Genome-Wide Identification and Evolutionary Analysis of Sarcocystis neurona Protein Kinases
Abstract
:1. Introduction
2. Results
2.1. Sarcocystis neurona Encodes 97 Putative Kinases
2.1.1. The AGC Group
2.1.2. The CAMK Group
2.1.3. The CK1 Group
2.1.4. The CMGC Group
2.1.5. The OPK Group
2.1.6. The STE Group
2.1.7. The TKL Group
2.1.8. The aPK Group
2.2. Evolution of S. neurona Protein Kinases
3. Discussion
4. Conclusions and Future Perspectives
5. Materials and Methods
5.1. Genome-Wide Identification of Putative S. neurona PKs
5.2. Phylogenetic Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dubey, J.P.; Lindsay, D.S.; Saville, W.J.; Reed, S.M.; Granstrom, D.E.; Speer, C.A. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 2001, 95, 89–131. [Google Scholar] [CrossRef]
- Reed, S.M.; Furr, M.; Howe, D.K.; Johnson, A.L.; MacKay, R.J.; Morrow, J.K.; Pusterla, N.; Witonsky, S. Equine protozoal myeloencephalitis: An updated consensus statement with a focus on parasite biology, diagnosis, treatment, and prevention. J. Vet. Intern. Med. 2016, 30, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Howe, D.K.; Furr, M.; Saville, W.J.; Marsh, A.E.; Reed, S.M.; Grigg, M.E. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM). Vet. Parasitol. 2015, 209, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.K.; MacKay, R.J.; Reed, S.M. Equine protozoal myeloencephalitis. Vet. Clin. N. Am. Equine Pract. 2014, 30, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Colahan, P.T.; Bailey, J.E.; Cheeksa, J.P.; Jones, G.L.; Yangc, M. Effect of sulfadiazine and pyrimethamine on selected physiologic and performance parameters in athletically conditioned thoroughbred horses during an incremental exercise stress test. Vet. Ther. 2002, 3, 49–63. [Google Scholar]
- McClure, S.R.; Palma, K.G. Treatment of equine protozoal myeloencephalitis with nitazoxanide. J. Equine Vet. Sci. 1999, 19, 639–641. [Google Scholar] [CrossRef]
- Bernard, W.V.; Beech, J. Neurological examination and neurological conditions causing gait deficits. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2003; pp. 135–145. [Google Scholar]
- Warschauer, B.A.; Sondhof, A. Equine protozoal myeloencephalitis. Iowa State Univ. Vet. 1998, 60, Article 10. [Google Scholar]
- Dubremetz, J.F.; Garcia-Reguet, N.; Conseil, V.; Fourmaux, M.N. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 1998, 28, 1007–1013. [Google Scholar] [CrossRef]
- Sadak, A.; Taghy, Z.; Fortier, B.; Dubremetz, J.F. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol. Biochem. Parasitol. 1988, 29, 203–211. [Google Scholar] [CrossRef]
- Cesbron-Delauw, M.F.; Gendrin, C.; Travier, L.; Ruffiot, P.; Mercier, C. Apicomplexa in mammalian cells: Trafficking to the parasitophorous vacuole. Traffic 2008, 9, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Hanks, S.K.; Hunter, T. Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 1995, 9, 576–596. [Google Scholar] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Plowman, G.D.; Hunter, T.; Sudarsanam, S. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 2002, 27, 514–520. [Google Scholar] [CrossRef]
- Hanks, S.K.; Quinn, A.M.; Hunter, T. The protein kinase family: Conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Saavedra, D.; Barton, G.J. Classification and functional annotation of eukaryotic protein kinases. Proteins Struct. Funct. Bioinform. 2007, 68, 893–914. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Saavedra, D.; Gabaldón, T.; Barton, G.J.; Langsley, G.; Doerig, C. The kinomes of apicomplexan parasites. Microbes Infect. 2012, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Equinet, L.; Packer, J.; Doerig, C. Protein kinases of the human malaria parasite Plasmodium falciparum: The kinome of a divergent eukaryote. BMC Genom. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, N.; Krupa, A. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins Struct. Funct. Bioinform. 2005, 58, 180–189. [Google Scholar]
- Talevich, E.; Tobin, A.B.; Kannan, N.; Doerig, C. An evolutionary perspective on the kinome of malaria parasites. Philos. Trans. R. Soc. B 2012, 367, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Straschil, U.; Bateman, A.; Böhme, U.; Cherevach, I.; Gong, P.; Pain, A.; Billker, O. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 2010, 8, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Talevich, E.; Mirza, A.; Kannan, N. Structural and evolutionary divergence of eukaryotic protein kinases in Apicomplexa. BMC Evol. Biol. 2011, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Talevich, E.; Kannan, N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol. Biol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Talevich, E.; Kannan, N.; Miranda-Saavedra, D. Computational analysis of apicomplexan kinomes. In Protein Phosphorylation in Parasites: Novel Targets for Antiparasitic Intervention, 5th ed.; Doerig, C., Späth, G., Wiese, M., Eds.; Wiley-Blackwell: Weinheim, Germany, 2014; pp. 1–36. [Google Scholar]
