Carbonic Anhydrase from Porphyromonas Gingivalis as a Drug Target
Abstract
:1. Introduction
1.1. Virulence of Porphyromonas Gingivalis
1.2. Carbonic Anhydrases
1.3. Carbonic Anhydrase Inhibitors
1.3.1. Anions
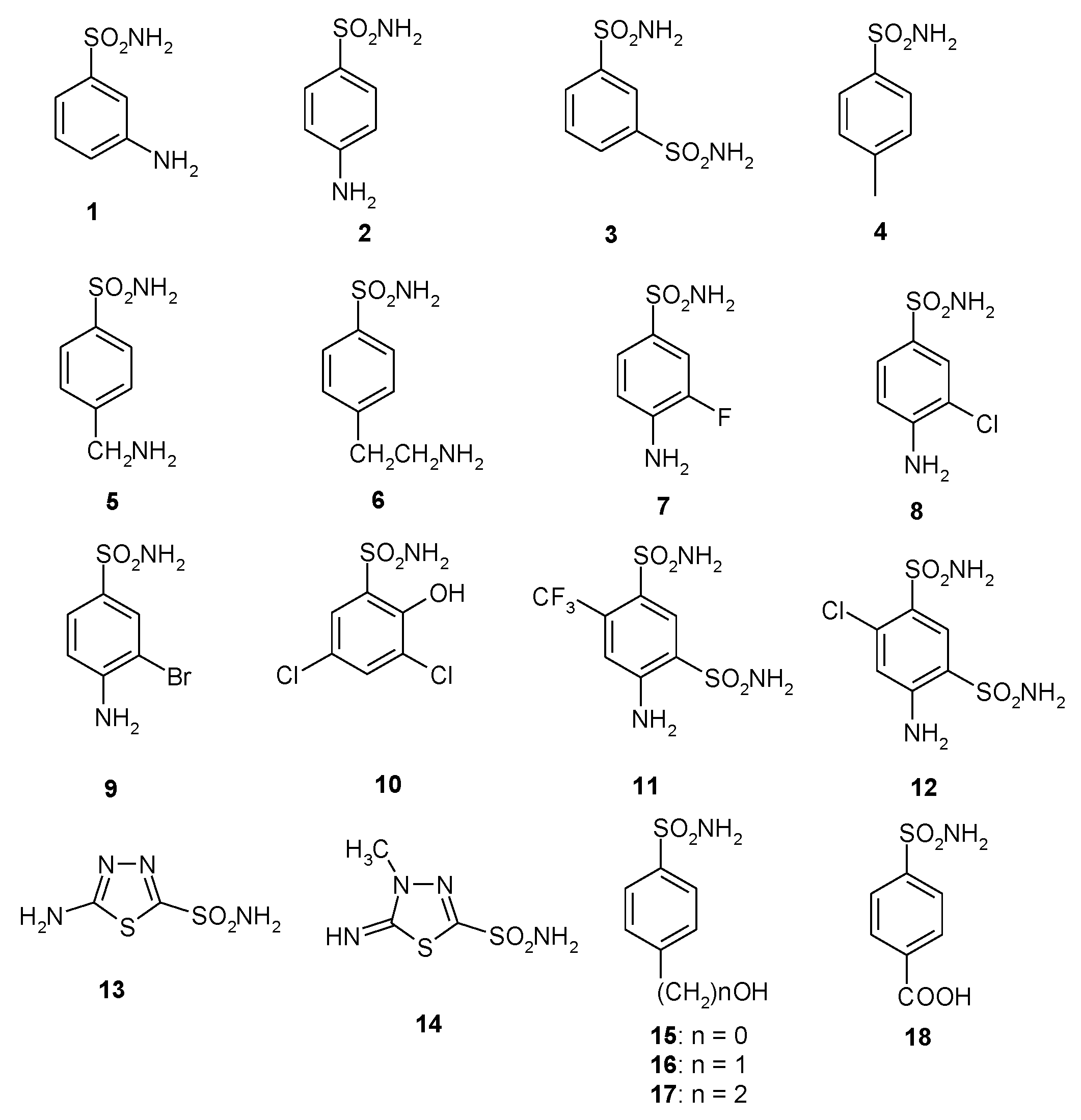
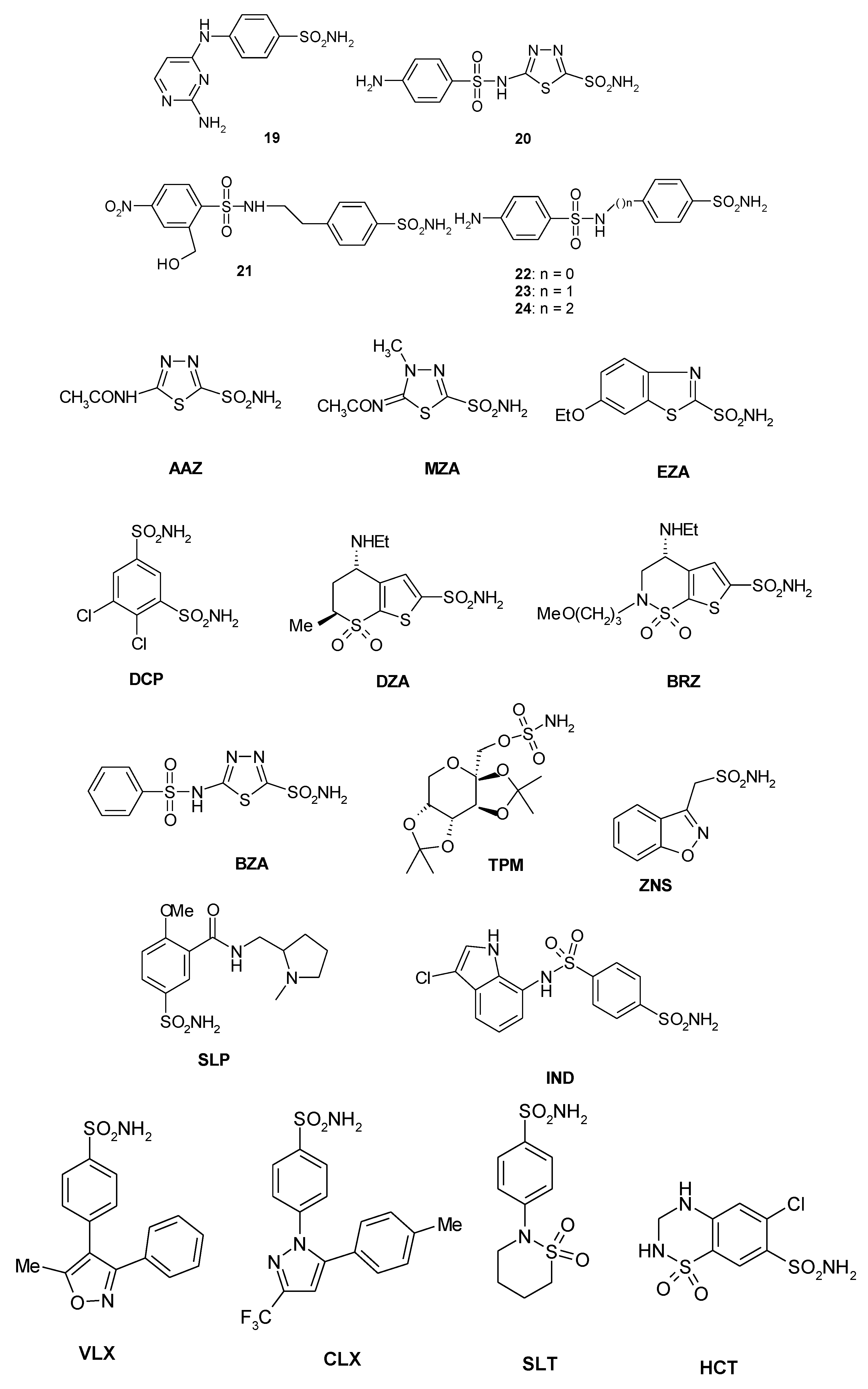
1.3.2. Sulfonamides
2. Sequence Analysis
3. Biochemical Characterization
4. Catalytic Properties
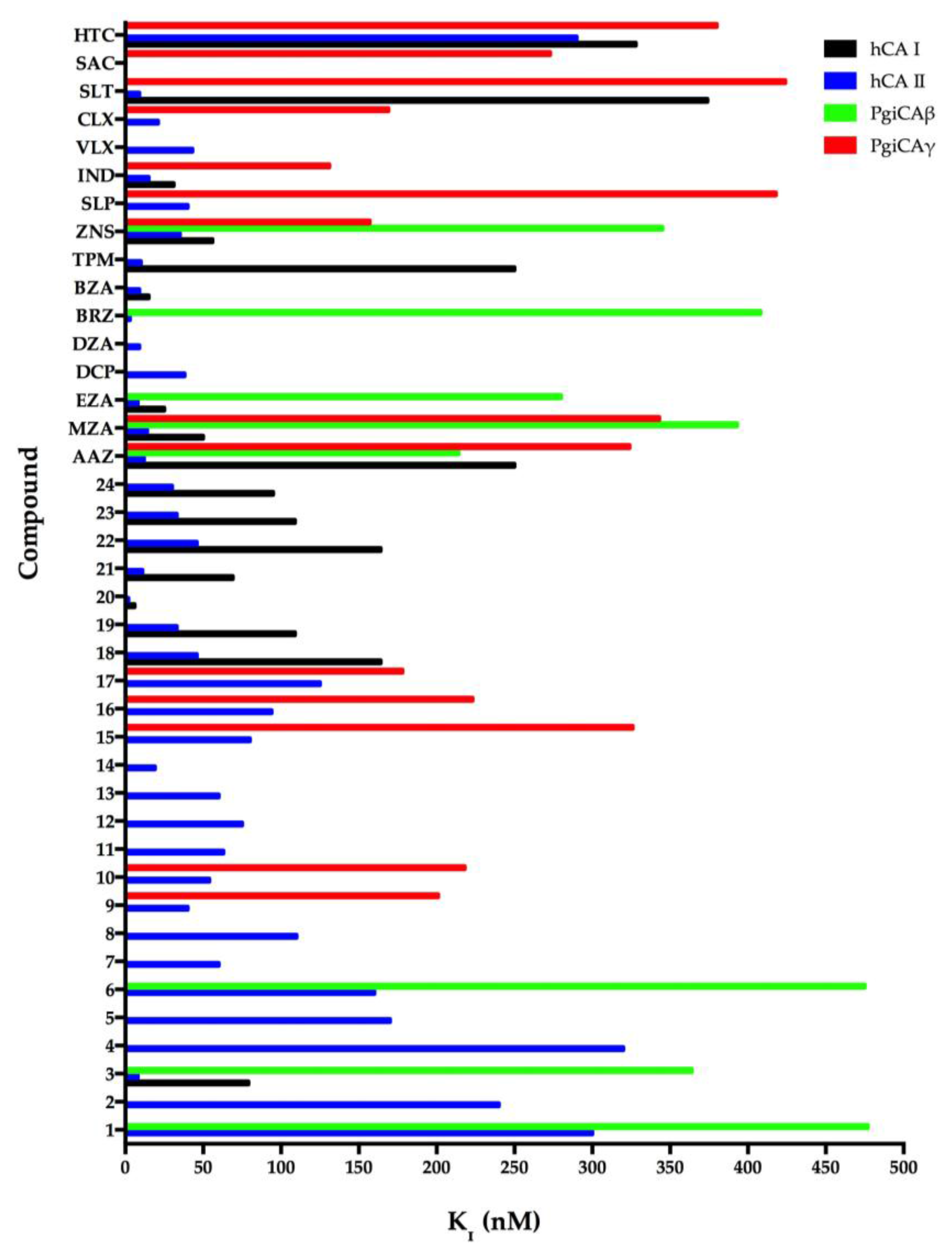
5. Sulfonamide Inhibition Studies
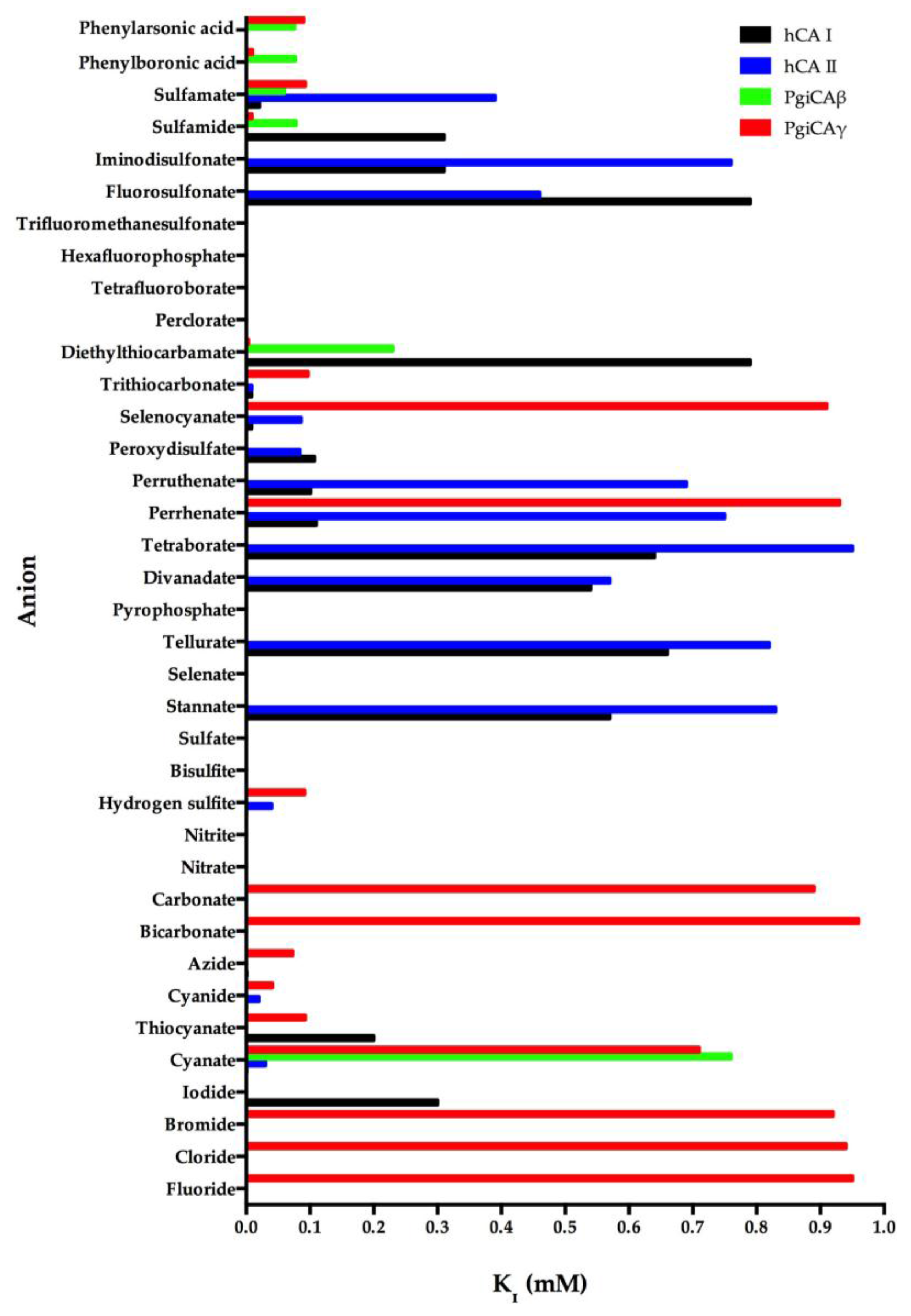
6. Anion Inhibition Studies
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Verstraeten, N.; Michiels, J. New approaches to combat porphyromonas gingivalis biofilms. J. Oral Microbiol. 2017, 9, 1300366. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Vullo, D.; Osman, S.M.; AlOthman, Z.; Carginale, V.; Supuran, C.T.; Capasso, C. A new procedure for the cloning, expression and purification of the β-carbonic anhydrase from the pathogenic yeast Malassezia globosa, an anti-dandruff drug target. J. Enzym. Inhib. Med. Chem. 2016, 31, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, expression, purification and sulfonamide inhibition profile of the complete domain of the eta-carbonic anhydrase from Plasmodium falciparum. Bioorgan. Med. Chem Lett. 2016, 26, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition profiles of the complete domain of the eta-carbonic anhydrase from Plasmodium falciparum. Bioorgan. Med. Chem. 2016, 24, 4410–4414. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J. Enzym. Inhib. Med. Chem. 2016, 31, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, G.; Angeli, A.; D’Alba, F.; Bruno, A.; Pieroni, M.; Vullo, D.; De Luca, V.; Capasso, C.; Supuran, C.T.; Costantino, G. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. Chemmedchem 2016, 11, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition profiles of alpha-, beta- and gamma-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorgan. Med. Chem. 2016, 24, 3413–3417. [Google Scholar] [CrossRef] [PubMed]
