Role of NAD+-Dependent Malate Dehydrogenase in the Metabolism of Methylomicrobium alcaliphilum 20Z and Methylosinus trichosporium OB3b
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Growth Conditions
2.2. DNA Manipulations
2.3. Expression of the Mdh Genes and Purification of Recombinant MDHs
2.4. Determination of MDH Molecular Masses
2.5. Enzyme Assays
2.6. Sequence Analysis
3. Results
3.1. Expression of the Mdh Genes and Purification of MDHs
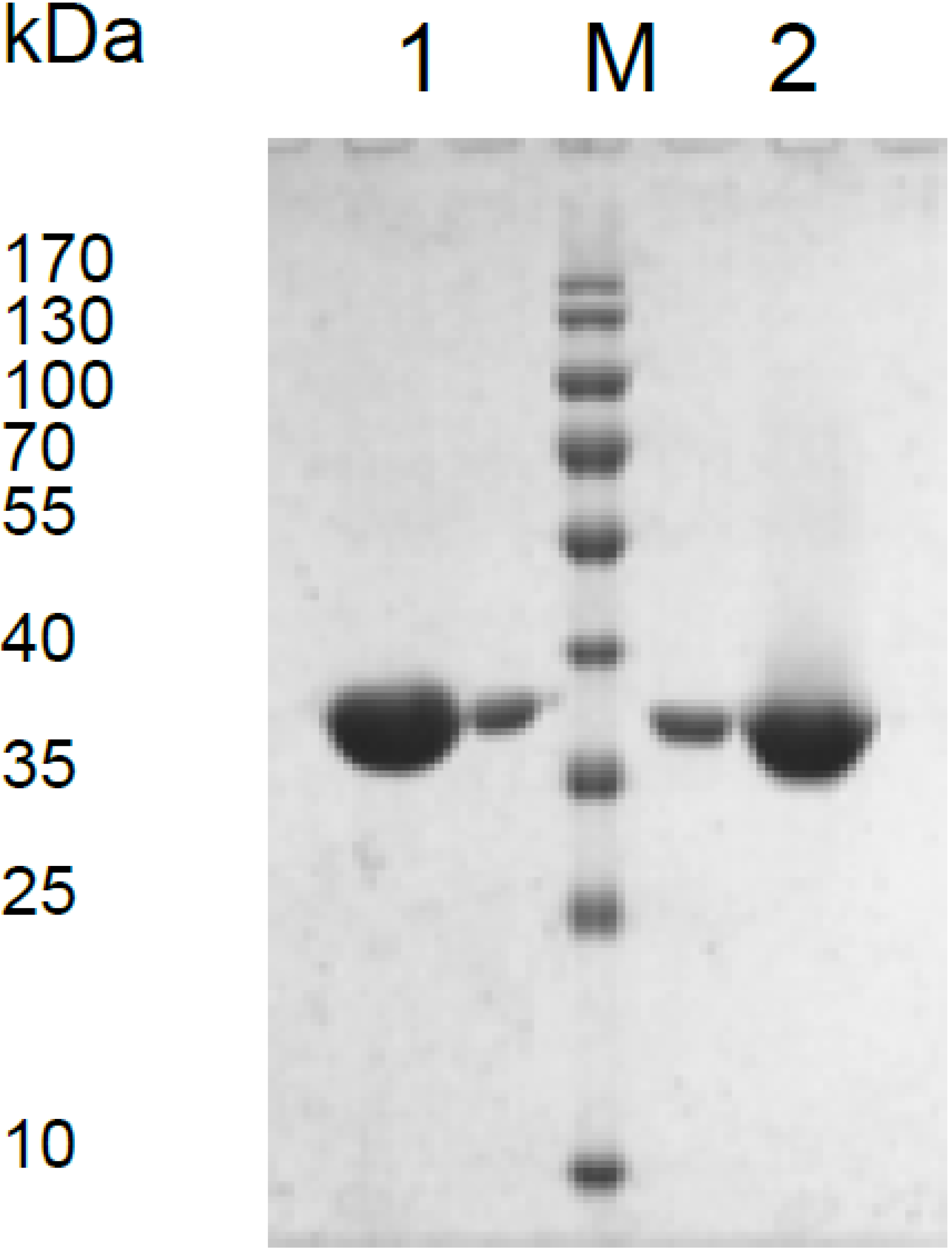
3.2. Catalytic Properties of Recombinant MDHs
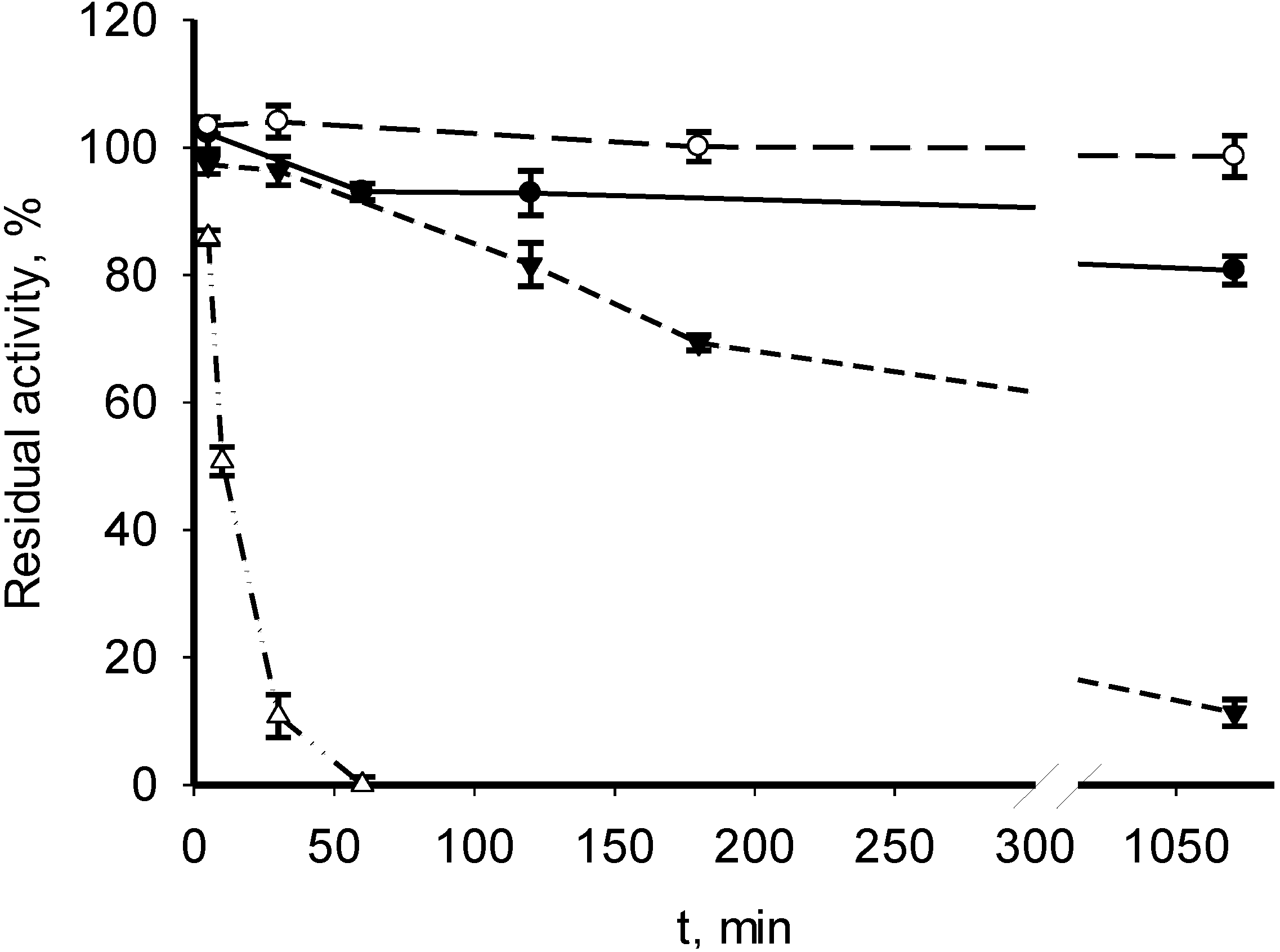
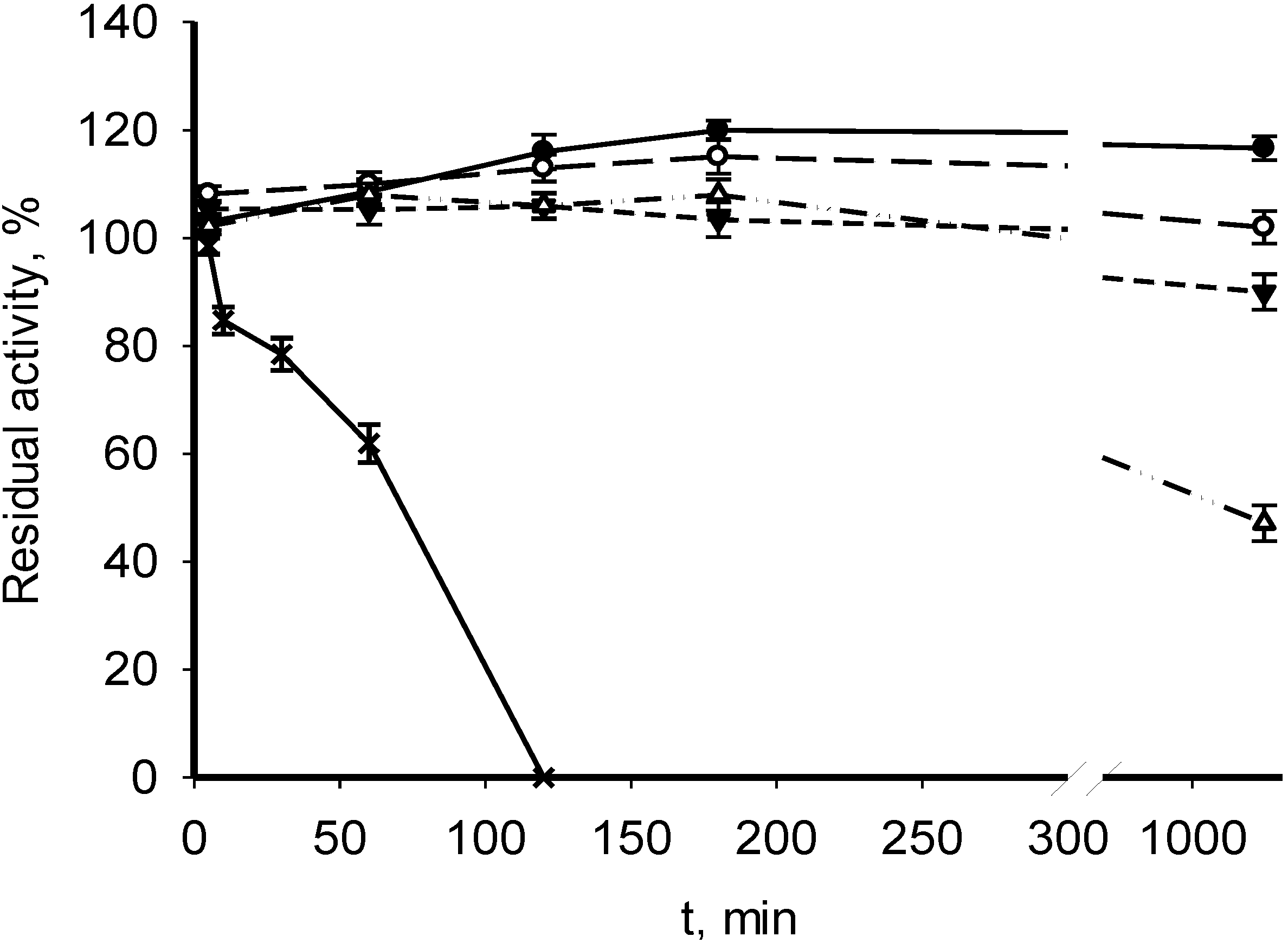
| Parameter | M. alcaliphilum 20Z | M. trichosporium OB3b | Streptomyces coelicolor A3(2) [18] | Nitrosomonas europaea [19] |
|---|---|---|---|---|
| Subunit molecular mass, kDa | 70 (35 × 2) | 170 (35 × 4) | 73 (36 × 2) | nd |
| pH opt for malate oxidation | 10 | 9.5 | nd | 8.5 |
| pH optimum for oxaloacetate reduction | 10 | 9.5 | 6.5/6.8 | 8.5 |
| Temperature optimum, °C | 60–65 | 60 | 30/50 | 55 |
| Km (mM) | ||||
| malate | 0.11 | 1.28 | 0.494 | 5 |
| oxaloacetate | 0.34 | 0.059 | 0.189 | 0.02 |
| NAD+ | 0.45 | 0.33 | 0.15 | 0.024 |
| NADH2 | 0.025 | 0.037 | 0.083 | 0.022 |