- Kumar, A.; Vaid, A.; Syin, C.; Sharma, P. PfPKB, a novel protein kinase B-like enzyme from Plasmodium falciparum I. Identification, characterization, and possible role in parasite development. J. Biol. Chem. 2004, 279, 24255–24264. [Google Scholar] [CrossRef] [PubMed]
- Billker, O.; Lourido, S.; Sibley, L.D. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 2009, 5, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Eschenlauer, S.; Reininger, L.; Doerig, C. SAM domain-dependent activity of PfTKL3, an essential tyrosine kinase-like kinase of the human malaria parasite Plasmodium falciparum. Cell. Mol. Life Sci. 2010, 67, 3355–3369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Wang, W.; Gu, Y.; Liu, H.; Wei, F.; Liu, Q. Targeted disruption of CK1a in Toxoplasma gondii increases acute virulence in mice. Eur. J. Protistol. 2016, 56, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kern, S.; Halbert, J.; Przyborski, J.M.; Baumeister, S.; Dandekar, T.; Doerig, C.; Pradel, G. Two nucleus-localized CDK-like kinases with crucial roles for malaria parasite erythrocytic replication are involved in phosphorylation of splicing factor. J. Cell. Biochem. 2011, 112, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.F.; Nahum, L.A.; Avelar, L.G.; Silva, L.L.; Zerlotini, A.; Ruiz, J.C.; Oliveira, G. Eukaryotic protein kinases (ePKs) of the helminth parasite Schistosoma mansoni. BMC Genom. 2011, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Low, H.; Lye, Y.M.; Sim, T.S. Pfnek3 functions as an atypical MAPKK in Plasmodium falciparum. Biochem. Biophys. Res. Commun. 2007, 361, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Dorin, D.; Le Roch, K.; Sallicandro, P.; Alano, P.; Parzy, D.; Poullet, P.; Meijer, L.; Doerig, C. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum. Eur. J. Biochem. 2001, 268, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Berry, L.; Chen, C.-T.; Reininger, L.; Carvalho, T.G.; El Hajj, H.; Morlon-Guyot, J.; Bordat, Y.; Lebrun, M.; Gubbels, M.-J.; Doerig, C. The conserved apicomplexan Aurora kinase TgArk3 is involved in endodyogeny, duplication rate and parasite virulence. Cell. Microbiol. 2016, 18, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.J.; Narasimhan, J.; Bhatti, M.M. Parasite-specific eIF2 (eukaryotic initiation factor-2) kinase required for stress-induced translation control. Biochem. J. 2004, 380, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Blazejewski, T.; Nursimulu, N.; Pszenny, V.; Dangoudoubiyam, S.; Namasivayam, S.; Chiasson, M.A.; Chessman, K.; Tonkin, M.; Swapna, L.S.; Hung, S.S.; et al. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a heteroxenous life cycle. mBio 2015, 6, e02445–14. [Google Scholar] [CrossRef] [PubMed]
- Dice, J.F.; Goldberg, A.L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc. Natl. Acad. Sci. USA 1975, 72, 3893–3897. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Sugi, T.; Takemae, H.; Takano, R.; Gong, H.; Ishiwa, A.; Horimoto, T.; Akashi, H. Characterization of a Toxoplasma gondii calcium calmodulin-dependent protein kinase homolog. Parasites Vectors 2016, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tang, J.; Qin, C.; Kim, K. Cyclin-dependent kinase TPK2 is a critical cell cycle regulator in Toxoplasma gondii. Mol. Microbiol. 2002, 45, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Solyakov, L.; Halbert, J.; Alam, M.M.; Semblat, J.P.; Dorin-Semblat, D.; Reininger, L.; Bottrill, A.R.; Mistry, S.; Abdi, A.; Fennell, C. Global kinomic and phospho-proteomic analyses of the human malaria parasite Plasmodium falciparum. Nat. Commun. 2011, 2, 565. [Google Scholar] [CrossRef] [PubMed]
- Gurnett, A.M.; Liberator, P.A.; Dulski, P.M.; Salowe, S.P.; Donald, R.G.; Anderson, J.W.; Wiltsie, J.; Diaz, C.A.; Harris, G.; Chang, B. Purification and molecular characterization of cGMP-dependent protein kinase from apicomplexan parasites a novel chemotherapeutic target. J. Biol. Chem. 2002, 277, 15913–15922. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, J.C.; Gajria, B.; Li, L.; Paulsen, I.T.; Roos, D.S. ToxoDB: Accessing the Toxoplasma gondii genome. Nucleic Acids Res. 2003, 31, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Lourido, S.; Shuman, J.; Zhang, C.; Shokat, K.M.; Hui, R.; Sibley, L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 2010, 465, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Donald, R.G.; Zhong, T.; Meijer, L.; Liberator, P.A. Characterization of two T. gondii CK1 isoforms. Mol. Biochem. Parasitol. 2005, 141, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Dorin-Semblat, D.; marta-Gatsi, C.; Hamelin, R.; Armand, F.; Carvalho, T.G.; Moniatte, M.; Doerig, C. Malaria parasite-Infected erythrocytes secrete PfCK1, the Plasmodium homologue of the pleiotropic protein kinase casein kinase 1. PLoS ONE 2015, 10, e0139591. [Google Scholar] [CrossRef] [PubMed]
