The Effect of Tellurite on Highly Resistant Freshwater Aerobic Anoxygenic Phototrophs and Their Strategies for Reduction
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Growth Conditions
2.2. Physiological and Biochemical Tests
2.3. Tellurite Reductase Expression, Activity, and Localization
3. Results
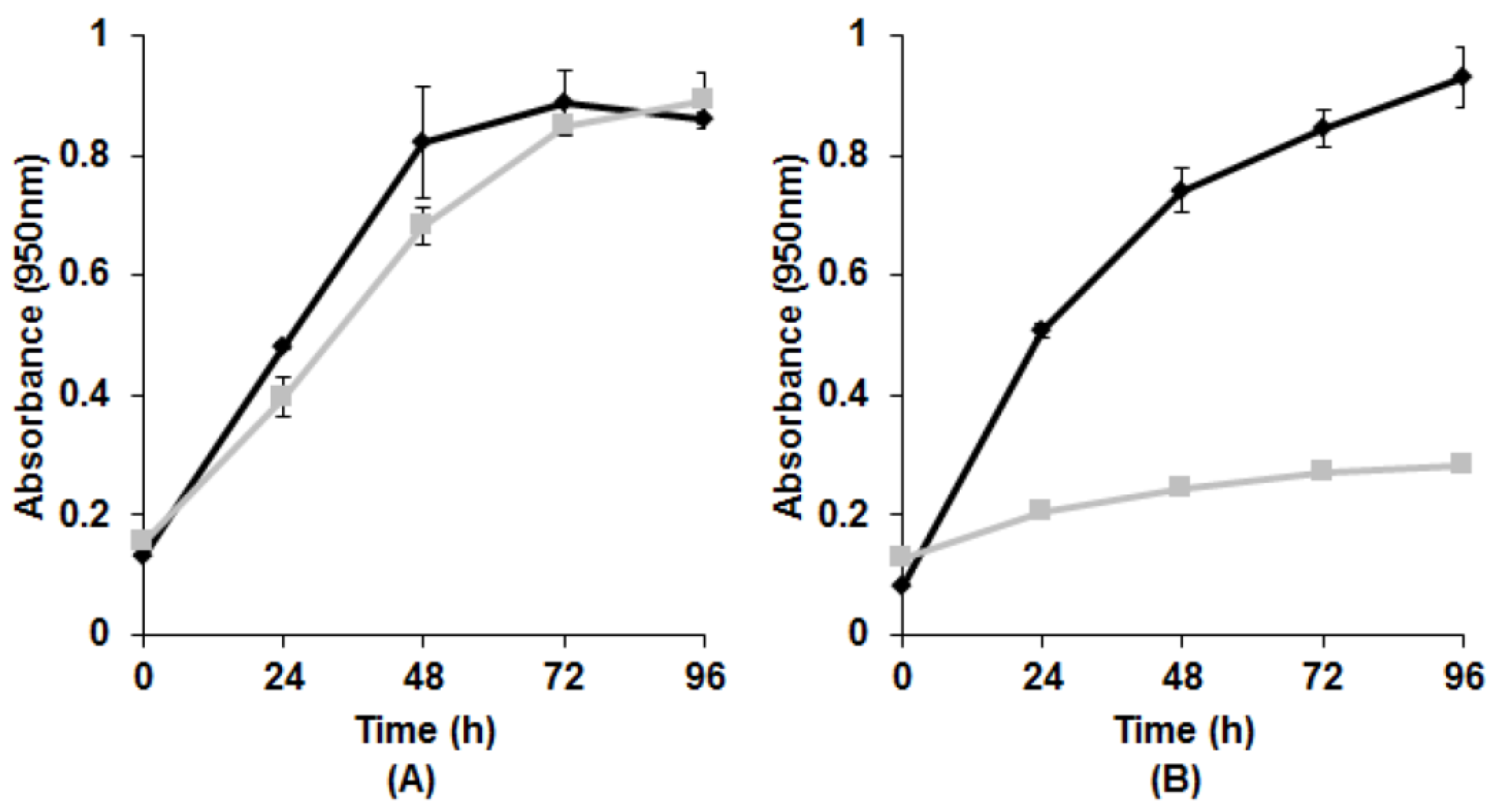
3.1. Growth with Tellurite
| Strain | pH | Aeration (rpm) | ||||
|---|---|---|---|---|---|---|
| 7.0 | 8.0 | 9.0 | 100 | 200 | 300 | |
| E1 1 | 78.4 ± 3.8 | 82.5 ± 4.8 | 100 ± 1.8 | 61.8 ± 5.9 | 100 ± 5.4 | 64.5 ± 4.9 |
| E4(1) 1 | 68.1 ± 5.1 | 77.6 ± 2.9 | 100 ± 0.7 | 54.2 ± 4.6 | 100 ± 2.2 | 67.4 ± 3.5 |
| E5 1 | 62.8 ± 4.5 | 74.9 ± 2.4 | 100 ± 4.7 | 81.7 ± 3.7 | 100 ± 2.7 | 72.1 ± 2.9 |
| KR99 1 | 77.7 ± 1.9 | 97.6 ± 1.1 | 100 ± 1.5 | 47.6 ± 2.2 | 100 ± 3.2 | 53.9 ± 4.4 |
| RB 16-17 2 | 70.8 ± 3.3 | 71.4 ± 4.6 | 100 ± 4.3 | 55.9 ± 2.9 | 100 ± 3.7 | 66.8 ± 3.3 |
| RB3 2 | 78.2 ± 3.6 | 76.6 ± 5.5 | 100 ± 3.2 | 59.7 ± 3.1 | 100 ± 4.3 | 62.1 ± 3.6 |
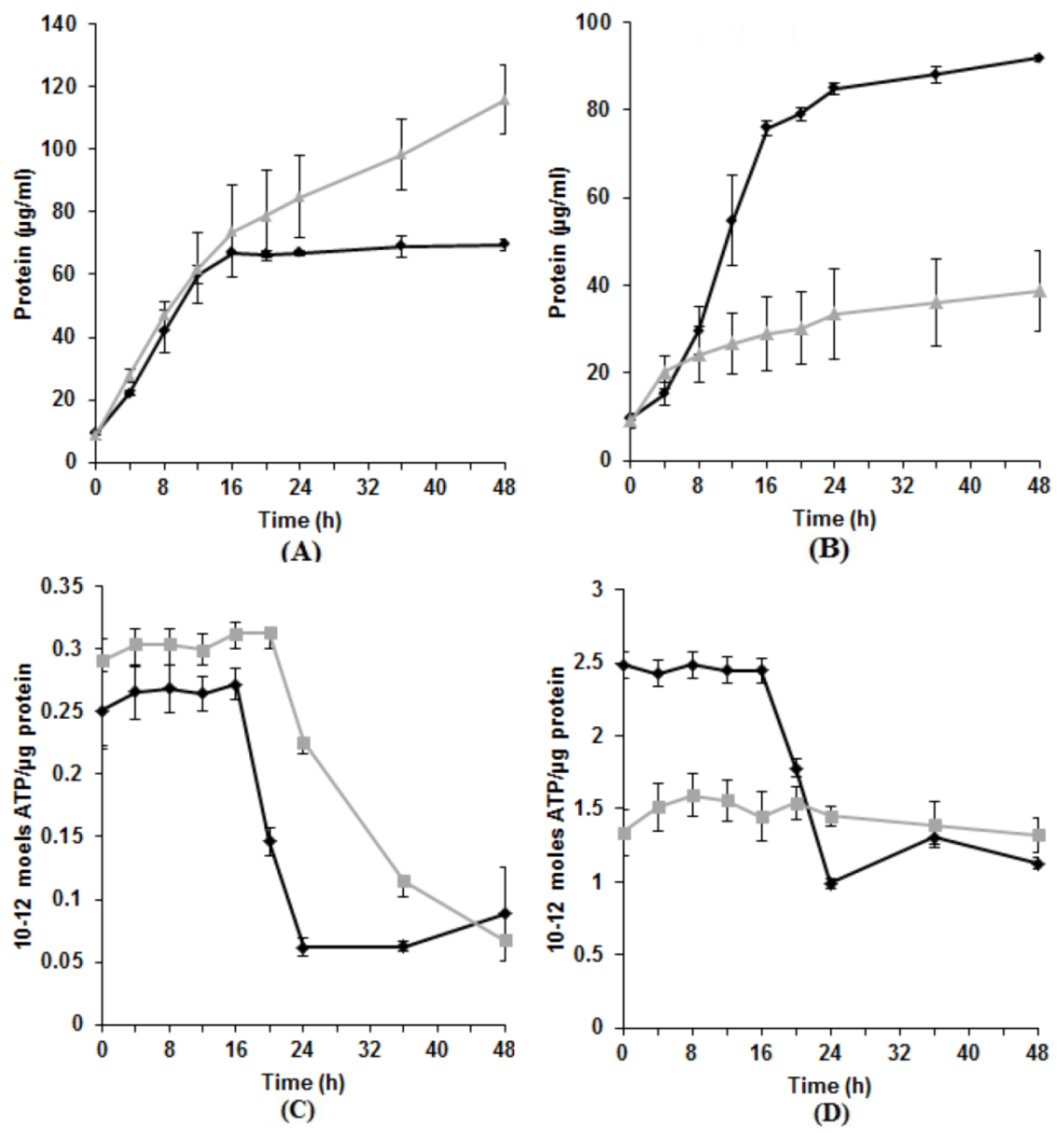
3.2. Effect of Tellurite on Protein and ATP Production
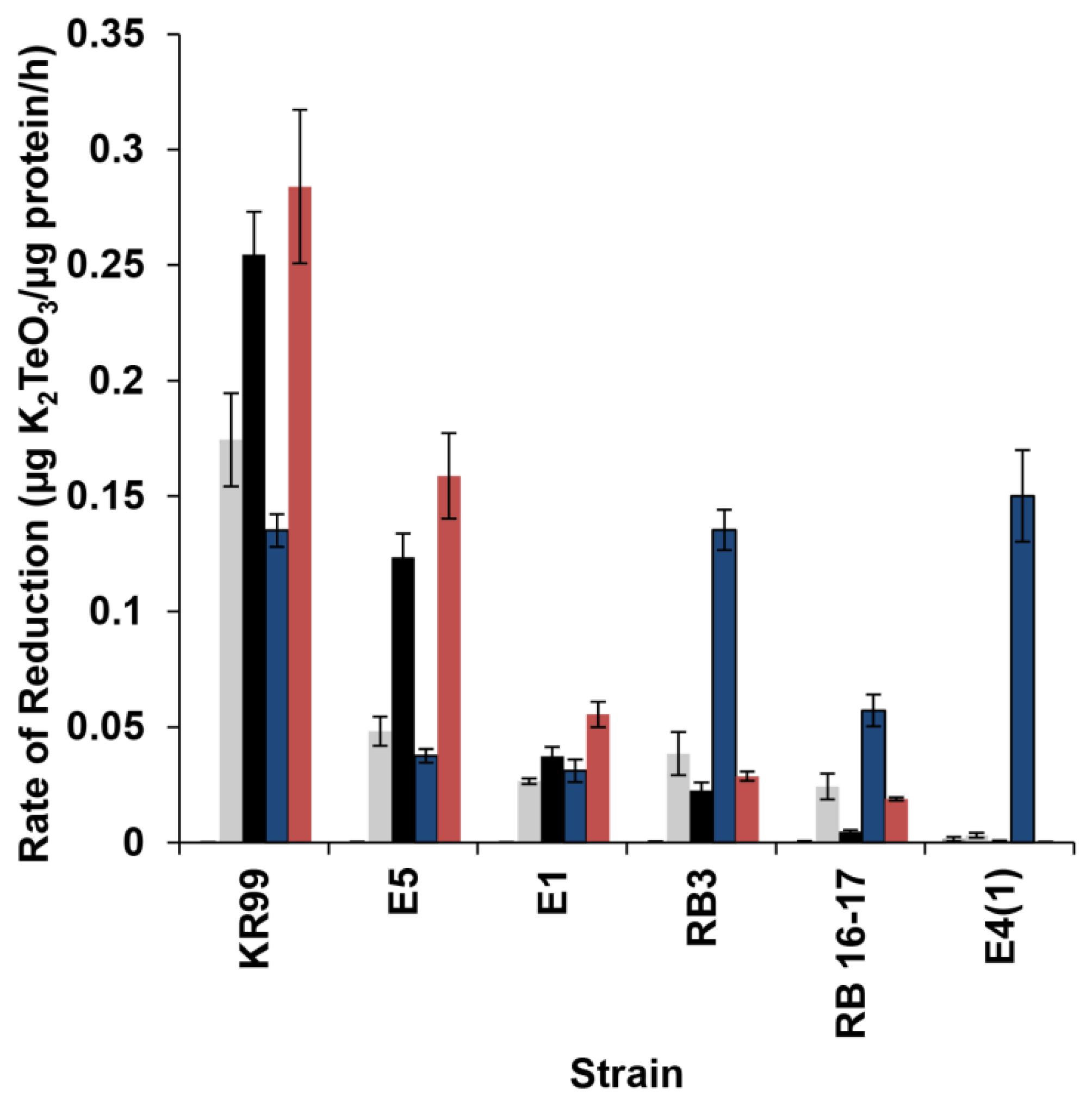
3.3. Characteristics of Tellurite Reductase Activity

3.4. Localization of Reductase Activity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, W.C. Tellurium; Van Nostrand Reinhold Company: New York, NY, USA, 1971. [Google Scholar]
- Yurkov, V.; Jappe, J.; Vermeglio, A. Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl. Environ. Microbiol. 1996, 62, 4195–4198. [Google Scholar] [PubMed]
- Pearion, C.; Jablonski, P. High level, intrinsic resistance of Natronococcus occultus to potassium tellurite. FEMS Microbiol. Lett. 1999, 174, 19–23. [Google Scholar] [CrossRef]
- Taylor, D.E. Bacterial tellurite resistance. Trends Microbiol. 1999, 7, 111–115. [Google Scholar] [CrossRef]
- Lloyd, J.; Mabbett, A.; Williams, D.; Macaskie, L. Metal reduction by sulfate-reducing bacteria: Physiological diversity and metal specificity. Hydrometallurgy 2001, 59, 327–337. [Google Scholar] [CrossRef]