| Vmax malate oxidation (U/mg) | 15 | 78 | 4.02 | nd * |
| Vmax oxaloacetate reduction (U/mg) | 21 | 188 | 1,600 | nd * |
| kcat malate (1/s) | 621 | 516 | 471 | nd |
| kcat oxaloacetate (1/s) | 870 | 1141 | 1870 | nd |
| kcat/Km malate (1/s mM) | 5648 | 403 | 9.53 | nd |
| kcat/Km oxaloacetate (1/s mM) | 2558 | 19,344 | 10,000 | nd |
| Inhibitors | No | No | Zn2+, Со2+, Fe2+ | Zn2+, Fe2+, Mn2+ |
| Activators | No | No | No | AMP, Cu2+ |
3.3. Phylogenetic Positions of MDHs from M. alcaliphilum 20Z and M. trichosporium OB3b
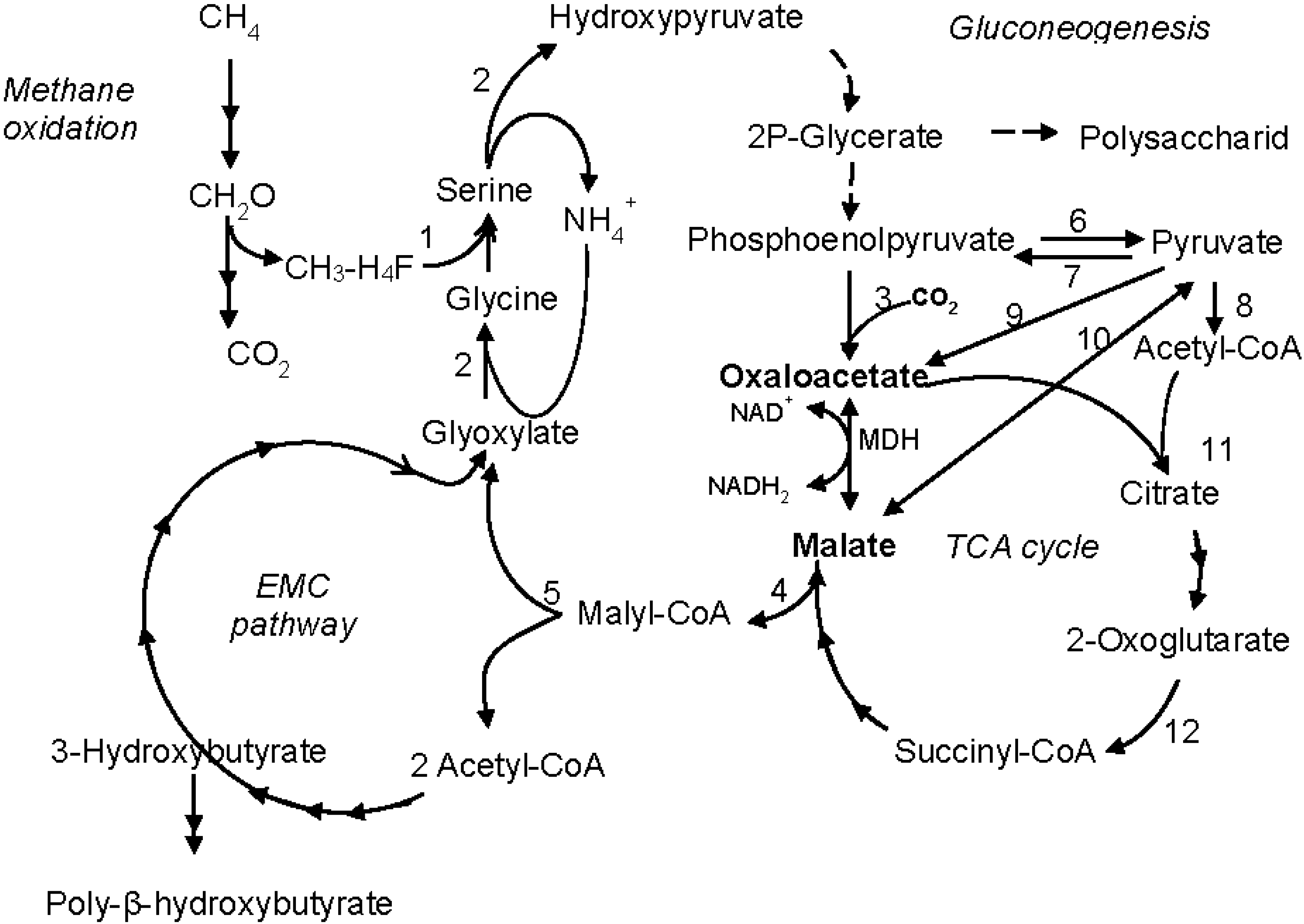
4. Discussion
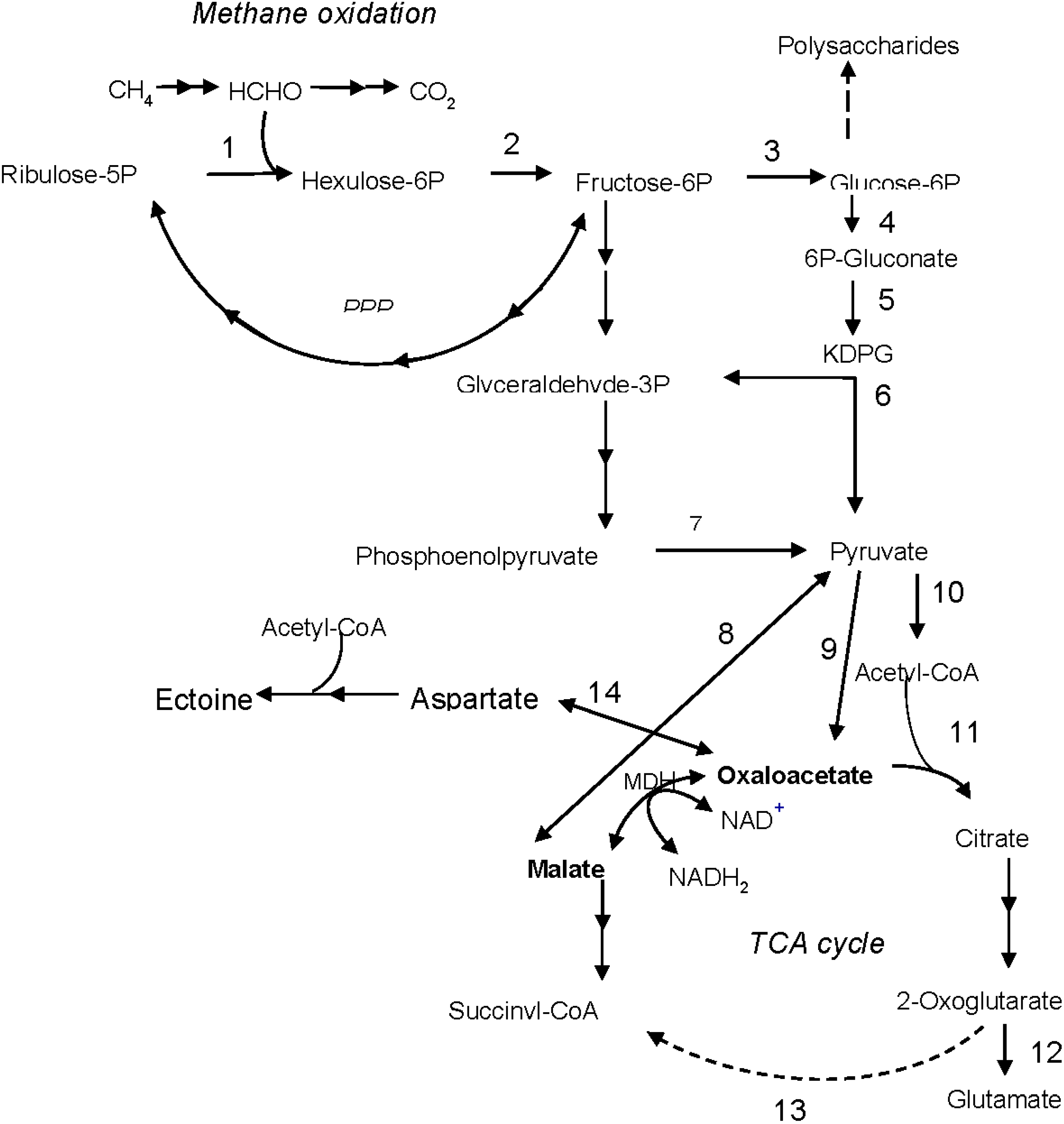
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [PubMed]
- Murrell, J.C.; Jetten, M.S.M. The microbial methane cycle. Environ. Microbiol. Rep. 2009, 1, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Trotsenko, Y.A.; Murrell, J.C. Metabolic aspects of obligate aerobic methanotrophy. Adv. Appl. Microbiol. 2008, 63, 183–229. [Google Scholar] [PubMed]
- Ward, N.; Larsen, Ø.; Sakwa, J.; Bruseth, L.; Khouri, H.; Durkin, A.S.; Dimitrov, G.; Jiang, L.; Scanlan, D.; Kang, K.H.; et al. Genomic insights into methanotrophy: The complete genome sequence of Methylococcus capsulatus (Bath). PLoS Biology. 2004, 2, 1616–1628. [Google Scholar]
