Metabolic Interaction of H. pylori and Gut Microbiota
10. Conclusions
Author Contributions
Conflicts of Interest
References
- Neish, A.S. Microbes in gastrointestinal health and disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A. Initial intestinal colonization in the human infant and immune homeostasis. Ann. Nutr. Metab. 2013, 63, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Reigstad, C.S.; Bäckhed, F. Intestinal microbiota during infancy and its implications for obesity. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010, 86, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.S.; Sheu, S.M.; Yang, H.B.; Huang, A.H.; Wu, J.J. Host gastric Lewis expression determines the bacterial density of Helicobacter pylori in babA2 genopositive infection. Gut 2003, 52, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.S.; Odenbreit, S.; Hung, K.H.; Liu, C.P.; Sheu, S.M.; Yang, H.B.; Wu, J.J. Interaction between host gastric Sialyl–Lewis X and H. pylori SabA enhances H. pylori density in patients lacking gastric Lewis B antigen. Am. J. Gastroenterol. 2006, 101, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ktsoyan, Z.A.; Beloborodova, N.V.; Sedrakyan, A.M.; Osipov, G.A.; Khachatryan, Z.A.; Kelly, D.; Manukyan, G.P.; Arakelova, K.A.; Hovhannisyan, A.I.; Olenin, A.Y.; et al. Profiles of Microbial Fatty Acids in the Human Metabolome are Disease-Specific. Front. Microbiol. 2011, 1, 148. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Ge, H.M.; Zheng, W.F.; Tan, R.X. NMR-based metabonomics for detection of Helicobacter pylori infection in gerbils: Which is more descriptive. Helicobacter 2008, 13, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Chervonsky, A.V. Influence of microbial environment on autoimmunity. Nat. Immunol. 2010, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Ann. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Englyst, H.N. Starch utilization by the human large intestinal microflora. J. Appl. Bacteriol. 1986, 60, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS. Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Gibson, P.R.; Young, G. Butyrate production from dietary fiber and protection against large bowel cancer in a rat model. Gut 1993, 34, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Ley, R.E.; Volchkov, P.Y.; Stranges, P.B.; Avanesyan, L.; Stonebraker, A.C.; Hu, C.; Wong, F.S.; Szot, G.L.; Bluestone, J.A.; et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008, 455, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Marshall, B.J.; Goodwin, C.S.; Warren, J.R.; Murray, R.; Blincow, E.D.; Blackbourn, S.J.; Phillips, M.; Waters, T.E.; Sanderson, C.R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet 1988, 2, 1437–1442. [Google Scholar] [CrossRef]
- Schistosomes, liver flukes, and Helicobacter pylori. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization, International Agency for Research on Cancer: Lyon, France, 1994; pp. 177–240.
- Queiroz, D.M.; Harris, P.R.; Sanderson, I.R.; Windle, H.J.; Walker, M.M.; Rocha, A.M.; Rocha, G.A.; Carvalho, S.D.; Bittencourt, P.F.; de Castro, L.P.; et al. Iron status and Helicobacter pylori infection in symptomatic children: An international multi-centered study. PLoS ONE 2013, 8, e68833. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, W.S.; Lee, K.H.; Bae, S.H.; Kim, M.K.; Joo, Y.D.; Zang, D.Y.; Jo, J.C.; Lee, S.M.; Lee, J.H.; et al. Efficacy of Helicobacter pylori eradication for the 1st line treatment of immune thrombocytopenia patients with moderate thrombocytopenia. Ann. Hematol. 2015, 94, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Sheu, B.S.; Yang, H.B.; Lu, C.C.; Chuang, C.C. Eradication of Helicobacter pylori increases childhood growth and serum acylated ghrelin levels. World J. Gastroenterol. 2012, 18, 2674–2681. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Forman, D.; Hunt, R.H.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g3174. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.D.; Melzer, E.; Attali, M.; Duek, G.; Yahav, J. Helicobacter pylori: Friend or foe? World J. Gastroenterol. 2014, 20, 8979–8985. [Google Scholar] [PubMed]
