1. Introduction
Pigs are the natural host of
Streptococcus (S.) suis and different mucosal surfaces are colonized by
S. suis in many healthy pigs. However, virulent
S. suis strains might cross different barriers of the host tissue and cause pathologies such as meningitis, arthritis and endocarditis [
1,
2]. Different serotypes have been identified in
S. suis and differences in virulence among strains of different serotypes and even within serotype 2 are well known [
3,
4,
5]. Serotypes 2 and 9 are very important in Europe and in China [
6,
7,
8].
S. suis serotype 2 is also an important zoonotic pathogen [
9]. Humans working with pigs or pork are at particular risk for
S. suis infections [
10]. In Vietnam, Thailand and Hong Kong,
S. suis is one of the three most important bacterial agents causing meningitis in adult human beings [
11,
12]. Importantly, it has been shown that consumption of raw or undercooked meat is a major risk factor of this zoonosis in Vietnam [
13].
The tonsils are considered to be an important entry site for invasive
S. suis strains [
2]. Recently it has been demonstrated that the swine influenza virus infection of respiratory epithelial cells promotes adhesion and invasion of
S. suis serotype 2, suggesting that influenza virus–infected respiratory epithelium is also an important port of entry [
14,
15]. Interestingly,
S. suis serotype 2 has also been demonstrated to reach mesenteric-intestinal lymph nodes of pigs after intestinal application or after oral application of gastric-acid fast capsules containing the bacteria [
16,
17]. These studies demonstrate that
S. suis might cross the intestinal epithelial barrier but leave unanswered how
S. suis might survive passage through the stomach. Thus, it is still not clear whether
S. suis might also frequently cause oro-gastrointestinal infections in pigs as proposed.
Salmonella enterica is an oro-gastrointestinal pathogen causing different diseases in various animals. Pigs are very important carriers of zoonotic
Salmonella enterica. Different studies have demonstrated that feed has a strong impact on the survival of salmonellae in the porcine stomach. A high water-binding capacity, high concentrations of organic acids and a pH below 4 in the stomach caused by coarsely ground meal feeding are associated with a prominent reduction of live salmonellae [
18,
19,
20]. On the other hand, uptake of finely ground feed leading to a gastric pH close to 5 and a separation of the stomach contents into a sediment and liquid phase allows salmonellae to survive and infect new hosts.
Salmonellae respond to acid shock through a complex adaptation mechanism called the acid tolerance response, which includes the synthesis of over 50 acid shock proteins [
21,
22]. However, the low pH of the gastric milieu is not the sole bactericidal mechanism in the porcine stomach [
22]. Notably, bacterial adaptation to the gastric milieu is similar but not identical to the acid shock response. This has been shown for
Campylobacter jejuni which exhibits a distinct gene expression in the porcine stomach including the downregulation of ribosomal proteins and the upregulation of heat-shock and nitrosative stress proteins [
23].
S. suis expresses an arginine deiminase system confering protection against acidity in the presence of high arginine concentrations [
24], but the relevance of this finding for putative oro-gastrointenstinal infections and survival in the stomach is not known.
In this study we evaluated the survival of S. suis in the stomach contents of differently fed growing piglets as well as in conventional compound feed and conducted an oral infection experiment to study the role of oro-gastrointestinal infection in piglets.