- Khmelenina, V.N.; Shchukin, V.N.; Reshetnikov, A.S.; Mustakhimov, I.I.; Suzina, N.E.; Eshinimaev, B.T.; Trotsenko, Y.A. Structural and functional features of methanotrophs from hypersaline and alkaline lakes. Microbiology 2010, 79, 472–482. [Google Scholar] [CrossRef]
- Vuilleumier, S.; Khmelenina, V.N.; Bringel, F.; Reshetnikov, A.S.; Lajus, A.; Mangenot, S.; Rouy, Z.; Op den Camp, H.J.M.; Jetten, M.S.M.; Dispirito, A.A.; et al. Genome sequence of the haloalkaliphilic methanotrophic bacterium Methylomicrobium alcaliphilum 20Z. J. Bacteriol. 2012, 194, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhnaya, M.G.; Yang, S.; Rozova, O.N.; Smalley, N.E.; Clubb, J.; Lamb, A.; Gowda, G.A.; Raftery, D.; Fu, Y.; Bringel, F.; et al. Highly efficient methane biocatalysis revealed in methanotrophic bacterium. Nat. Commun. 2013, 4, 2785. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, V.N.; Trotsenko, Y.A. Multiple enzymic lesions in obligate methanotrophic bacteria. FEMS Microbiol. Lett. 1982, 13, 237–242. [Google Scholar] [CrossRef]