- Perez, J.; Calderon, I.; Arenas, F.; Fuentes, D.; Pradenas, G.; Fuentes, E.; Sandoval, J.; Castro, M.; Elias, A.; Vasquez, C. Bacterial toxicity of potassium tellurite: Unveiling an ancient enigma. PLoS ONE 2007, 2, e211. [Google Scholar] [CrossRef] [PubMed]
- Calderon, I.; Arenas, F.; Perez, J.; Fuentes, D.; Araya, M.; Saavedra, C.; Tantalean, J.; Pichuantes, S.; Youderian, P.; Vasquez, C. Catalases are NAD(P)H-dependent tellurite reductases. PLoS ONE 2006, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Avazeri, C.; Turner, J.; Weiner, J.; Giordano, G.; Vermeglio, A. Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 1997, 143, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Sabaty, M.; Avazeri, C.; Pignol, D.; Vermeglio, A. Characterization of the reduction of selenate and tellurite by nitrate reductases. Appl. Environ. Microbiol. 2001, 67, 5122–5126. [Google Scholar] [CrossRef] [PubMed]
- Borsetti, F.; Francia, F.; Turner, R.J.; Zannoni, D. The thiol:disulfide oxidoreductase DsbB mediates the oxidizing effects of the toxic metalloid tellurite (TeO32−) on the plasma membrane redox system of the facultative phototroph Rhodobacter capsulatus. J. Bacteriol. 2007, 189, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, J.; Dubow, M. A novel selenite- and tellurite-inducible gene in Escherichia coli. Appl. Environ. Microbiol. 2000, 66, 4972–4978. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Lou, Y.; Chen, C. NMR solution of KP-TerB, a tellurite-resistance protein from Klebsiella pneumoniae. Protein Sci. 2008, 17, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.; Gonzalez, E.; Barra, R.; Vasquez, C. Purification and biochemical characterization of tellurite-reducing activities from Thermus thermophilus HB8. J. Bacteriol. 1988, 170, 3269–3273. [Google Scholar] [PubMed]
- Kabiri, M.; Amoozegar, M.; Tabebordbar, M.; Gilany, K.; Salekdeh, G. Effects of selenite and tellurite on growth, physiology, and proteome of a moderately halophilic bacterium. J. Proteome Res. 2009, 8, 3098–3108. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, H.; Saavedra, C.; Loyola, C.; Pichuantes, S.; Vasquez, C. Biochemical characterization of tellurite-reducing activities of Bacillus stearothermophilus V. Res. Microbiol. 1998, 149, 389–397. [Google Scholar] [CrossRef]