- Abdel Gawad, N.M.; Amin, N.H.; Elsaadi, M.T.; Mohamed, F.M.; Angeli, A.; De Luca, V.; Capasso, C.; Supuran, C.T. Synthesis of 4-(thiazol-2-ylamino)-benzenesulfonamides with carbonic anhydrase I, II and IX inhibitory activity and cytotoxic effects against breast cancer cell lines. Bioorgan. Med. Chem. 2016, 24, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr. Top. Med. Chem. 2016, 16, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Comparison of the sulfonamide inhibition profiles of the alpha-, beta- and gamma-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorgan. Med. Chem. Lett. 2016, 26, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr. Med. Chem. 2015, 22, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Bacterial, fungal and protozoan carbonic anhydrases as drug targets. Expert Opin. Ther. Targets 2015, 19, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta- and gamma-carbonic anhydrases from bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Iandolo, E.; Supuran, C.T.; Capasso, C. Protonography, a powerful tool for analyzing the activity and the oligomeric state of the gamma-carbonic anhydrase identified in the genome of Porphyromonas gingivalis. Bioorgan. Med. Chem. 2015, 23, 3747–3750. [Google Scholar] [CrossRef] [PubMed]
- Prete, S.D.; Vullo, D.; Osman, S.M.; Scozzafava, A.; AlOthman, Z.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition study of the carbonic anhydrases from the bacterial pathogen Porphyromonas gingivalis: The beta-class (PgiCAb) versus the gamma-class (pgica) enzymes. Bioorgan. Med. Chem. 2014, 22, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Ceruso, M.; Del Prete, S.; AlOthman, Z.; Osman, S.M.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Synthesis of sulfonamides with effective inhibitory action against Porphyromonas gingivalis gamma-carbonic anhydrase. Bioorgan. Med. Chem. Lett. 2014, 24, 4006–4010. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. A highly catalytically active gamma-carbonic anhydrase from the pathogenic anaerobe Porphyromonas gingivalis and its inhibition profile with anions and small molecules. Bioorgan. Med. Chem. Lett. 2013, 23, 4067–4071. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Osman, S.M.; De Luca, V.; Scozzafava, A.; Alothman, Z.; Supuran, C.T.; Capasso, C. Sulfonamide inhibition studies of the gamma-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis. Bioorgan. Med. Chem. Lett. 2014, 24, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Osman, S.M.; Scozzafava, A.; Alothman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition study of the beta-class carbonic anhydrase (PgiCAb) from the oral pathogen Porphyromonas gingivalis. Bioorgan. Med. Chem. Lett. 2014, 24, 4402–4406. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Ceruso, M.; Al-Tamimi, A.M.; Del Prete, S.; Supuran, C.T.; Capasso, C. Inhibition studies of quinazoline-sulfonamide derivatives against the gamma-CA (PgiCA) from the pathogenic bacterium, porphyromonas gingivalis. J. Enzym. Inhib. Med. Chem. 2015, 30, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Vullo, D.; Scozzafava, A.; Carginale, V.; Supuran, C.T.; Capasso, C. Biochemical characterization of the gamma-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis, PgiCAa. J. Enzym. Inhib. Med. Chem. 2014, 29, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. Inhibition of Bacterial Carbonic Anhydrases as a Novel Approach to Escape Drug Resistance. Curr. Top. Med. Chem. 2017, 17, 1237–1248. [Google Scholar]
- Del Prete, S.; Vullo, D.; De Luca, V.; AlOthman, Z.; Osman, S.M.; Supuran, C.T.; Capasso, C. Biochemical characterization of recombinant beta-carbonic anhydrase (PgiCAb) identified in the genome of the oral pathogenic bacterium Porphyromonas gingivalis. J. Enzym. Inhib. Med. Chem. 2015, 30, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ozensoy Guler, O.; Capasso, C.; Supuran, C.T. A magnificent enzyme superfamily: Carbonic anhydrases, their purification and characterization. J. Enzym. Inhib. Med. Chem. 2016, 31, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; Ferraroni, M.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Sulfonamide inhibition studies of the beta-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorgan. Med. Chem. 2016, 24, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; De Simone, G.; Supuran, C.T.; Capasso, C. Cloning, expression and purification of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum. J. Enzym. Inhib. Med. Chem. 2016, 31, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. The eta-class carbonic anhydrases as drug targets for antimalarial agents. Expert Opin. Ther. Targets 2015, 19, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Supuran, C.T.; Capasso, C. Protonography, a technique applicable for the analysis of eta-carbonic anhydrase activity. J. Enzym. Inhib. Med. Chem. 2015, 30, 920–924. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Di Fiore, A.; Capasso, C.; Supuran, C.T. The zinc coordination pattern in the eta-carbonic anhydrase from Plasmodium falciparum is different from all other carbonic anhydrase genetic families. Bioorgan. Med. Chem. Lett. 2015, 25, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Del Prete, S.; Supuran, C.T.; Capasso, C. Protonography, a new technique for the analysis of carbonic anhydrase activity. J. Enzym. Inhib. Med. Chem. 2015, 30, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M.; Abdel-Aziz, H.A.; Vullo, D.; Al-Tamimi, A.M.; Awaad, A.S.; Mohamed, M.A.; Capasso, C.; Supuran, C.T. Inhibition of human carbonic anhydrase isozymes I, II, IX and XII with a new series of sulfonamides incorporating aroylhydrazone-, [1,2,4]triazolo[3,4-b][1,3,4]thiadiazinyl- or 2-(cyanophenylmethylene)-1,3,4-thiadiazol-3(2H)-yl moieties. J. Enzym. Inhib. Med. Chem. 2015, 30, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, S.; Nakajima, K.; Nagasato, C.; Tsuji, Y.; Miyatake, A.; Matsuda, Y. Thylakoid luminal theta-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. USA 2016, 113, 9828–9833. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; Fisher, G.M.; Andrews, K.T.; Poulsen, S.A.; Capasso, C.; Supuran, C.T. Discovery of a new family of carbonic anhydrases in the malaria pathogen plasmodium falciparum–The η-carbonic anhydrases. Bioorgan. Med. Chem. Lett. 2014, 24, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Pinard, M.A.; Lotlikar, S.R.; Boone, C.D.; Vullo, D.; Supuran, C.T.; Patrauchan, M.A.; McKenna, R. Structure and inhibition studies of a type ii beta-carbonic anhydrase PsCA3 from Pseudomonas aeruginosa. Bioorgan. Med. Chem. 2015, 23, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; Del Prete, S.; Vullo, D.; Capasso, C.; Supuran, C.T. Crystal structure and kinetic studies of a tetrameric type ii beta-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Monti, S.M.; Alterio, V.; Buonanno, M.; De Luca, V.; Rossi, M.; Carginale, V.; Supuran, C.T.; Capasso, C.; Di Fiore, A. Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, sazca from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorgan. Med. Chem. Lett. 2015, 25, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Zolnowska, B.; Slawinski, J.; Pogorzelska, A.; Chojnacki, J.; Vullo, D.; Supuran, C.T. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series n-substituted n'-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes i and ii and the transmembrane tumor-associated isozymes IX and XII. Eur. J. Med. Chem. 2014, 71, 135–147. [Google Scholar] [PubMed]