- Khmelenina, V.N.; Kalyuzhnaya, M.G.; Sakharovsky, V.G.; Suzina, N.E.; Trotsenko, Y.A.; Gottschalk, G. Osmoadaptation in halophilic and alkaliphilic methanotrophs. Arch. Microbiol. 1999, 172, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 2001. [Google Scholar]
- Kalyuzhnaya, M.; Khmelenina, V.N.; Kotelnikova, S.; Holmquist, L.; Pedersen, K.; Trotsenko, Y.A. Methylomonas scandinavica sp. nov., a new methanotrophic psychrotrophic bacterium isolated from deep igneous rock ground water of Sweden. Syst. Appl. Microbiol. 1999, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, A.S.; Rozova, O.N.; Khmelenina, V.N.; Mustakhimov, I.I.; Beschastny, A.P.; Murrell, J.C.; Trotsenko, Y.A. Characterization of the pyrophosphate-dependent 6-phosphofructokinase from Methylococcus capsulatus Bath. FEMS Microbiol. Lett. 2008, 288, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.G. Stable pattern formation and determination of molecular size by pore-limit electrophoresis. Anal. Chem. 1969, 41, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Boil. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Madern, D. Molecular evolution within the l-malate and l-lactate dehydrogenase super-family. J. Mol. Evol. 2002, 54, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, J.H.; Cho, E.J.; Youn, H.D. A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress. Cell Death Differ. 