2. Results
2.1. Evaluation of Survival of S. suis in Porcine Stomach Contents ex Vivo
This part of the study was designed to investigate the survival of
S. suis serotypes 2 and 9 in porcine stomach contents ex vivo in comparison to the survival of
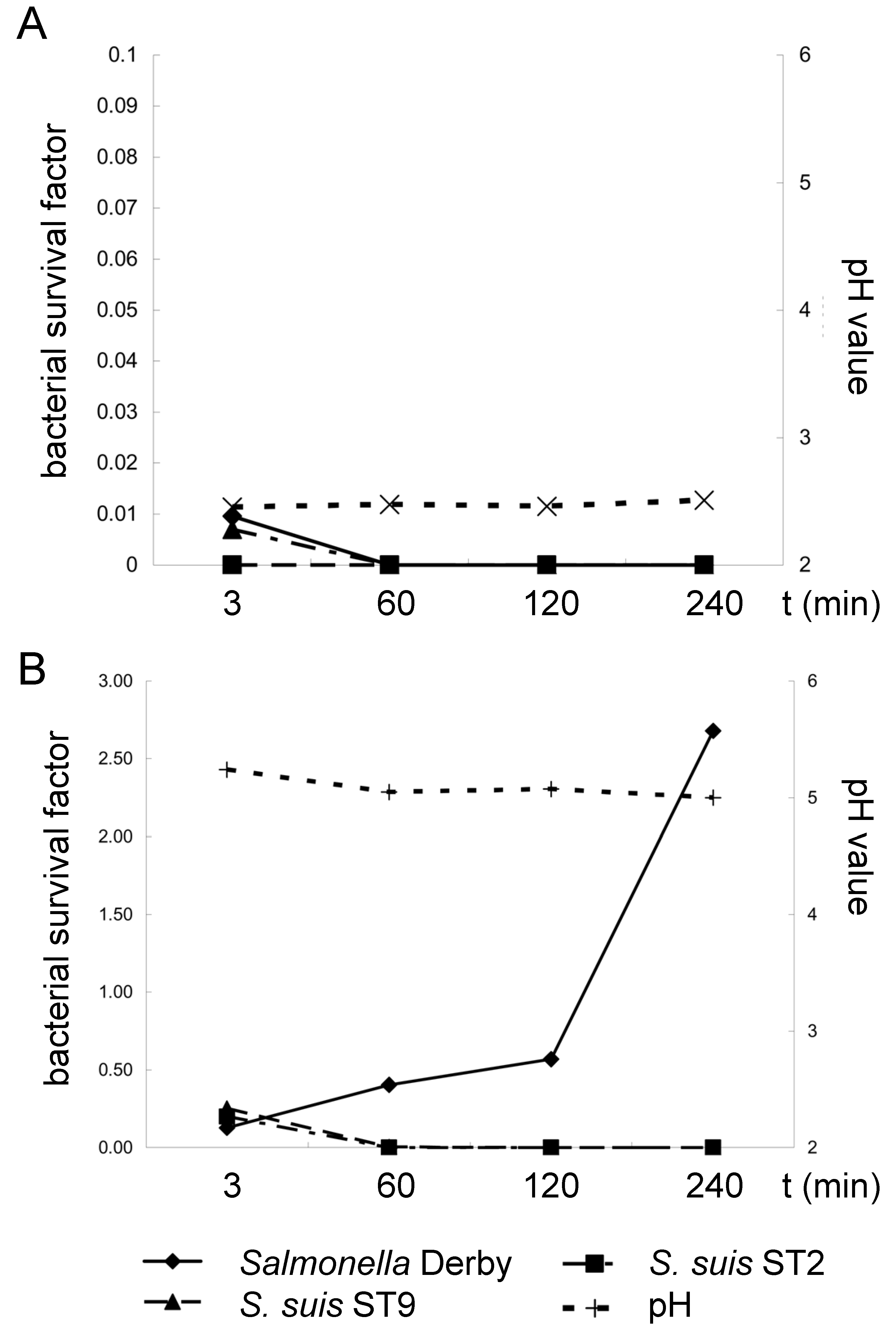
Salmonella enterica (here Serovar Derby), a pathogen known to survive the gastric passage under feeding conditions, leading only to moderate acidic conditions in the stomach. Stomach contents were either collected from piglets fed coarse meal or fine pellets, leading to a mean gastric pH of 2.5 and 5.0, respectively (
Figure 1). The content of culturable
S. suis and
Salmonella Derby bacteria were determined 3, 60, 120 and 240 min after mixing with the stomach contents.
S. suis serotypes 2 and 9 showed mean survival/detection rates of 0.20 (±0.20) and 0.25 (±0.27) 3 min after mixing with the stomach contents of piglets fed fine pellets. The survival/detection rates were calculated by dividing the specific bacterial content determined for a specific time point by the inoculum used for this sample (inoculum in relation to the mass of the respective stomach contents). Of note, determination of the specific bacterial content of the
S. suis serotypes was not hampered by the bacteria present in the stomach contents, as other typical α-hemolytic streptococci were not recorded and the selective media did not show overgrowth. After incubation of the mixed stomach contents for 60, 120 and 240 min at 37 °C,
S. suis was not detectable anymore. In contrast,
Salmonella Derby proliferated under these conditions, leading to a survival rate of 2.6 ± 1.3 after 240 min, significantly higher than the survival rates at 120 min and 60 min (
Figure 1B). Three minutes after the mixing of bacteria with stomach contents with a pH below 3 of piglets fed coarse meal, the survival/detection rates of
S. suis and
Salmonella Derby were below 0.1. Incubation of these mixtures at 37 °C for 60 min or longer resulted in negative results for
S. suis and
Salmonella Derby (
Figure 1B).