- Moore, M.D.; Kaplan, S. Members of the family Rhodospirillaceae reduce heavy-metal oxyanions to maintain redox poise during photosynthetic growth. ASM News 1994, 60, 17–23. [Google Scholar]
- Borghese, R.; Zannoni, D. Acetate permease (ActP) is responsible for tellurite (TeO32−) uptake and resistance in cells of the facultative phototroph Rhodobacter capsulatus. Appl. Environ. Microbiol. 2010, 76, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Borghese, R.; Marchetti, D.; Zannoni, D. The highly toxic oxyanion tellurite (TeO3−2) enters the phototrophic bacterium Rhodobacter capsulatus via an as yet uncharacterized monocarboxylate transport system. Arch. Microbiol. 2008, 189, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Kay, W. Tellurite susceptibility and non-plasmid-mediated resistance in Escherichia coli. Antimicrob. Agents Chemother. 1986, 30, 127–131. [Google Scholar] [CrossRef]
- Ollivier, P.; Bahrou, A.; Marcus, S.; Cox, T.; Church, T.; Hanson, T. Volatilization and precipitation of tellurium by aerobic tellurite-resistant marine microbes. Appl. Environ. Microbiol. 2008, 74, 7163–7173. [Google Scholar] [CrossRef] [PubMed]
- Schroder, I.; Rech, S.; Krafft, T.; Macy, J. Purification and characterization of the selenate reductase from Thauera selenatis. J. Biol. Chem. 1997, 272, 23765–23768. [Google Scholar] [CrossRef] [PubMed]
- Etezad, S.; Khajeh, K.; Soudi, M.; Ghazvini, P.; Dabirmanesh, B. Evidence on the presence of two distinct enzymes responsible for the reduction of selenate and tellurite in Bacillus sp. STG-83. Enzym. Microb. Technol. 2009, 45, 1–6. [Google Scholar] [CrossRef]