2009, 16, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.D.; Song, P.; Cao, Z.Y.; Wang, P.; Zhu, G.P. Alteration of coenzyme specificity of malate dehydrogenase from Streptomyces coelicolor A3(2) by site-directed mutagenesis. Genet. Mol. Res. 2014, 13, 5758–5766. [Google Scholar] [CrossRef] [PubMed]
- Deutch, C.E. l-malate dehydrogenase activity in the reductive arm of the incomplete citric acid cycle of Nitrosomonas europaea. Antonie van Leeuwenhoek 2013, 104, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Keithly, J.S. α-proteobacterial relationship of apicomplexan lactate and malate dehydrogenases. J. Eukaryot. Microbiol. 2002, 49, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Roger, A.J.; Morrison, H.G.; Sogin, M.L. Primary structure and phylogenetic relationships of a malate dehydrogenase gene from Giardia lamblia. J. Mol. Evol. 1999, 6, 750–755. [Google Scholar] [CrossRef]
- Yang, S.; Matsen, J.B.; Konopka, M.; Green-Saxena, A.; Clubb, J.; Sadilek, M.; Orphan, V.J.; Beck, D.; Kalyuzhnaya, M.G. Global molecular analyses of methane metabolism in methanotrophic Alphaproteobacterium, Methylosinus trichosporium OB3b. Part II. Metabolomics and 13C-labeling study. Front. Microbiol. 2013, 4, 70. [Google Scholar] [PubMed]