- Niemelä, S.; Karttunen, T.; Korhonen, T.; Läärä, E.; Karttunen, R.; Ikäheimo, M.; Kesäniemi, Y.A. Could Helicobacter pylori infection increase the risk of coronary heart disease by modifying serum lipid concentrations? Heart 1996, 75, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Buzás, G.M. Metabolic consequences of Helicobacter pylori infection and eradication. World J. Gastroenterol. 2014, 20, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Saijo, Y.; Yoshioka, E.; Tsutsui, H. Helicobacter pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J. Atheroscler. Thromb. 2010, 17, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Jia, E.Z.; Zhao, F.J.; Hao, B.; Zhu, T.B.; Wang, L.S.; Chen, B.; Cao, K.J.; Huang, J.; Ma, W.Z.; Yang, Z.J.; et al. Helicobacter pylori infection is associated with decreased serum levels of high density lipoprotein, but not with the severity of coronary atherosclerosis. Lipids Health Dis. 2009, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Wang, S.S.; Hsieh, Y.T.; Kuo, F.C.; Soon, M.S.; Wu, D.C. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur. J. Clin. Investig. 2013, 43, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Blaser, M.J. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J. Infect. Dis. 2012, 205, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Naja, F.; Nasreddine, L.; Hwalla, N.; Moghames, P.; Shoaib, H.; Fatfat, M.; Sibai, A.; Gali-Muhtasib, H. Association of H. pylori infection with insulin resistance and metabolic syndrome among Lebanese adults. Helicobacter 2012, 17, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, W.; Gu, M.; Zhou, H.; Zhang, G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J. Gastroenterol. 2015, 50, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Vafaeimanesh, J.; Rajabzadeh, R.; Ahmadi, A.; Moshtaghi, M.; Banikarim, S.; Hajiebrahimi, S.; Seyyedmajidi, M. Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab. J. Gastroenterol. 2013, 14, 55–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bégué, R.E.; Gómez, R.; Compton, T.; Vargas, A. Effect of Helicobacter pylori eradication in the glycemia of children with type 1 diabetes: a preliminary study. South. Med. J. 2002, 95, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Stearns, J.C.; Lynch, M.D.; Senadheera, D.B.; Tenenbaum, H.C.; Goldberg, M.B.; Cvitkovitch, D.G.; Croitoru, K.; Moreno-Hagelsieb, G.; Neufeld, J.D. Bacterial biogeography of the human digestive tract. Sci. Rep. 2011, 1, 170. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Bhatnagar, S.; George, M.D.; Paster, B.J.; Canfield, D.R.; Eisen, J.A.; Solnick, J.V. The impact of Helicobacter pylori infection on the gastric microbiota of the rhesus macaque. PLoS ONE 2013, 8, e76375. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.N.; Wang, C.L.; Liu, X.W.; Cui, Y.; Xie, N.; Yu, Q.F.; Li, F.J.; Lu, F.G. Gastric and duodenum microflora analysis after long-term Helicobacter pylori infection in Mongolian Gerbils. Helicobacter 2011, 16, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Fischer, A.; Plickert, R.; Wiedemann, T.; Loddenkemper, C.; Göbel, U.B.; Bereswill, S.; Rieder, G. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. PLoS ONE 2014, 9, e100362. [Google Scholar]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011, 5, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Sheh, A.; Fox, J.G. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 2013, 4, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Franceschi, F.; Gasbarrini, A. The gastrointestinal microbiome-functional interference between stomach and intestine. Best. Pract. Res. Clin. Gastroenterol. 2014, 28, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Kuzuoka, H.; Tsujikawa, T.; Nakamura, S.; Hirai, F.; Suzuki, Y.; Matsui, T.; Fujiyama, Y.; Matsumoto, T. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J. Gastroenterol. 2012, 47, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Fujita, Y. Determination of the discriminant score of intestinal microbiota as a biomarker of disease activity in patients with ulcerative colitis. BMC Gastroenterol. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Maukonen, J.; Kolho, K.L.; Paasela, M.; Honkanen, J.; Klemetti, P.; Vaarala, O.; Saarela, M. Altered fecal microbiota in paediatric inflammatory bowel disease. J. Crohn’s Colitis 2015, 9, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Kolho, K.L.; Korpela, K.; Jaakkola, T.; Pichai, M.V.; Zoetendal, E.G.; Salonen, A.; de Vos, W.M. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am. J. Gastroenterol. 2015, 110, 92–130. [Google Scholar] [CrossRef] [PubMed]