- Masch, A.; Kunick, C. Selective inhibitors of Plasmodium falciparum glycogen synthase-3 (PfGSK-3): New antimalarial agents? Biochim. Biophys. Acta Proteins Proteom. 2015, 1854, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Chen, F.; Harb, O.S.; Davis, P.H.; Beiting, D.P.; Brownback, C.S.; Ouloguem, D.; Roos, D.S. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host. Microbe 2010, 8, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.D.; Qiu, W.; Fentress, S.; Taylor, S.J.; Khan, A.; Hui, R. Forward genetics in Toxoplasma gondii reveals a family of rhoptry kinases that mediates pathogenesis. Eukaryot. Cell 2009, 8, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, M.; Scholz, A.; Melzer, I.M.; Schmetz, C.; Wiese, M. Interacting protein kinases involved in the regulation of flagellar length. Mol. Biol. Cell 2006, 17, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Kõhler, S. Multi-membrane-bound structures of Apicomplexa: II. the ovoid mitochondrial cytoplasmic (OMC) complex of Toxoplasma gondii tachyzoites. Parasitol. Res. 2006, 98, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.D.; Wernimont, A.K.; lali-Hassani, A.; Zhao, Y.; Amani, M.; Lin, Y.H.; Senisterra, G.; Wasney, G.A.; Fedorov, O.; King, O. The Cryptosporidium parvum kinome. BMC Genom. 2011, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Solyakov, L.; Bottrill, A.R.; Flueck, C.; Siddiqui, F.A.; Singh, S.; Mistry, S.; Viskaduraki, M.; Lee, K.; Hopp, C.S. Phosphoproteomics reveals malaria parasite Protein Kinase G as a signalling hub regulating egress and invasion. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Morlon-Guyot, J.; Berry, L.; Chen, C.-T.; Gubbels, M.-J.; Lebrun, M.; Daher, W. The Toxoplasma gondii calcium-dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell. Microbiol. 2014, 16, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Sugi, T.; Kobayashi, K.; Takemae, H.; Gong, H.; Ishiwa, A.; Murakoshi, F.; Recuenco, F.C.; Horimoto, T.; Akashi, H. Characterization of Plasmodium falciparum cdc2-related kinase and the effects of a CDK inhibitor on the parasites in erythrocytic schizogony. Parasitol. Int. 2013, 62, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Aubol, B.E.; Adams, J.A. Recruiting a silent partner for activation of the protein kinase SRPK1. Biochemistry 2014, 53, 4625–4634. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.; Andrée, N.; Razanau, A.; Zock-Emmenthal, S.; Lützelberger, M.; Plath, S.; Schmidt, H.; Guerra-Moreno, A.; Cozzuto, L.; Ayté, J. Prp4 kinase grants the license to splice: Control of weak splice sites during spliceosome activation. PLoS Genet. 2016, 12, e1005768. [Google Scholar] [CrossRef] [PubMed]
- Gazarini, M.L.; Garcia, C.R.S. Interruption of the blood-stage cycle of the malaria parasite, Plasmodium chabaudi, by protein tyrosine kinase inhibitors. Braz. J. Med. Biol. Res. 2003, 36, 1465–1469. [Google Scholar] [CrossRef]
- Kern, S.; Agarwal, S.; Huber, K.; Gehring, A.P.; Strõdke, B.; Wirth, C.C.; Brügl, T.; Abodo, L.O.; Dandekar, T.; Doerig, C. Inhibition of the SR protein-phosphorylating CLK kinases of Plasmodium falciparum impairs blood stage replication and malaria transmission. PLoS ONE 2014, 9, e105732. [Google Scholar] [CrossRef] [PubMed]
- Rüben, K.; Wurzlbauer, A.; Walte, A.; Sippl, W.; Bracher, F.; Becker, W. Selectivity profiling and biological activity of novel ß-carbolines as potent and selective DYRK1 kinase inhibitors. PLoS ONE 2015, 10, e0132453. [Google Scholar] [CrossRef] [PubMed]
- Brumlik, M.J.; Pandeswara, S.; Ludwig, S.M.; Murthy, K.; Curiel, T.J. Parasite mitogen-activated protein kinases as drug discovery targets to treat human protozoan pathogens. J. Signal Transduct. 2011. [Google Scholar] [CrossRef] [PubMed]
- Brumlik, M.J.; Pandeswara, S.; Ludwig, S.M.; Jeansonne, D.P.; Lacey, M.R.; Murthy, K.; Daniel, B.J.; Wang, R.F.; Thibodeaux, S.R.; Church, K.M. TgMAPK1 is a Toxoplasma gondii MAP kinase that hijacks host MKK3 signals to regulate virulence and interferon-g-mediated nitric oxide production. Exp. Parasitol. 2013, 134, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.A.; Rommereim, L.M.; Guevara, R.B.; Falla, A.; Triana, M.A.H.; Sun, Y.; Bzik, D.J. The Toxoplasma gondii rhoptry kinome is essential for chronic infection. mBio 2016, 7, e00193–16. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.G.; Hendrix, R.W.; Casjens, S. Where are the pseudogenes in bacterial genomes? Trends Microbiol. 2001, 9, 535–540. [Google Scholar] [CrossRef]