- Rathgeber, C.; Yurkova, N.; Stackebrandt, E.; Schumann, P.; Humphrey, E.; Beatty, T.; Yurkov, V. Metalloid reducing bacteria isolated from deep ocean hydrothermal vents of the Juan de Fuca ridge, Pseudoalteromonas telluritireducens sp. nov. and Pseudoalteromonas spiralis sp. nov. Curr. Microbiol. 2006, 53, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V.; Krieger, S.; Stackebrandt, E.; Beatty, T. Citromicrobium bathyomarinum, a novel aerobic bacterium isolated form deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [PubMed]
- Yurkov, V.; Csotonyi, J. Aerobic anoxygenic phototrophs and heavy metalloid reducers from extreme environments. Recent Res. Dev. Bacteriol. 2003, 1, 247–300. [Google Scholar]
- Yurkov, V.; Gorlenko, V. Erythrobacter sibiricus sp. nov., a new freshwater aerobic bacterial species containing bacteriochlorophyll a. Microbiology 1990, 59, 85–89. [Google Scholar]
- Yurkov, V.; Gorlenko, V. New species of aerobic bacteria from the genus Erythromicrobium containing bacteriochlorophyll a. Mikrobiologiya 1992, 61, 163–168. [Google Scholar]
- Yurkov, V.; Gorlenko, V.; Kompantseva, E. A new genus of orange-coloured bacteria containing bacteriochlorophyll a; Erythromicrobium gen. nov. Mikrobiologiya 1992, 61, 256–260. [Google Scholar]
- Yurkov, V.; Lysenko, A.; Gorlenko, V. Hybridization analysis of the classification of bacteriochlorophyll a-containing freshwater aerobic bacteria. Microbiology 1991, 60, 362–366. [Google Scholar]
- Yurkov, V.; Stackebrandt, E.; Holmes, A.; Fuerst, J.; Hugenholtz, P.; Golecki, J.; Gad’on, N.; Gorlenko, V.; Kompantseva, E.; Drews, G. Phylogenetic positions of novel aerobic bacteriochlorophyll a-containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp. nov. Int. J. Syst. Bacteriol. 1994, 44, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Drews, G. Mikrobiologisches Praktikum; Springer-Verlag: Berlin, Germany, 1983; p. 11. [Google Scholar]
- Redman, M.; Harvey, W. The precipitation behaviour of group IIB cations with oxyanions of selenium and tellurium. J. Less Common Met. 1967, 12, 395–404. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Stanly, P.E.; Williams, S.G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal. Biochem. 1969, 29, 381–392. [Google Scholar] [CrossRef]