- De Luca, L.; Ferro, S.; Damiano, F.M.; Supuran, C.T.; Vullo, D.; Chimirri, A.; Gitto, R. Structure-based screening for the discovery of new carbonic anhydrase VII inhibitors. Eur. J. Med. Chem. 2014, 71, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Capasso, C.; De Luca, V.; Monti, S.M.; Carginale, V.; Supuran, C.T.; Scozzafava, A.; Pedone, C.; Rossi, M.; De Simone, G. X-ray structure of the first `extremo-alpha-carbonic anhydrase', a dimeric enzyme from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases--an overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Mahon, B.P.; Cruzeiro, V.W.; Cornelio, B.; Laronze-Cochard, M.; Ceruso, M.; Sapi, J.; Rance, G.A.; Khlobystov, A.N.; Fontana, A.; et al. Structure-activity relationships of benzenesulfonamide-based inhibitors towards carbonic anhydrase isoform specificity. Chembiochem Eur. J. Chem. Biol. 2017, 18, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Langella, E.; Viparelli, F.; Vullo, D.; Ascione, G.; Dathan, N.A.; Morel, F.M.; Supuran, C.T.; De Simone, G.; Monti, S.M. Structural and inhibition insights into carbonic anhydrase cdca1 from the marine diatom Thalassiosira weissflogii. Biochimie 2012, 94, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Buzas, G.M.; Supuran, C.T. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puscas (1932–2015). J. Enzym. Inhib. Med. Chem. 2016, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Sulfa and trimethoprim-like drugs–antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J. Enzym. Inhib. Med. Chem. 2014, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Anti-infective carbonic anhydrase inhibitors: A patent and literature review. Expert Opin. Ther. Pat. 2013, 23, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Onishi, S.; Takeuchi, H.; Supuran, C.T. The alpha and beta classes carbonic anhydrases from Helicobacter pylori as novel drug targets. Curr. Pharm. Des. 2008, 14, 622–630. [Google Scholar] [PubMed]