- Templeton, T.J.; Iyer, L.M.; Anantharaman, V.; Enomoto, S.; Abrahante, J.E.; Subramanian, G.M.; Hoffman, S.L.; Abrahamsen, M.S.; Aravind, L. Comparative analysis of apicomplexa and genomic diversity in eukaryotes. Genome Res. 2004, 14, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Sugi, T.; Ma, Y.F.; Tomita, T.; Murakoshi, F.; Eaton, M.S.; Yakubu, R.; Han, B.; Tu, V.; Kato, K.; Kawazu, S.I. Toxoplasma gondii cyclic AMP-dependent protein kinase subunit 3 is involved in the switch from tachyzoite to bradyzoite development. mBio 2016, 7, e00755–16. [Google Scholar] [CrossRef] [PubMed]
- Gaji, R.Y.; Johnson, D.E.; Treeck, M.; Wang, M.; Hudmon, A.; Arrizabalaga, G. Phosphorylation of a myosin motor by TgCDPK3 facilitates rapid Initiation of motility during Toxoplasma gondii egress. PLoS Pathog. 2015, 11, e1005268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.; Wang, Q.; Sibley, L.D. Analysis of noncanonical calcium-dependent protein kinases in Toxoplasma gondii by targeted gene deletion using CRISPR/Cas9. Infect. Immun. 2016, 84, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Larson, E.T.; Keyloun, K.R.; Castaneda, L.J.; DeRocher, A.E.; Inampudi, K.K.; Kim, J.E.; Arakaki, T.L.; Murphy, R.C.; Zhang, L. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010, 17, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, Z.; Wang, S.; Li, J.; Wang, X.; Wei, F.; Liu, Q. Deletion of mitogen-activated protein kinase 1 inhibits development and growth of Toxoplasma gondii. Parasitol. Res. 2016, 115, 797–805. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q.; Liu, Q. TgERK7 is involved in the intracellular proliferation of Toxoplasma gondii. Parasitol. Res. 2016, 115, 3419–3424. [Google Scholar] [CrossRef] [PubMed]
- Behnke, M.S.; Fentress, S.J.; Mashayekhi, M.; Li, L.X.; Taylor, G.A.; Sibley, L.D. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 2012, 8, e1002992. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.G.; Wang, Q.; Sibley, L.D. Secreted protein kinases regulate cyst burden during chronic toxoplasmosis. Cell. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Knoll, L.J. Functional analysis of the rhoptry kinome during chronic Toxoplasma gondii infection. mBio 2016, 7, e00842–16. [Google Scholar] [CrossRef] [PubMed]
- Doerig, C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim. Biophys. Acta Proteins Proteom. 2004, 1697, 155–168. [Google Scholar] [CrossRef]
- Zhang, M.; Chavchich, M.; Waters, C. Targeting protein kinases in the malaria parasite: Update of an antimalarial drug target. Curr. Top. Med. Chem. 2012, 12, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The regulation of protein function by multisite phosphorylation—A 25 year update. Trends Biochem. Sci. 2000, 25, 596–601. [Google Scholar] [CrossRef]
- Eglen, R.M.; Reisine, T. The current status of drug discovery against the human kinome. Assay Drug Dev. Technol. 2009, 7, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Ojo, K.K.; Dangoudoubiyam, S.; Verma, S.K.; Scheele, S.; DeRocher, A.E.; Yeargan, M.; Choi, R.; Smith, T.R.; Rivas, K.L.; Hulverson, M.A. Selective inhibition of Sarcocystis neurona calcium-dependent protein kinase 1 for equine protozoal myeloencephalitis therapy. Int. J. Parasitol. 2016, 46, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Dissous, C.; Grevelding, C.G. Piggy-backing the concept of cancer drugs for schistosomiasis treatment: A tangible perspective? Trends Parasitol. 2011, 27, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Miranda-Saavedra, D.; Barton, G.J. Kinomer v. 1.0: A database of systematically classified eukaryotic protein kinases. Nucleic Acids Res. 2009, 37, D244–D250. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Sundarsanam, S.; Bingham, J.; Manning, G.; Charydczak, G.; Chen, M.J. Kinase.com—Genomics, Evolution and Function of Protein Kinases. 1999. Available online: www.kinase.com (accessed on 30 January 2017).
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook, 1st ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Bailey, T.L.; Bodén, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2: A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der, M.P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]

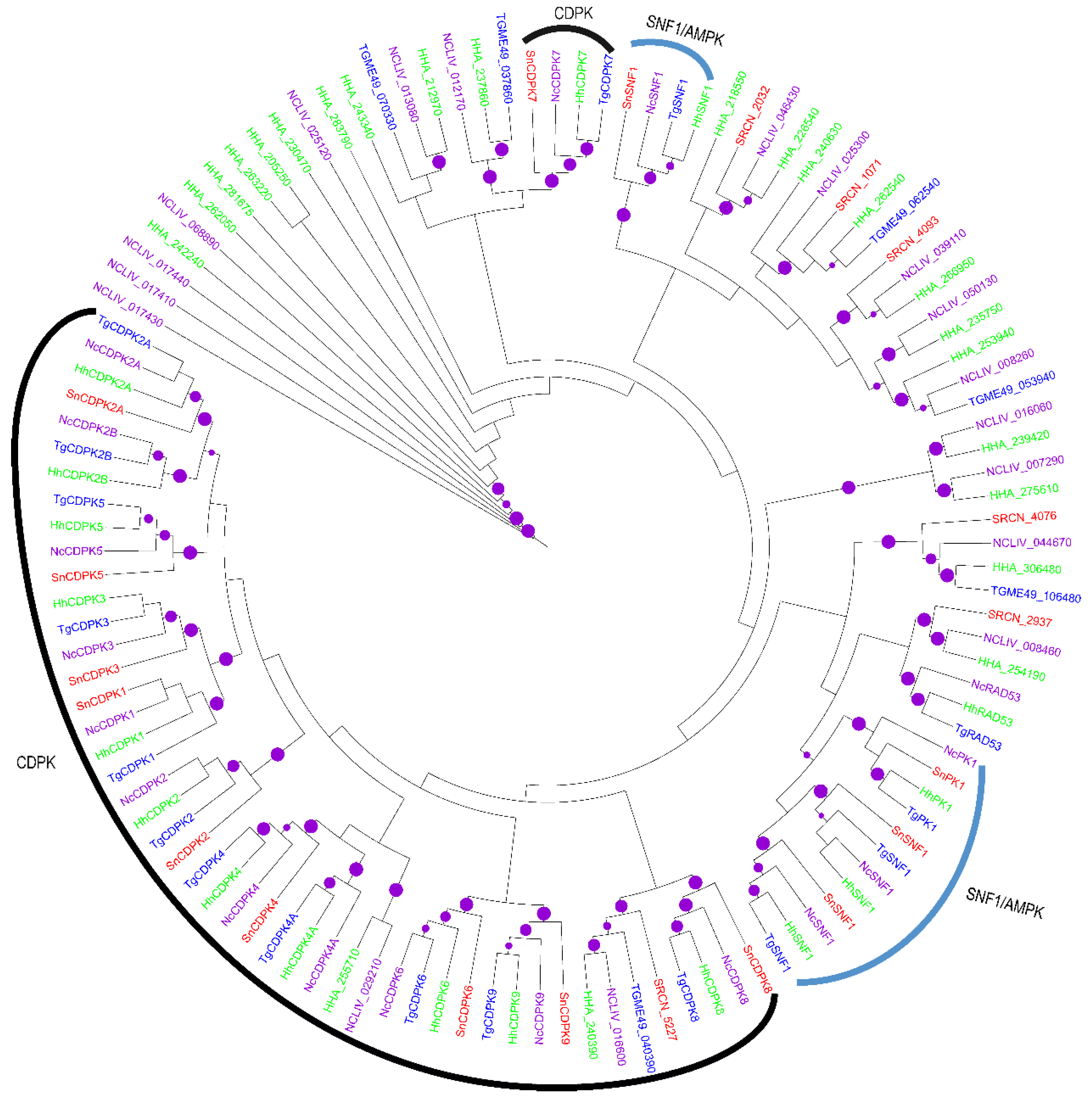
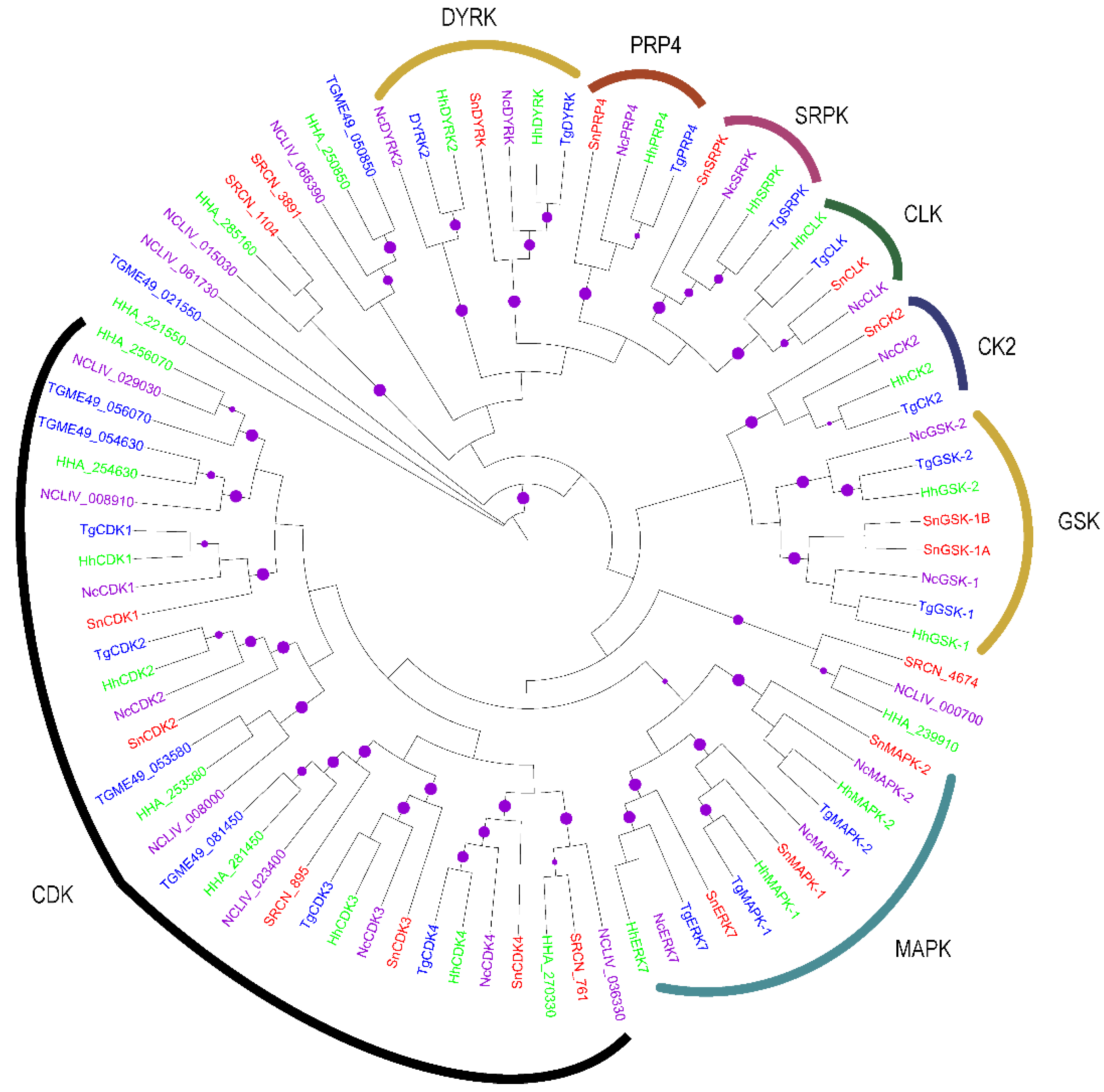
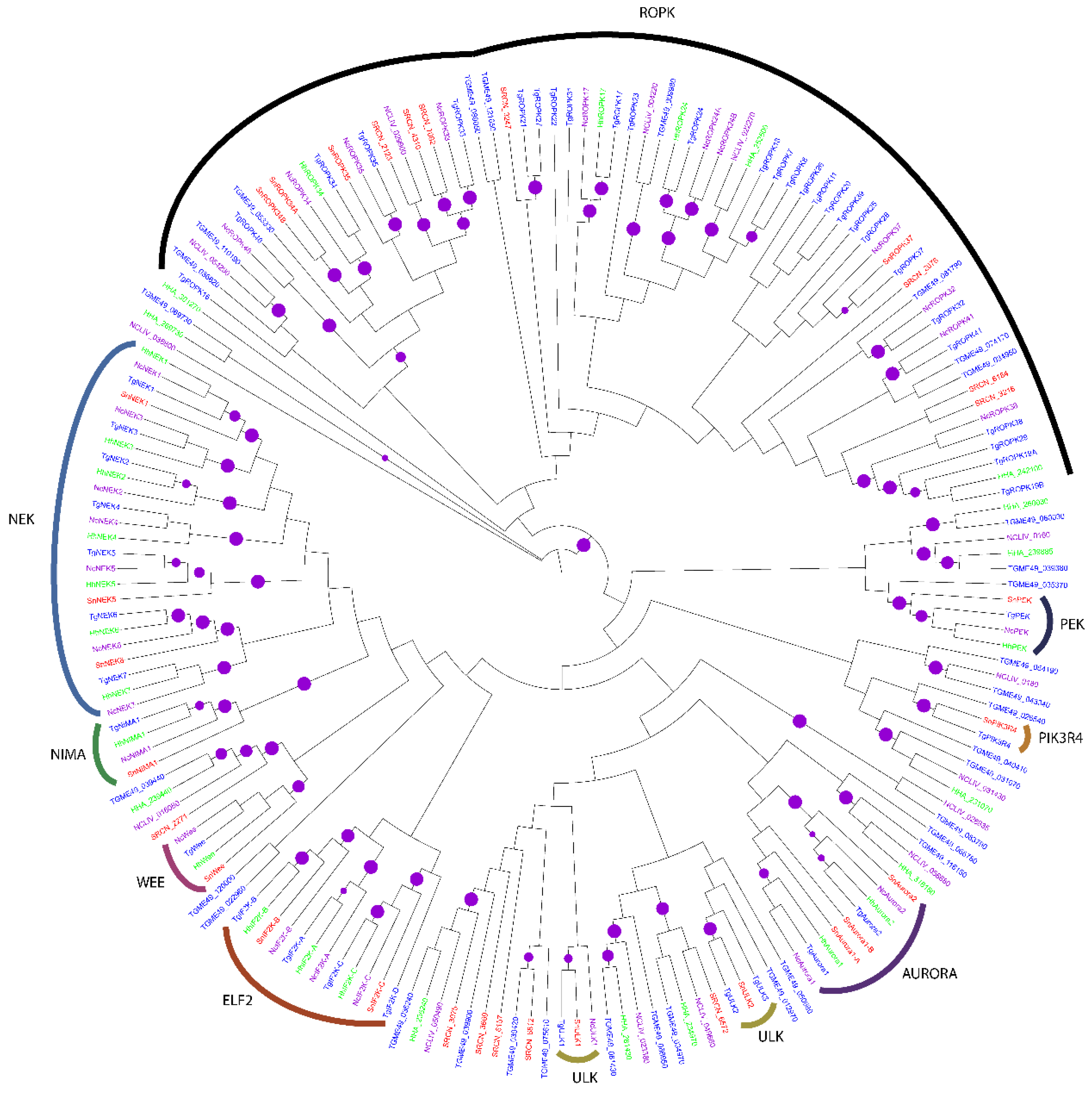