- Matsen, J.B.; Yang, S.; Stein, L.Y.; Beck, D.; Kalyuzhnaya, M.G. Global molecular analyses of methane metabolism in methanotrophic Alphaproteobacterium, Methylosinus trichosporium OB3b. Part I. Transcriptomic study. Front. Microbiol. 2013, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Falaleeva, M.I.; Klimova, M.A.; Kompantseva, E.I. Physicochemical properties of malate dehydrogenase from the bacterium Rhodopseudomonas palustris strain f8pt. Biochemistry 2006, 71, 692–695. [Google Scholar] [PubMed]
- Eprintsev, A.T.; Falaleeva, M.I.; Klimova, M.A.; Parfenova, N.V. Isolation and properties of malate dehydrogenase from meso- and thermophilic bacteria. Appl. Biokhem. Microbiol. 2006, 42, 274–278. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Falaleeva, M.I.; Arabtseva, M.A.; Parfenova, I.V. Structural-functional transformation of the malate dehydrogenase system of the bacterium Sphaerotilus sp. strain D-507 depending on nutritional mode. Izv. Akad. Nauk. Ser. Biol. 2009, 3, 269–275. [Google Scholar] [PubMed]
- Ge, Y.D.; Cao, Z.Y.; Wang, P.; Song, P.; Zhu, G.P.; Zhu, Y.M. Identification and biochemical characterization of thermostable malate dehydrogenase from the mesophilic Streptomyces coelicolor A3(2). Biocsi. Biochem. Biotechnol. 2010, 74, 2194–2201. [Google Scholar] [CrossRef]
- Langelandsvik, A.S.; Steen, I.H.; Birkeland, N.-K.; Lien, T. Properties and primary structure of a thermostable l-malate dehydrogenase from Archaeoglobus fulgidus. Arch. Microbiol. 1997, 168, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Wang, B.J.; Ge, Y.D.; Pan, W.; Wang, J.; Xu, L.; Liu, A.M.; Zhu, G.P. Expression and identification of a thermostable malate dehydrogenase from multicellular prokaryote Streptomyces avermitilis MA-4680. Mol. Biol. Rep. 2011, 38, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, A.S.; Khmelenina, V.N.; Trotsenko, Y.A. Characterization of the ectoine biosynthesis genes of haloalkalitolerant obligate methanotroph “Methylomicrobium alcaliphilum 20Z”. Arch. Microbiol. 2006, 184, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Madern, D.; Ebel, C.; Dale, H.A.; Lien, T.; Steen, I.H.; Birkeland, N.K.; Zaccai, G. Differences in the oligomeric states of the (LDH-like) l-MalDH from the hyperthermophilic archaea Methanococcus jannaschii and Archaeoglobus fulgidus. Biochemistry 2001, 40, 10310–10316. [Google Scholar] [CrossRef] [PubMed]