- Morishita, S.; Nishimori, I.; Minakuchi, T.; Onishi, S.; Takeuchi, H.; Sugiura, T.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Cloning, polymorphism, and inhibition of beta-carbonic anhydrase of Helicobacter pylori. J. Gastroenterol. 2008, 43, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Kohsaki, T.; Onishi, S.; Takeuchi, H.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: The beta-carbonic anhydrase from helicobacter pylori is a new target for sulfonamide and sulfamate inhibitors. Bioorgan. Med. Chem. Lett. 2007, 17, 3585–3594. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Vullo, D.; Minakuchi, T.; Morimoto, K.; Onishi, S.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Cloning and sulfonamide inhibition studies of a carboxyterminal truncated alpha-carbonic anhydrase from Helicobacter pylori. Bioorgan. Med. Chem. Lett. 2006, 16, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Morimoto, K.; Sano, S.; Onishi, S.; Takeuchi, H.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: DNA cloning and inhibition studies of the alpha-carbonic anhydrase from Helicobacter pylori, a new target for developing sulfonamide and sulfamate gastric drugs. J. Med. Chem. 2006, 49, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Isik, S.; Del Prete, S.; De Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. Anion inhibition studies of the alpha-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorgan. Med. Chem. Lett. 2013, 23, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; De Luca, V.; Scozzafava, A.; Carginale, V.; Supuran, C.T.; Capasso, C. Biochemical properties of a new alpha-carbonic anhydrase from the human pathogenic bacterium, vibrio cholerae. J. Enzym. Inhib. Med. Chem. 2014, 29, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Isik, S.; Vullo, D.; De Luca, V.; Carginale, V.; Scozzafava, A.; Supuran, C.T.; Capasso, C. DNA cloning, characterization, and inhibition studies of an alpha-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. J. Med. Chem. 2012, 55, 10742–10748. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Moshfegh, A.P.; Sachs, G.; Scott, D.R. The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J. Bacteriol. 2005, 187, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Weeks, D.L.; Wen, Y.; Marcus, E.A.; Scott, D.R.; Melchers, K. Acid acclimation by Helicobacter pylori. Physiology 2005, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Cobaxin, M.; Martinez, H.; Ayala, G.; Holmgren, J.; Sjoling, A.; Sanchez, J. Cholera toxin expression by El Tor Vibrio cholerae in shallow culture growth conditions. Microb. Pathog. 