2.2. The Influence of the Growth Phase on the Survival of S. suis in Porcine Stomach Contents
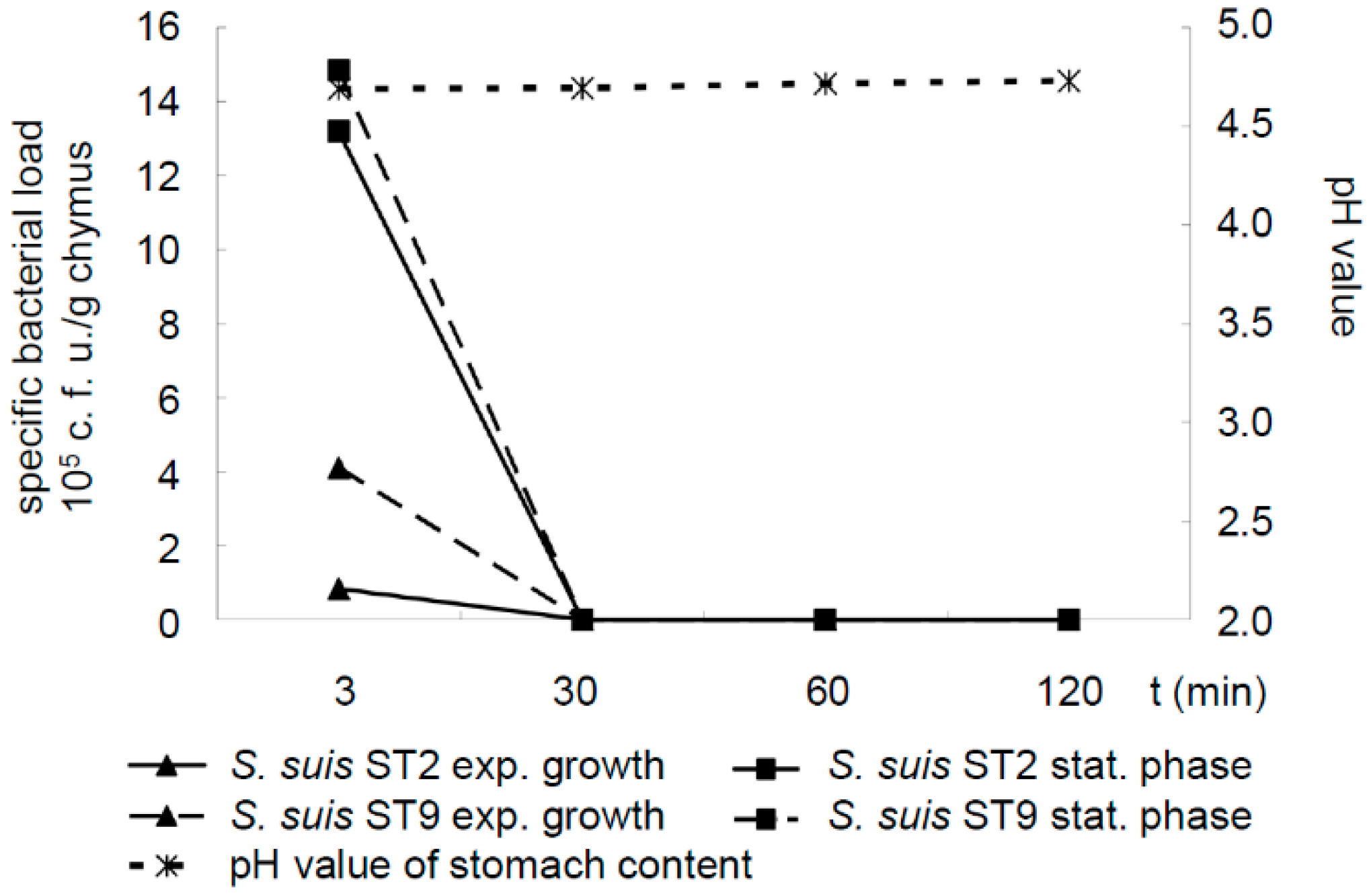
The arginine deiminase system, which is highly expressed in the stationary but not in the exponential growth phase, has been shown to protect
S. suis against low pH [
25,
26]. Thus, we investigated the survival of the
S. suis serotype 2 and 9 strains grown either to the exponential or the stationary phase in stomach contents with a moderate pH around 4.7 due to feeding fine pellets. However, live
S. suis bacteria were only detectable 3 min but not 30, 60 or 120 min after 37 °C incubation in these stomach contents, independently of the growth phase chosen for collecting streptococci. In conclusion, we did not identify conditions leading to the detectable survival of
S. suis in porcine stomach contents for at least 30 min incubation (
Figure 2).