| Description of the Putative Protein Kinases (PKs) in the Genome of S. neurona | Description of Protein Kinase (PK) Homologies (BLASTp) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein ID a | Sequence Annotations; Description b | Family; (Subfamily) c | Length (aa) | pI | MW (kDa) | PK Domain Coordinates | Sequence Name; (Apicomplexan) | Bit Score | E-Value | Identity (%) | Accession Number |
| 1. Kinase Group AGC (Protein kinases A (PKA), G (PKG) and C (PKC) families) | |||||||||||
| SRCN_1312 | AGC kinase | 3-phosphoinositide dependent PK-1 (PDK1) | 903 | 5.52 | 101.13 | 137–481 | PDPK; (T. gondii RUB) | 417 | 6.00 × 10−135 | 59 | KFG61374.1 |
| SRCN_3339 | AGC kinase | PKA | 1428 | 8.85 | 147.52 | 1102–1417 | Putative AGC kinase; (N. caninum L) | 611 | 0.0 | 81 | CEL65574.1 |
| SRCN_4249 | AGC kinase | Nuclear dbf2-related (NDR) | 152 | 8.91 | 17.47 | 6–141 | Putative AGC kinase; (N. caninum L) | 219 | 2.00 × 10−68 | 76 | XP_003883757.1 |
| SRCN_4518 | PK G AGC kinase family member PKG | Ciliate-E2 | 425 | 5.83 | 48.78 | 97–399 | AGC kinase TgPKG1; (T. gondii ME49) | 830 | 0.0 | 92 | EPR61116.1 |
| SRCN_3990 | cAMP-dependent kinase | CAMKL; (MELK) | 1907 | 9.55 | 217.55 | 782–1634 | cAMP-dependent protein kinase (T. gondii VEG) | 75.9 | 1 × 10−12 | 30 | ESS31194.1 |
| SRCN_5165 | cAMP-dependent PK, catalytic chain | PKA | 343 | 8.99 | 39.36 | 20–338 | AGC kinase; (T. gondii ARI) | 425 | 3.00 × 10−150 | 90 | KYF43224.1 |
| SRCN_4913 | Putative PK | PKD | 2330 | 6.42 | 244.64 | 1093–1737 | Putative PK; (E. tenella) | 107 | 9.00 × 10−22 | 60 | XP_013228294.1 |
| SRCN_5430 | AGC kinase | Ribosomal protein S6 Kinases (RSK; (p70)) | 1378 | 5.54 | 139.28 | 824–1344 | AGC kinase; (T. gondii MAS) | 223 | 3.00 × 10−60 | 59 | KFH07588.1 |
| SRCN_5610 | cAMP-dependent PK, catalytic chain | PKA | 333 | 9.00 | 37.96 | 12–318 | cAMP-dependent PK, catalytic subunit; (T. gondii ME49) | 641 | 0.0 | 92 | XP_002366464.1 |
| 2. Kinase Group calcium (Ca2+)-/calmodulin-regulated kinases (CAMK) | |||||||||||
| SRCN_1071 | Ca2+-dependent kinase | CAMK1 | 1495 | 9.47 | 152.66 | 1085–1401 | Putative PK; (T. gondii VEG) | 188 | 1.00 × 10−47 | 67 | ESS31884.1 |
| SRCN_2032 | Putative PK | Ciliate-C1 | 297 | 6.22 | 33.26 | 15–297 | PK; (H. hammondi) | 424 | 1.00 × 10−146 | 68 | XP_008882026.1 |
| SRCN_2165 | Ca2+-dependent kinase CDPK2B | CDPK | 692 | 7.33 | 75.65 | 101–401 | Ca2+-dependent PK CDPK2A; (T. gondii ARI) | 674 | 0.0 | 90 | KYF44522.1 |
| SRCN_2257 | Histone kinase | CAMKL; (AMP-activated protein kinase (AMPK)) | 1800 | 8.98 | 187.71 | 1159–1448 | Putative CAM kinase, SNF1 family; (E. acervulina) | 377 | 7.00 × 10−105 | 64 | XP_013252246.1 |
| SRCN_2544 | CAM SNF1 AMK1 family | CAMKL;(AMPK-regulated kinase novel kinase (NUAK)) | 333 | 5.71 | 37.85 | 62–333 | CAM kinase, SNF1/AMK1 family ToxPK1; (N. caninum L) | 480 | 9.00 × 10−167 | 79 | XP_003882065.1 |
| SRCN_2937 | Ca2+-signaling kinase MARK | CAMKL; (microtubule affinity regulating kinase (MARK)) | 278 | 9.62 | 31.29 | 1–250 | Putative Ca2+ signaling PK MARK; (T. gondii GT1) | 356 | 5.00 × 10−124 | 72 | EPR59053.1 |
| SRCN_3011 | Calmodulin-dependent PK (CAM) CDPK6 | CDPK | 1435 | 9.25 | 154.44 | 1238–1435 | Cdpk kinase domain; (T. gondii) | 179 | 6.00 × 10−49 | 75 | 3IS5_A |
| SRCN_3314 | A Chain crystal Structure of TgCDPK1 with inhibitor bound | CDPK1 | 519 | 5.99 | 58.89 | 37–335 | Calmodulin-domain PK 1; (T. gondii) | 536 | 0.0 | 97 | 3MA6_A |
| SRCN_3583 | Ca2+-dependent kinase CDPK5 | CDPK | 454 | 6.09 | 50.38 | 35–308 | Ca2+-dependent PK CDPK5; (T. gondii ARI) | 776 | 0.0 | 89 | KYF43137.1 |
| SRCN_3701 | Ca2+-dependent kinase CDPK3 | CDPK | 560 | 5.91 | 62.07 | 77–362 | Ca2+-dependent Kinase; (T. gondii) | 493 | 1.00 × 10−172 | 87 | 3DXN_A |
| SRCN_4076 | CAM CDPK family | CDPK | 1701 | 5.93 | 181.42 | 1079–1663 | CAM kinase, CDPK family; (H. hammondi) | 239 | 2.00 × 10−62 | 69 | XP_008884897.1 |
| SRCN_4093 | PK | CAMKL; (AMP-activated protein kinase (AMPK)) | 1155 | 9.02 | 118.33 | 1–261 | Putative atypical MEK-related kinase; (N. caninum L) | 222 | 1.00 × 10−59 | 70 | XP_003880869.1 |
| SRCN_4390 | Ca2+-dependent kinase CDPK2 | CDPK | 790 | 6.24 | 85.47 | 280–556 | Ca2+-dependent PK, related; (N. caninum L) | 1130 | 0.0 | 74 | XP_003884321.1 |
| SRCN_4815 | Histone kinase (partial) | CAMKL; (AMP-activated protein kinase (AMPK)) | 711 | 6.53 | 75.98 | 1–314 | SNF1-related PK catalytic-α KIN10, 5 AMP-activated PK; (N. caninum L) | 568 | 0.0 | 48 | CEL67550.1 |
| SRCN_5227 | CAM CDPK CDPK8-like | CDPK | 2748 | 8.98 | 282.12 | 796–885 | Putative CAM kinase, CDPK family; (N. caninum L) | 117 | 3.00 × 10−24 | 59 | XP_003881901.1 |
| SRCN_5410 | Calmodulin-dependent PK (CAM-SNF1 family) | CAMK1 | 467 | 8.94 | 52.04 | 168–446 | CAM kinase, SNF1 family; (H. hammondi) | 431 | 5.00 × 10−134 | 78 | XP_008883430.1 |