- Maltman, C.; Yurkov, V. The impact of tellurite on highly resistant marine bacteria and strategies for its reduction. Int. J. Environ. Eng. Nat. Resour. 2014, 1, 109–119. [Google Scholar]
- Turner, R.; Weiner, J.; Taylor, D. Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology 1999, 145, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Baseman, S.; Bullen, T.; Dewald, J.; Zhang, D.; Curran, S.; Islam, F.; Beveridge, T.; Oremland, R. Formation of tellurium nanocrystals during anaerobic growth of bacteria that use Te oxyanions as respiratory electron acceptors. Appl. Environ. Microbiol. 2007, 73, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Baseman, S.; Stolz, J.; Kulp, T. Enrichment and isolation of Bacillus beveridgei sp. Nov., a facultative anaerobic haloalkaliphile from Mono Lake, California, that respires oxyanions of tellurium, selenium, and arsenic. Extremophiles 2009, 13, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier-Vogel, E.M.; Ung, S.; Turner, R.J. In vivo 31P nuclear magnetic resonance investigation of tellurite toxicity in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 7324–7347. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.; Rice, C.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.; Alston, M.; Stringer, M.; Betts, R.; Baranyi, J.; Peck, M.; Hinton, J. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2011, 194, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Spear, J.; Figueroa, L.; Honeyman, B. Modeling the removal of uranium U(VI) from aqueous solutions in the presence on sulfate reducing bacteria. Environ. Sci. Technol. 1999, 33, 2667–2675. [Google Scholar] [CrossRef]
- Yurkov, V.; Beatty, T. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 695–724. [Google Scholar]
- Yurkov, V.; Hughes, E. Genes associated with the peculiar phenotypes of the aerobic anoxygenic phototrophs. In The Genome Evolution of Photosynthetic Bacteria; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 66, pp. 327–358. [Google Scholar]