2.3. Survival of S. suis in Compound Pig Feed
As S. suis is a common bacterium in the oral and nasal cavity of piglets, contamination of feed with S. suis is a likely process during feeding. We monitored the survival of S. suis in two kinds of compound feed in comparison to the survival of Salmonella Derby, as salmonellae are known to be transmitted via contaminated feed. Though feed might be contaminated with the two pathogens by different mechanisms, a comparison with salmonellae was included to demonstrate bacterial survival in the stomach contents under specific conditions.
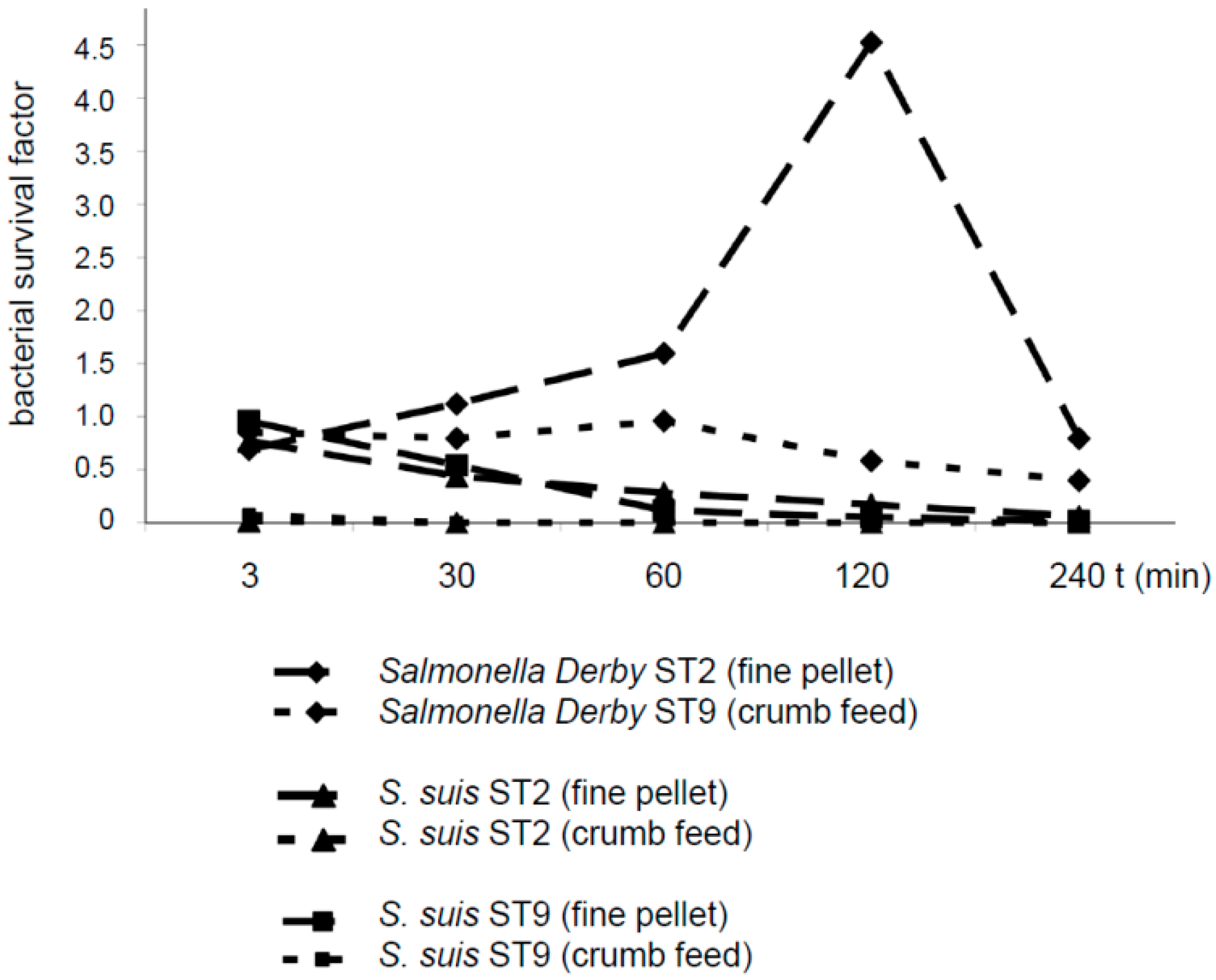
S. suis serotypes 2 and 9 were reduced in number in diet 1 (fine pellet not including formic acid) after an incubation period ranging from 30 to 240 min. After incubation in diet 2 (crumb feed including formic acid), neither
S. suis serotypes 2 or 9 were detectable at any time point after mixing (
Figure 3). In contrast,
Salmonella Derby increased in number in diet 1, reaching a survival rate of 4.5 after 120 min. In diet 2,
Salmonella Derby showed a survival factor close to 1 after incubation for the first 60 min, followed by a numerical reduction to a survival factor of approximately 0.4 at 240 min.
2.4. Determination of Acid Sensitivity of S. suis
We considered the low pH in the stomach contents and the feed to be bactericidal to S. suis. To address this further, formic acid was added to Todd-Hewitt broth (THB) in different concentrations to obtain pH values of 4.0, 4.3 and 5.3, and streptococci grown to exponential or stationary phase were added to these solutions. S. suis serotypes 2 and 9 were completely killed in THB plus formic acid with pH 4.0 within 1 h. At pH 4.3, S. suis serotypes 2 and 9 grown to the stationary but not to the exponential growth phase survived with low numbers of about 103 CFU/mL after 1 h incubation. Interestingly, S. suis serotypes 2 and 9 grown to stationary phase survived for 1 h at a pH of 5.3 in THB without a detectable reduction in the bacterial numbers.
2.5. Oral Infection Experiment of Piglets with S. suis
The in vitro results of this study indicated the clearance of S. suis serotypes 2 and 9 in different stomach contents, which was most likely related at least in part to the low pH. Because of data obtained in other studies suggesting oro-gastrointestinal infection, we nevertheless conducted an oral infection experiment with piglets designed to test whether S. suis causes intestinal infection after passage through the stomach. The bacteria were mixed with individual portions of feed to best mimic putative oro-gastrointestinal infection in the field. None of the piglets showed specific clinical signs of S. suis infection such as lameness or central nervous system dysfunction after oral application of 1 × 1010 CFU S. suis serotypes 2 or 9, a dose almost 10-fold higher than the dose used for inducing mortality after intranasal application in serotype 2 infection experiments. However, numerous piglets in each group including the control group demonstrated elevated body temperatures at different time points. The white blood cell count on days 5, 8, 11 and 15 post S. suis application revealed values below 22 × 109/l except for one animal in the serotype 2– and the serotype 9–infected group. The reason for the elevated body temperatures is unclear, but an association with S. suis infection is less likely, as control piglets (kept in a separate unit) were also affected and the high body temperature was not associated with leucocytosis or the detection of fibrinosuppurative lesions (see below).