| SRCN_5812 | Ca2+-dependent kinase CDPK9 | CDPK | 760 | 8.37 | 84.23 | 254–573 | Ca2+-dependent PK CDPK9; (H. hammondi) | 1139 | 0.0 | 81 | XP_008889286.1 |
| SRCN_5948 | Ca2+-dependent kinase CDPK8 | CDPK | 3298 | 7.11 | 345.85 | 208–860 | EF-hand domain-containing protein; (T. gondii ME49) | 114 | 1.00 × 10−23 | 58 | XP_002368547.1 |
| SRCN_6597 | Ca2+ dependent kinase CDPK7 | CAMK1 | 1374 | 9.09 | 138.28 | 365–623 | PK-PH domain-containing protein; (T. gondii ME49) | 813 | 0.0 | 80 | XP_002366487.1 |
| SRCN_6606 | Ca2+-dependent kinase CDPK4 | CDPK | 1632 | 9.42 | 170.93 | 813–1236 | Ca2+-dependent PK; (T. gondii) | 731 | 0.0 | 58 | CAD32376.2 |
| 3. Kinase Group casein kinase 1 (cell kinase 1) | |||||||||||
| SRCN_3445 | Casein kinase I | CK1-D | 323 | 9.34 | 37.65 | 6–290 | Casein kinase 1; (T. gondii ME49) | 603 | 0.0 | 94 | XP_002366683.1 |
| SRCN_4587 | Casein kinase I | CK1-D | 137 | 7.78 | 15.94 | 1–137 | Casein kinase I; (H. hammondi) | 226 | 3.00 × 10−71 | 81 | XP_008883809.1 |
| SRCN_4645 | Casein kinase I | CK1-D | 229 | 9.51 | 25.66 | 38–229 | Casein kinase I; (T. gondii GAB2-2007-GAL-DOM2) | 259 | 1.00 × 10−86 | 79 | KFG42638.1 |
| 4. Kinase Group CMGC (including cyclin-dependent kinases, mitogen-activated PKs, glycogen synthase kinases and CDK-like kinases) | |||||||||||
| SRCN_1104 | Cyclin-dependent kinase family 5 | Ca2+-dependent PK-L (CDKL) | 372 | 9.23 | 42.71 | 1–318 | Cyclin-dependent kinase family 5 protein; (H. hammondi) | 490 | 2.00 × 10−173 | 76 | XP_008884207.1 |
| SRCN_1236 | Cell-cycle-associated kinase (SRPK) | Serine-arginine rich PK (SRPK) | 2911 | 5.37 | 302.90 | 713–1837 | PK; (T. gondii ME49) | 476 | 9.00 × 10−141 | 76 | XP_002369401.1 |
| SRCN_1479 | CMGC Lammer | CLK | 748 | 10.23 | 79.09 | 485–748 | Cell-cycle-associated PK CLK; (T. gondii FOU) | 288 | 2.00 × 10−81 | 74 | KFG33061.1 |
| SRCN_1611 | CMGC Dual-specificity tyrosine-regulated kinase (DYRK) | DYRK; (DYRKP) | 1504 | 5.86 | 160.34 | 551–1498 | Cell-cycle-associated PK DYRK; (T. gondii VEG) | 223 | 2.00 × 10−57 | 63 | ESS33160.1 |
| SRCN_1731 | Cell-cycle-associated kinase GSK | Glycogen synthase kinase (GSK) | 219 | 6.59 | 24.39 | 1–175 | Cell-cycle-associated PK GSK; (H. hammondi) | 330 | 2.00 × 10−112 | 82 | XP_008887193.1 |
| SRCN_1732 | Cell-cycle-associated kinase GSK | Glycogen synthase kinase (GSK) | 203 | 10.78 | 20.72 | 86–203 | CMGC kinase, GSK family TgPK3; (E. brunetti) | 114 | 1.00 × 10−28 | 91 | CDJ46527.1 |
| SRCN_2759 | Cell-cycle-associated kinase partial | Ca2+-dependent PK (CDK); (CRK7) | 1122 | 6.14 | 118.67 | 541–1122 | Cell-cycle-associated PK CDK; (T. gondii VAND) | 118 | 1.00 × 10−25 | 79 | KFH12036.1 |
| SRCN_2845 | CMGC DYRK PRP4 kinase | DYRK; (PRP4) | 1665 | 9.76 | 177.08 | 1267–1596 | Putative PK (CLK3); (P. malariae) | 330 | 1.00 × 10−102 | 69 | SBS85334.1 |
| SRCN_3891 | CMGC kinase | DYRK; (DYRK2) | 674 | 8.85 | 73.85 | 399–674 | Putative CMGC kinase; (T. gondii ME49) | 80.1 | 6.00 × 10−14 | 67 | EPT25192.1 |
| SRCN_4209 | CMGC MAPK family (ERK) MAPK-1 | Mitogen-activated PK (MAPK); (ERK)) | 2361 | 6.73 | 247.71 | 94–754 | CMGC, MAPK/ (ERK) TgMAPK-1; (E. brunetti) | 137 | 1.00 × 10−30 | 74 | CDJ49492.1 |
| SRCN_4674 | Cyclin-dependent kinase | Ca2+-dependent PK (CDK); (CDK7) | 138 | 7.80 | 15.50 | 1–138 | Cyclin-dependent kinase; (T. gondii GT1) | 108 | 7.00 × 10−27 | 58 | EPR60430.1 |
| SRCN_4801 | Cell-cycle-associated kinase | Ca2+-dependent PK (CDK); (CDK5) | 300 | 6.08 | 34.33 | 1–289 | CMGC kinase, CDK family TgPK2; (N. caninum L) | 576 | 0.0 | 91 | XP_003885801.1 |
| SRCN_5365 | Cell-cycle-associated kinase MAPK | Mitogen-activated PK (MAPK; (ERK)) | 417 | 6.77 | 48.32 | 7–363 | Cell-cycle-associated PK MAPK; (H. hammondi) | 823 | 0.0 | 93 | XP_008886907.1 |
| SRCN_6346 | Cell-cycle-associated kinase CDK | Ca2+-dependent PK (CDK); (CDK5) | 690 | 9.55 | 80.90 | 208–603 | Putative cell-cycle-associated PK CDK; (T. gondii ARI) | 390 | 2.00 × 10−122 | 87 | KYF45878.1 |
| SRCN_6427 | CMGC CK2 kinase | Cell Kinase 2 (CK2) | 1395 | 10.29 | 144.86 | 885–1356 | CMGC kinase, CK2 family; (T. gondii MAS) | 241 | 6.00 × 10−73 | 98 | KFH07655.1 |
| SRCN_6472 | Cell-cycle-associated kinase ERK7 | Mitogen-activated PK (MAPK; (ERK)) | 983 | 9.28 | 104.95 | 7–317 | Cell-cycle-associated PK ERK7; (T. gondii ARI) | 647 | 0.0 | 81 | KYF46268.1 |
| SRCN_761 | Cell-cycle-associated kinase | Ca2+-dependent PK (CDK); (CDK7) | 577 | 9.34 | 58.39 | 144–490 | Cell-cycle-associated PK; (H. hammondi) | 283 | 3.00 × 10−88 | 68 | XP_008882409.1 |
| SRCN_895 | Cell-cycle-associated kinase | Ca2+-dependent PK (CDK); (CDK10) | 340 | 8.93 | 38.57 | 1–307 | Cell-cycle-associated PK; (T. gondii ARI) | 234 | 6.00 × 10−75 | 76 | KYF44017.1 |