- Mattila, E.; Uusitalo-Seppälä, R.; Wuorela, M.; Lehtola, L.; Nurmi, H.; Ristikankare, M.; Moilanen, V.; Salminen, K.; Seppälä, M.; Mattila, P.S.; et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012, 142, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015, 149, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.; Dave, M.; Higgins, P.D.; Kao, J.Y. Association between Helicobacter pylori infection and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Inflamm. Bowel Dis. 2010, 16, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Konstantopoulos, P.; Mantzaris, G.J. Helicobacter pylori infection and inflammatory bowel disease: Is there a link? World J. Gastroenterol. 2014, 20, 6374–6385. [Google Scholar] [CrossRef] [PubMed]
- Rad, R.; Brenner, L.; Bauer, S.; Schwendy, S.; Layland, L.; da Costa, C.P.; Reindl, W.; Dossumbekova, A.; Friedrich, M.; Saur, D.; et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 2006, 131, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Chuang, C.C.; Yang, H.B.; Lu, C.C.; Sheu, B.S. Susceptibility to pediatric Helicobacter pylori infection correlates with the host responses of regulatory and effector T cells. Pediatr. Infect. Dis. J. 2014, 33, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Parronchi, P.; Romagnani, P.; Annunziato, F.; Sampognaro, S.; Becchio, A.; Giannarini, L.; Maggi, E.; Pupilli, C.; Tonelli, F.; Romagnani, S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am. J. Pathol. 1997, 150, 823–832. [Google Scholar] [PubMed]
- Luther, J.; Owyang, S.Y.; Takeuchi, T.; Cole, T.S.; Zhang, M.; Liu, M.; Erb-Downward, J.; Rubenstein, J.H.; Chen, C.C.; Pierzchala, A.V.; et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut 2011, 60, 1479–1486. [Google Scholar] [PubMed]
- Higgins, P.D.; Johnson, L.A.; Luther, J.; Zhang, M.; Sauder, K.L.; Blanco, L.P.; Kao, J.Y. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: Mucosal crosstalk between stomach and distal intestine. Inflamm. Bowel Dis. 2011, 17, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Engler, D.B.; Leonardi, I.; Hartung, M.L.; Kyburz, A.; Spath, S.; Becher, B.; Rogler, G.; Müller, A. Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm. Bowel Dis. 2015, 21, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Hänninen, M.L. Helicobacter spp. other than H. pylori. Helicobacter 2012, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Zhang, L.; Day, A.S.; Leach, S.; Mitchell, H. Detection of enterohepatic and gastric Helicobacter species in fecal specimens of children with Crohn’s disease. Helicobacter 2008, 13, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, B.; Chao, K.; Xiao, Y.; Cui, Y.; Gao, X.; Chen, B.; He, Y.; Hu, P.; Chen, M.; et al. Role of Helicobacter species in Chinese patients with inflammatory bowel disease. J. Clin. Microbiol. 2011, 49, 1987–1989. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, N.A.; Robinson, C.J.; Bergin, I.L.; Eaton, K.A.; Huffnagle, G.B.; Young, V.B. The effects of intestinal microbial community structure on disease manifestation in IL-10−/− mice infected with Helicobacter hepaticus. Microbiome 2013, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Y.; Seow, S.W.; Amoyo, A.A.; Chiow, K.H.; Tan, T.L.; Wong, W.Y.; Poh, Q.H.; Sentosa, I.M.; Bunte, R.M.; Pettersson, S.; et al. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci. Rep. 2015, 5, 8731. [Google Scholar] [PubMed]
- Yang, Y.J.; Sheu, B.S. Probiotics-containing yogurts suppress H. pylori load, and modify immune response & intestinal microbiota in the H. pylori-infected children. Helicobacter 2012, 17, 297–304. [Google Scholar]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-J.; Sheu, B.-S. Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota. Microorganisms 2016, 4, 15. https://doi.org/10.3390/microorganisms4010015
Yang Y-J, Sheu B-S. Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota. Microorganisms. 2016; 4(1):15. https://doi.org/10.3390/microorganisms4010015
Chicago/Turabian StyleYang, Yao-Jong, and Bor-Shyang Sheu. 2016. "Metabolic Interaction of Helicobacter pylori Infection and Gut Microbiota" Microorganisms 4, no. 1: 15. https://doi.org/10.3390/microorganisms4010015