As the incubation time of oro-gastrointenstinal S. suis infection is completely unknown, piglets were sacrificed at different time points post oral S. suis application (5, 8, 11, 15 dpi). As our aim was to demonstrate oro-gastrointestinal infection, we investigated numerous intestinal lymph nodes (Lnn. jejunales, ileocolici and colici) in addition to various internal organs bacteriologically and histologically. Neither S. suis serotypes 2 or 9 were detected in any of the internal organs or in any of the investigated intestinal lymph nodes. Furthermore, none of the piglets which had received feed mixed with either S. suis serotype 2 or serotype 9 showed a fibrinous or suppurative lesion in any of the investigated tissues including various mesenteric intestinal lymph nodes with the exception of one piglet with a focal suppurative jejunal lymphadenitis. The latter was, however, not positive for the challenge strain and other pathogenic bacteria were also not detected in this lymph node. It is worth noting that the animal experiment was designed to reveal intestinal infection leading at least to infection of the intestinal lymph nodes. Colonization or passage of the intestinal tract was not investigated. In conclusion, the oral infection experiment did not indicate intestinal infection after uptake of compound feed mixed with 1010 CFU S. suis serotype 2 or 9.
3. Discussion
The stomach acts as a barrier preventing harmful bacteria from entering and proliferating in the lower part of the gastrointestinal tract. One important defence mechanism of this barrier is its low pH. In this study we demonstrated that a virulent
S. suis serotype 2 and a virulent serotype 9 strain were killed in the stomach contents of pigs fed different diets, including a finely ground and pelleted diet leading to a comparably high pH of 5 in the stomach. The latter allowed salmonellae, a well-known oro-gastrointestinal pathogen, not only to survive but even to proliferate, indicating that salmonellae pass the stomach efficiently under these conditions in contrast to
S. suis. Furthermore, we did not detect our challenge strains after oral application in any internal organ or mesenteric intestinal lymph node. In contrast, Ferrando et al. [
16] recorded
S. suis serotypes 2 and 9 after oral application in mesenteric intestinal lymph nodes and translocation across a porcine intestinal epithelial cell barrier in vitro, indicating that
S. suis might pass the intestinal epithelial barrier. However, the authors applied
S. suis in gastric acid–resistant capsules. Thus, intestinal infection by
S. suis might occur once the pathogen has passed the stomach, but the conditions leading to sufficient passage through the stomach are unclear. It appears unlikely that oro-gastrointestinal infection constitutes a main infection route in growing piglets fed diets similar to the ones used in this study, as
S. suis did not survive in compound feed with commonly used additives such as formic acid, and was rapidly killed in stomach contents of differently fed growing piglets and infection was not observed after administration of very high oral doses. However, the putative passage of
S. suis through the stomach during the suckling period merits further investigation.
The findings of this study are surprising with regard to an earlier publication describing
S. suis as the predominant bacterial species in the stomach of 21-day-old weaning piglets [
27]. Though this difference might be related to the change in diet during weaning, it is also worth mentioning that the authors used a real-time PCR assay for
S. suis detection. They did not confirm live
S. suis bacteria by culture, which is very important as porcine saliva contains
S. suis in high numbers. Thus, their results do not confirm the passage of live
S. suis through the porcine stomach.
S. suis is thought to colonize the intestinal mucosa of pigs efficiently. This hypothesis is mainly based on the detection of the 16S rRNA gene in intestinal contents [
28,
29]. However, to the best of our knowledge, the role of the porcine intestinal tract as a putative reservoir and site of infection for important
S. suis pathotypes has not been investigated at all. Interestingly, Devriese et al. [
30] showed that most of the intestinal
S. suis bacteria belonged to a beta-glucuronidase–negative biotype, which is only rarely detected in invasive
S. suis infections. It is important to characterize putative
S. suis isolates of the intestinal tract in future studies using state-of-the-art phenotyping and genotyping.
Based on our results,
S. suis serotypes 2 and 9 did not survive in stomach contents with a pH = 5 for 60 min, though stable numbers of viable, stationary phase bacteria were recorded in THB with a pH = 5. Thus, porcine gastric fluid exhibited further bactericidal effects on
S. suis in addition to a low pH. This has also been shown for
Salmonella Typhimurium mutants [
22] and
Listeria monocytogenes [
31].