- Commichau, F.; Gunka, K.; Landmann, J.; Stulk, J. Glutamate metabolism in Bacillus subtilis: Gene expression and enzyme activities evolved to avoid futile cycles and to allow rapid responses to perturbations of the system. J. Bacteriol. 2008, 190, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Csotonyi, J.; Maltman, C.; Swiderski, J.; Stackenbrandt, E.; Yurkov, V. Extremely “vanadiphilic” multiply metal-resistant and halophilic aerobic anoxygenic phototrophs, strains EG13 and EG8, from hypersaline springs in Canada. Extremophiles 2015, 19, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Kaplan, S. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: Characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J. Bacteriol. 1992, 174, 1505–1514. [Google Scholar] [PubMed]
- Borloo, J.; Vergauwen, B.; de Smet, L.; Brige, A.; Motte, B.; Devreese, B.; Van Beeumen, J. A kinetic approach to the dependence of dissimilatory metal reduction by Shewanella oneidensis MR-1 on the outer membrane cytochromes c OmcA and OmcB. FEBS J. 2007, 274, 3728–3738. [Google Scholar] [CrossRef] [PubMed]
- Van Marwijk, J.; Opperman, D.; Piater, L.; Van Heerden, E. Reduction of vanadium(V) by Enterobacter cloacae EV-SA01 isolated from a South African deep gold mine. Biotechnol. Lett. 2009, 31, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Silver, S. Bacterial resistances to toxic metal ions—A review. Gene 1996, 179, 9–19. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltman, C.; Yurkov, V. The Effect of Tellurite on Highly Resistant Freshwater Aerobic Anoxygenic Phototrophs and Their Strategies for Reduction. Microorganisms 2015, 3, 826-838. https://doi.org/10.3390/microorganisms3040826
Maltman C, Yurkov V. The Effect of Tellurite on Highly Resistant Freshwater Aerobic Anoxygenic Phototrophs and Their Strategies for Reduction. Microorganisms. 2015; 3(4):826-838. https://doi.org/10.3390/microorganisms3040826
Chicago/Turabian StyleMaltman, Chris, and Vladimir Yurkov. 2015. "The Effect of Tellurite on Highly Resistant Freshwater Aerobic Anoxygenic Phototrophs and Their Strategies for Reduction" Microorganisms 3, no. 4: 826-838. https://doi.org/10.3390/microorganisms3040826