| SRCN_977 | Cell-cycle-associated kinase CDK | Ca2+-dependent PK (CDK); (PITSLRE/CDK11) | 1502 | 7.38 | 156.35 | 1114–1429 | Cell-cycle-associated PK CDK; (T. gondii p89) | 454 | 3.00 × 10−135 | 92 | KFG28420.1 |
| 5. Kinase Group ‘Other’ (OPK; i.e., kinases with conventional PK (ePK) domains that do not fit into any of the other major groups of kinases) | |||||||||||
| SRCN_108 | Unc-51-like autophagy activating kinase 1 (ULK1) | ULK | 343 | 7.13 | 38.90 | 1-223 | ULK kinase; (T. gondii VAND) | 376 | 2.00 × 10−130 | 75 | KFH07419.1 |
| SRCN_1606 | eIF2 kinase IF2K-C | PEK; (general control nonderepressible 2 (GCN2)) | 4034 | 8.98 | 406.57 | 1235–2178 | eIF2 kinase IF2K-C; (T. gondii VAND) | 259 | 4.00 × 10−67 | 35 | KFH07289.1 |
| SRCN_2076 | Rhoptry kinase family ROP30 | Conserved hypothetical protein | 1276 | 9.18 | 134.73 | 812–1260 | ROP30 (T. gondii VEG) | 230 | 3.00 × 10−63 | 53 | CEL76436.1 |
| SRCN_2123 | Rhoptry kinase family ROP35 | PLK; (PLK-Unclassified) | 291 | 9.30 | 33.50 | 53–265 | ROP35; (T. gondii RUB) | 207 | 2.00 × 10−61 | 43 | KFG59037.1 |
| SRCN_3216 | Rhoptry kinase family ROP32 | CAMK-Unique | 523 | 7.08 | 57.00 | 214–520 | Putative PK; (T. gondii VAND) | 167 | 1.00 × 10−42 | 30 | KFH00232.1 |
| SRCN_2183 | Rhoptry kinase family ROP35 | Aurora-like | 226 | 6.36 | 25.84 | 1–212 | ROP35; (T. gondii VEG) | 198 | 8.00 × 10−59 | 48 | ESS33297.1 |
| SRCN_2271 | Putative PK (incomplete catalytic triad) | NimA (Never in mitosis gene A)-related Kinase (NEK) | 1463 | 9.02 | 157.18 | 437–1145 | Putative PK; (N. caninum L) | 327 | 5.00 × 10−90 | 68 | XP_003881849.1 |
| SRCN_2403 | Aurora kinase (incomplete catalytic triad) | PLK; (SAK/Plk4) | 778 | 9.79 | 79.92 | 492–778 | Putative Aurora kinase; (N. caninum L) | 127 | 3.00 × 10−28 | 44 | XP_003880644.1 |
| SRCN_2630 | NimA related kinase (NEK) family protein | NEK | 351 | 8.70 | 38.38 | 1–336 | NEK kinase; (T. gondii ME49) | 242 | 8.00 × 10−75 | 52 | XP_018638598.1 |
| SRCN_286 | Wee kinase | Inhibitory regulator of the RAS-cAMP (IRA1) kinase suppressor (IKS) | 1019 | 6.20 | 106.67 | 598–959 | Wee kinase; (H. hammondi) | 445 | 5.00 × 10−141 | 58 | XP_008882669.1 |
| SRCN_3075 | Tyrosine kinase-like (TKL) protein | Numb-associated kinase (NAK) | 1571 | 8.41 | 164.18 | 16–500 | TKL; (T. gondii TgCatPRC2) | 138 | 1.00 × 10−32 | 73 | KYK64203.1 |
| SRCN_3142 | PIK3R4 kinase-related | Aurora | 997 | 8.72 | 106.54 | 548–899 | Putative PIK3R4 kinase-related protein; (N. caninum L) | 449 | 2.00 × 10−137 | 60 | XP_003885774.1 |
| SRCN_3151 | NimA related kinase (NEK) family protein | NEK | 3186 | 7.96 | 318.69 | 352–656 | NEK kinase; (T. gondii VEG) | 468 | 7.00 × 10−131 | 73 | CEL78174.1 |
| SRCN_3247 | Rhoptry kinase family ROP27 | Ciliate-D | 345 | 8.94 | 38.81 | 23–325 | ROP27; (T. gondii p89) | 163 | 4.00 × 10−43 | 31 | KFG37427.1 |
| SRCN_3417 | Aurora kinase | Aurora | 438 | 7.65 | 48.49 | 14–289 | Aurora kinase; (T. gondii TgCatPRC2) | 490 | 3.00 × 10−155 | 76 | KYK63669.1 |
| SRCN_3444 | Unc-51-like Autophagy activating kinase 1 (ULK1) | ULK | 406 | 6.52 | 44.69 | 12–406 | ULK kinase; (T. gondii RUB) | 232 | 3.00 × 10−71 | 61 | KFG59767.1 |
| SRCN_3669 | CMGC kinase | ULK | 1803 | 8.41 | 189.65 | 736–1200 | Putative CMGC kinase; (N. caninum L) | 624 | 0.0 | 62 | CEL65030.1 |
| SRCN_4410 | Rhoptry kinase family ROP35 | PKA-like | 204 | 9.44 | 23.50 | 1–166 | ROP35; (H. hammondi) | 107 | 1.00E × 10−25 | 39 | XP_008885989.1 |
| SRCN_4503 | eIF2 kinase IF2K-B | PEK; (general control nonderepressible 2 (GCN2)) | 158 | 5.76 | 17.59 | 1–158 | eIF2 kinase IF2K-B (T. gondii TgCatPRC2) | 149 | 5.00 × 10−41 | 74 | KYK69938.1 |
| SRCN_4528 | NimA related kinase (NEK) family protein | NEK | 187 | 8.20 | 21.23 | 1–186 | NEK kinase; (H. hammondi) | 177 | 4.00 × 10−54 | 64 | XP_008885186.1 |
| SRCN_2404 | Aurora kinase (incomplete catalytic triad) | Serum and glucocorticoid induced Kinase (SGK) | 295 | 8.81 | 31.42 | 1–249 | Putative Aurora kinase; (N. caninum L) | 126 | 7.00 × 10−31 | 43 | CEL65223.1 |
| SRCN_5653 | PEK kinase | Aurora | 626 | 8.27 | 60.75 | 513–626 | PEK kinase (T. gondii TgCatPRC2) | 251 | 1.00 × 10−76 | 60 | KYK62422.1 |
| SRCN_5943 | NIMA-related kinase NIMA1 | NEK | 2842 | 9.04 | 295.44 | 73–383 | NIMA-related PK NIMA1; (T. gondii MAS) | 486 | 2.00 × 10−140 | 67 | KFH05809.1 |
| SRCN_6157 | Unc-51-like autophagy activating kinase 1 (ULK1) | ULK | 2420 | 9.38 | 250.58 | 1380–1672 | ULK kinase (T. gondii ME49) | 99.8 | 3 × 10−21 | 38 | XP_018635814.1 |
| SRCN_6184 | Myosin-light-chain kinase | Ciliate-E2-Unclassified | 478 | 5.42 | 53.65 | 177–474 | ROP19A (T. gondii ME49) | 127 | 3.00 × 10−30 | 27 | XP_018637476.1 |