Interestingly, Grüning et al. [
26] demonstrated the survival of
S. suis at a pH of 4 in the presence of 25 mM l-arginine. This phenotype was reduced in an isogenic mutant of the
arcA gene encoding the arginine deiminase. Thus, the arginine deiminase system might be important to cope with the low pH in the stomach. However, free arginine is very limited in the diets of pigs [
32,
33]. As free l-arginine might reach higher concentrations after the uptake of food of animal origin,
S. suis might still pass through the human stomach in high numbers after eating “high-risk dishes”, which were identified in a case-control study conducted in Vietnam [
13]. Thus, the results of this study do not at all exclude oro-gastrointestinal infection as an important infection route in humans eating undercooked meat, blood and intestine of
S. suis ST2–infected pigs in Vietnam and other countries. However, colonization of the human intestinal tract by
S. suis seems to be uncommon, as rectal samples of more than 1500 healthy individuals were negative in a PCR for
S. suis [
13].
The presented data suggests that the gastric passage of live S. suis bacteria is an uncommon process in growing piglets, the age class most often and most severely affected by S. suis diseases. This does not rule out that S. suis diseases in growing piglets develop after intestinal translocation of bacteria that have colonized the intestinal tract of these piglets in the previous weeks and that have passed through the stomach under very different conditions in the neonatal, suckling or weaning periods. Thus, it is important to conduct further studies on putative oro-gastrointestinal S. suis infections in piglets. This should include experimental designs to identify conditions leading to passage of the stomach, which is a very important barrier against S. suis infection, as shown in this study.
4. Materials and Methods
4.1. Bacteria
The
S. suis reference strains 10 and A3286/94 were used in this study and are referred to as serotype 2 and 9 strain, respectively. Strain 10 is an MRP+ EF+ SLY+ serotype 2 strain which has been used successfully by different groups to induce disease experimentally in either intranasal or intravenous application models with piglets [
34,
35,
36,
37,
38,
39]. The MRP* SLY+ serotype 9
S. suis strain A3286/94 caused disease after intravenous injection but only subclinical pathological findings after intranasal application [
40]. Cultivation of
S. suis was conducted on Columbia agar with 6% sheep blood supplemented in appropriate cases with StaphStrep selective Supplement or in Todd-Hewitt broth (THB, all Oxoid, Wesel, Germany) at 37 °C for 24 h.
The Salmonella enterica ssp. enterica Serovar Derby (Salmonella Derby) strain A147/85 (O 1,4, 12; H:f,g) was cultivated on Columbia agar with 6% sheep blood, on BrillianceTMSalmonella agar, on Brilliant Green agar (all Oxoid, Wesel, Germany) or in lysogeny broth as appropriate.
4.2. Virulence-Associated Gene Profiling of Putative S. suis Isolates by PCR
A previously described multiplex (MP) PCR was used to detect the virulence-associated genes
mrp,
epf,
sly,
gdh,
arcA,
cps1J,
cps2J,
cps7H and
cps9H in
S. suis isolates [
8].
4.3. Determination of Bacterial Survival in Porcine Stomach Contents ex Vivo
Stomach contents of the gastric fundus region were collected 6 h postprandial and directly after killing from male growing piglets (n = 5 per feed) with a body weight of 33.3 ± 6.0 kg. These piglets were from an SPF herd known to be free of salmonellae and mrp+ epf+ sly+ cps2 as well as cps9 S. suis strains. They were either fed a finely ground and pelleted (“fine pellets”; n = 5) or coarsely ground meal diet (“coarse meal”; n = 5) with a dry matter content of 914 g/kg and 884 g/kg, respectively. The particle size distribution after wet sieve analysis of fine pellets showed 5% larger than 1 mm and 54% smaller than 0.2 mm resulting in a geometric mean diameter (GMD) of 217 µm. Corresponding parameters for the coarse meal were 53% larger 1 mm and 26% smaller than 0.2 mm and 671 µm GMD. The energy contents of the fine pellets and coarse meal were 13.9 MJ/kg and 13.5 MJ/kg, respectively. The different stomach contents resulting from the feeding of these diets were used for comparative analysis of survival of S. suis serotypes 2 and 9 as well as Salmonella Derby.
For analysis of survival of S. suis serotypes 2 and 9 grown to exponential versus stationary phase contents of the gastric fundus region were collected 6 h postprandial from 6 piglets with a body weight of 22.3 ± 2.7 kg fed fine pellets with 42.4% of particles smaller than 0.2 mm and 292 µm GMD. After necropsy of piglets stomach contents were transferred to sterile plastic containers and kept at −20 °C until use.