2014, 66, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Abuaita, B.H.; Withey, J.H. Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing toxt activity. Infect. Immun. 2009, 77, 4111–4120. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.; Ouahrani-Bettache, S.; Montero, J.L.; Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Winum, J.Y.; Kohler, S.; Supuran, C.T. A new beta-carbonic anhydrase from Brucella suis, its cloning, characterization, and inhibition with sulfonamides and sulfamates, leading to impaired pathogen growth. Bioorgan. Med. Chem. 2011, 19, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Modak, J.K.; Liu, Y.C.; Machuca, M.A.; Supuran, C.T.; Roujeinikova, A. Structural basis for the inhibition of Helicobacter pylori alpha-carbonic anhydrase by sulfonamides. PLoS ONE 2015, 10, e0127149. [Google Scholar] [CrossRef]
- Vullo, D.; De Luca, V.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Osman, S.M.; AlOthman, Z.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of the gamma-carbonic anhydrase from the antarctic bacterium Colwellia psychrerythraea. Bioorgan. Med. Chem. Lett. 2016, 26, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Otten, H. Domagk and the development of the sulphonamides. J. Antimicrob. Chemother. 1986, 17, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.; Somers, D.O.; Champness, J.N.; Bryant, P.K.; Rosemond, J.; Stammers, D.K. Crystal structure of the anti-bacterial sulfonamide drug target dihydropteroate synthase. Nat. Struct. Biol. 1997, 4, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev. Neurother. 2015, 15, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Zimmerman, S.A.; Scozzafava, A.; Ferry, J.G.; Supuran, C.T. Carbonic anhydrase activators: Activation of the archaeal beta-class (Cab) and gamma-class (CAM) carbonic anhydrases with amino acids and amines. Bioorgan. Med. Chem. Lett. 2008, 18, 6194–6198. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.A.; Ferry, J.G.; Supuran, C.T. Inhibition of the archaeal beta-class (Cab) and gamma-class (CAM) carbonic anhydrases. Curr. Top. Med. Chem. 2007, 7, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Tripp, B.C.; Bell, C.B., 3rd; Cruz, F.; Krebs, C.; Ferry, J.G. A role for iron in an ancient carbonic anhydrase. J. Biol. Chem. 2004, 279, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.; Innocenti, A.; Casini, A.; Ferry, J.G.