| SRCN_6572 | Tyrosine kinase-like (TKL) | ULK | 622 | 6.13 | 68.80 | 1–345 | TKL; (T. gondii VAND) | 181 | 1.00 × 10−46 | 74 | KFH00338.1 |
| SRCN_6812 | PK | ULK | 199 | 6.74 | 22.72 | 1–183 | PK; (H. hammondi) | 172 | 3.00 × 10−48 | 53 | XP_008887491.1 |
| SRCN_7083 | Rhoptry kinase family ROP35 | PKA-like | 262 | 9.62 | 30.26 | 1–242 | ROP35; (H. hammondi) | 127 | 6.00 × 10−32 | 39 | XP_008885989.1 |
| SRCN_4310 | Rhoptry kinase family ROP33 | Kinase Homologous to SPS1/STE20 (KHS) | 1591 | 9.85 | 169.63 | 1265–1578 | ROP33; (H. hammondi) | 306 | 2.00 × 10−87 | 39 | XP_008887632.1 |
| SRCN_7082 | Rhoptry kinase family ROP33 | Kinase Homologous to SPS1/STE20 (KHS) | 403 | 9.59 | 45.92 | 77–390 | ROP33 (T. gondii p89) | 277 | 3.00 × 10−89 | 40 | KFG45248.1 |
| SRCN_7084 | Rhoptry kinase family ROP37 | Ribosomal protein S6 Kinases (RSK; (RSK)) | 339 | 5.41 | 38.09 | 19–334 | ROP37; (N. caninum L) | 144 | 1.00 × 10−36 | 36 | CEL64242.1 |
| 6. Kinase Group “Sterile” serine/threonine kinase, or sterile-phenotype kinases (STE) | |||||||||||
| SRCN_1328 | Serine threonine kinase | Conserved hypothetical protein | 1461 | 9.29 | 158.74 | 559–671 | Hypothetical protein, conserved; (E. maxima) | 88.6 | 5.00 × 10−16 | 68 | XP_013335801.1 |
| SRCN_5172 | “Sterile” serine/threonine kinase (STE) | Mammalian Sterile 20-like (MST)) | 6552 | 6.14 | 671.51 | 3410–4122 | STE kinase; (T. gondii TgCatPRC2) | 412 | 1.00 × 10−114 | 54 | KYK71951.1 |
| 7. Kinase Group Tyrosine Kinase-Like (TKL) | |||||||||||
| SRCN_1435 | Tyrosine kinase-like (TKL) | Mixed lineage kinase (MLK); (Leucine Zipper-bearing Kinase (LZK)) | 3064 | 8.05 | 306.74 | 2540–3060 | Tyrosine kinase-like (TKL) protein; (N. caninum L) | 278 | 3.00 × 10−73 | 72 | CEL64955.1 |
| SRCN_1571 | Tyrosine kinase-like (TKL) | Microtubule-associated S/T kinase (MAST) | 550 | 9.76 | 59.91 | 135–501 | Conserved hypothetical protein; (E. praecox) | 76.3 | 6.00 × 10−13 | 53 | CDI87140.1 |
| SRCN_3466 | Tyrosine kinase-like (TKL) | TKL-Unique | 3002 | 9.87 | 320.97 | 2342–2997 | Tyrosine kinase-like (TKL) protein; (H. hammondi) | 202 | 3.00 × 10−50 | 65 | XP_008887506.1 |
| SRCN_3928 | Tyrosine kinase-like (TKL) | LISK - LIMK (LIM kinase) and TESK (Testicular protein Kinase); (DD1) | 5842 | 8.78 | 608.80 | 3639–4268 | Tyrosine kinase-like (TKL) protein; (T. gondii TgCatPRC2) | 216 | 2.00 × 10−61 | 79 | KYK63216.1 |
| SRCN_4277 | Kinase domain-containing protein | TKL-ciliate1 | 2256 | 8.43 | 240.25 | 1570–2256 | Tyrosine kinase-like (TKL) protein; (N. caninum L) | 204 | 8.00 × 10−51 | 61 | CEL67693.1 |
| SRCN_811 | Tyrosine kinase-like (TKL) | TKL-Unique | 1099 | 9.18 | 119.26 | 814–1083 | Putative tyrosine kinase-like (TKL) protein; (E. acervulina) | 403 | 6.00 × 10−125 | 59 | XP_013252162.1 |
| 8. Kinase Group Atypical (aPKs) | |||||||||||
| SRCN_3601 | Atypical MEK-related kinase | Muscle-associated kinase TRIO | 950 | 7.20 | 103.14 | 381–850 | Atypical MEK-related kinase; (T. gondii GT1) | 171 | 1.00 × 10−42 | 32 | EPR62774.1 |
| SRCN_5962 | Atypical MEK-related kinase | Rho-associated protein kinase (ROCK)-like | 805 | 5.01 | 87.71 | 525–805 | Atypical MEK-related kinase; (H. hammondi) | 127 | 4.00 × 10−29 | 56 | XP_008884362.1 |
| SRCN_3988 | Phosphatidylinositol 3-/4-kinase (PI3K) | Atypical/PIKK/ATM | 4251 | 5.95 | 440.5 | 3671–3772 | PI3K, ; (H. hammondi) | 268 | 1 × 10−69 | 55 | XP_008886631.1 |
| SRCN_6465 | Phosphatidylinositol 3-/4-kinase (PI3K) | Atypical PIKK/ATM | 207 | 5.2 | 23.24 | 5-122 | Phosphatidylinositol 4-kinase, partial; (T. gondii p89) | 272 | 4 × 10−93 | 96 | KFG28404.1 |
| SRCN_1259 | Phosphatidylinositol 3-/4-kinase (PI3K) | Atypical/PIKK/FRAP | 1362 | 9.56 | 142.85 | 1140–1311 | PI3K; (T. gondii MAS) | 317 | 3 × 10−94 | 76 | KFH10008.1 |
| SRCN_6464 | Phosphatidylinositol 3-/4-kinase (PI3K) | No hits found | 2108 | 8.95 | 209.68 | 1626–1722 | Phosphatidylinositol 3-4-kinase; (T. gondii p89) | 244 | 1 × 10−63 | 66 | KFG28409.1 |
| SRCN_1743 | Pyruvate dehydrogenase kinase | Atypical/PDHK/BCKDK | 930 | 6.50 | 101.41 | 291–426 | PDHK, isoenzyme-2; (N. caninum L) | 458 | 6 × 10−146 | 45 | CEL70411.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murungi, E.K.; Kariithi, H.M. Genome-Wide Identification and Evolutionary Analysis of Sarcocystis neurona Protein Kinases. Pathogens 2017, 6, 12. https://doi.org/10.3390/pathogens6010012
Murungi EK, Kariithi HM. Genome-Wide Identification and Evolutionary Analysis of Sarcocystis neurona Protein Kinases. Pathogens. 2017; 6(1):12. https://doi.org/10.3390/pathogens6010012
Chicago/Turabian StyleMurungi, Edwin K., and Henry M. Kariithi. 2017. "Genome-Wide Identification and Evolutionary Analysis of Sarcocystis neurona Protein Kinases" Pathogens 6, no. 1: 12. https://doi.org/10.3390/pathogens6010012