- Khmelenina, V.I.; Kalyuzhnaya, M.G.; Starostina, N.G.; Suzina, N.E.; Trotsenko, Y.A. Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda lakes. Curr. Microbiol. 1997, 35, 257–261. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozova, O.N.; Khmelenina, V.N.; Bocharova, K.A.; Mustakhimov, I.I.; Trotsenko, Y.A. Role of NAD+-Dependent Malate Dehydrogenase in the Metabolism of Methylomicrobium alcaliphilum 20Z and Methylosinus trichosporium OB3b. Microorganisms 2015, 3, 47-59. https://doi.org/10.3390/microorganisms3010047
Rozova ON, Khmelenina VN, Bocharova KA, Mustakhimov II, Trotsenko YA. Role of NAD+-Dependent Malate Dehydrogenase in the Metabolism of Methylomicrobium alcaliphilum 20Z and Methylosinus trichosporium OB3b. Microorganisms. 2015; 3(1):47-59. https://doi.org/10.3390/microorganisms3010047
Chicago/Turabian StyleRozova, Olga N., Valentina N. Khmelenina, Ksenia A. Bocharova, Ildar I. Mustakhimov, and Yuri A. Trotsenko. 2015. "Role of NAD+-Dependent Malate Dehydrogenase in the Metabolism of Methylomicrobium alcaliphilum 20Z and Methylosinus trichosporium OB3b" Microorganisms 3, no. 1: 47-59. https://doi.org/10.3390/microorganisms3010047