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the prokariotic beta and gamma-class enzymes from archaea with sulfonamides. Bioorgan. Med. Chem. Lett. 2004, 14, 6001–6006. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Fisher, G.M.; Andrews, K.T.; Poulsen, S.A.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of the eta-class carbonic anhydrase from the malaria pathogen Plasmodium falciparum. Bioorgan. Med. Chem. 2015, 23, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; De Luca, V.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of the gamma-carbonic anhydrase from the antarctic bacterium Pseudoalteromonas haloplanktis. Bioorgan. Med. Chem. Lett. 2015, 25, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; De Luca, V.; Del Prete, S.; Carginale, V.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition studies of the gamma-carbonic anhydrase from the antarctic cyanobacterium Nostoc commune. Bioorgan. Med. Chem. 2015, 23, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, N.; DeLuca, V.; Isik, S.; Yildirim, H.; Kockar, F.; Capasso, C.; Supuran, C.T. Sulfonamide inhibition study of the beta-class carbonic anhydrase from the caries producing pathogen Streptococcus mutans. Bioorgan. Med. Chem. Lett. 2015, 25, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
| Organism | Enzyme Acronym | Class | kcat (s−1) | kcat/Km (M−1 × s−1) |
|---|---|---|---|---|
| Homo sapiens | hCA I | α | 2.0 × 105 | 5.0 × 107 |
| hCA II | α | 1.4 × 106 | 1.5 × 108 | |
| Flaveria bidentis | FbCA1 | β | 1.2 × 105 | 7.5 × 106 |
| Sulfurihydrogenibium azorense | SazCA | α | 4.4 × 106 | 3.5 × 108 |
| Porphyromonas gingivalis | PgiCAβ | β | 2.8 × 105 | 1.5 × 107 |
| PgiCAγ | γ | 4.1 × 105 | 5.4 × 107 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supuran, C.T.; Capasso, C. Carbonic Anhydrase from Porphyromonas Gingivalis as a Drug Target. Pathogens 2017, 6, 30. https://doi.org/10.3390/pathogens6030030
Supuran CT, Capasso C. Carbonic Anhydrase from Porphyromonas Gingivalis as a Drug Target. Pathogens. 2017; 6(3):30. https://doi.org/10.3390/pathogens6030030
Chicago/Turabian StyleSupuran, Claudiu T., and Clemente Capasso. 2017. "Carbonic Anhydrase from Porphyromonas Gingivalis as a Drug Target" Pathogens 6, no. 3: 30. https://doi.org/10.3390/pathogens6030030