For determination of bacterial survival 10 g stomach content was mixed with 1 mL of a bacteria-PBS suspension containing a calculated specific bacterial load of 2 × 108 CFU of either Salmonella Derby A147/85 (grown to OD600 = 0.6), S. suis serotype 2 strain 10 or S. suis serotype 9 strain A3286/94 (both grown to OD600 = 0.8) or as negative control PBS without bacteria. Accompanying bacteria of the stomach contents did not overgrow the Columbia blood and StaphStrep selective agar plates and did not include similar α-haemolytic streptococci. All typical α-haemolytic streptococci were screened in the MP-PCR for virulence-associated genes to confirm the genotype used for inoculation. This was conducted for each of the indicated time points of analysis in a separate air tight sealed bag. Bags were incubated at 37 °C in a shaking water bath after mixing. The pH in the stomach content was measured at every sampling. A 10-fold dilution series in PBS was made for determination of the specific bacterial load. The first dilution was homogenized in an air tight sealed bag using a stomacher (Stomacher 400 Circulator, Fa. Seward, Worthing, West Sussex, UK). Dilutions were plated onto selective media for streptococci and salmonellae as well as on Columbia blood agar as specified above. The survival factor was calculated by dividing the specific bacterial content at a specific time point (CFU/g) by the inoculation dose. The second experiment investigating survival of S. suis grown to different ODs comparatively was conducted alike only that 1.8 × 108 CFU of S. suis serotype 2 strain 10 or S. suis serotype 9 strain A3286/94 grown to OD600 = 0.6 or 1.2 (as indicated) were mixed with the stomach content.
4.4. Determination of Bacterial Survival in Pig Feed
Ten g of conventional compound feed (dry starter diet, either as fine pellet or as crumb feed) based on wheat, barley and soybean was mixed with 1 mL PBS solution containing 1.9 × 108 CFU Salmonella Derby or 7.5 × 109 CFU S. suis serotype 2 or 6.8 × 109 S. suis serotype 9 and incubated for the indicated time points (3, 30, 60, 120 and 240 min) at room temperature. The compound feed in crumb form contained also 3.5 g formic acid/kg uS. The specific bacterial load was determined using the stomacher for homogenization and a 10-fold dilution series in PBS as described above. Quantification was possible as the accompanying bacteria of the feed did not overgrow the media used for cultivation and did not include α-haemolytic streptococci. The S. suis genotypes were confirmed in the MP-PCR for virulence-associated genes.
4.5. Determination of Survival of S. suis in Culture Medium after Addition of Formic Acid
Formic acid (98%–100%, Sigma-Aldrich-Chemie GmbH, Taufkirchen) was added at concentrations of 3.5, 2.4 and 1.2 g/kg to 10 ml THB immediately after addition of 1 × 108 CFU S. suis serotypes 2 or 9 grown as indicated to OD600 = 0.6 or 1.2. This led to a pH of 4.0, 4.3 and 5.3, respectively. THB-medium without formic acid was included as positive control.
For addition of defined numbers of bacteria the S. suis strain was grown to OD600 = 0.6 and 1.2. Aliquots of these cultures were frozen via liquid nitrogen after addition of 10% glycerol. The specific bacterial content of these aliquots was determined using serial dilutions.
4.6. Oral S. suis Infection of Piglets
The piglets used in this study were cared for in accordance with the principles outlined in the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes and the German Animal Protection Law. The animal experiment was approved by the Lower Saxonian State Office for Consumer Protection and Food Safety (permit no. 33.14-42502-04-12/0803, LAVES). The 27 piglets used in this study were from a herd that is free of cps7, cps9 and mrp+ epf+ sly+ cps2 S. suis strains. After weaning the piglets were trained to feed from individual plastic containers allowing application of a distinct oral dosage of bacteria through feeding. Commercially available compound feed in crumb form (deuka primo pro gran, Deutsche Tierernährung Cremer GmbH & Co. KG, Bramsche, Germany) with 15.5 MJ ME/kg feed including 3.5 g formic acid/kg uS was used for restricted feeding after weaning. The feed’s particle size distribution was 28% larger than 1 mm and 42% smaller than 0.2 mm. After 18 h abrosia each piglet received 50 g feed prior to application of the infection dose to increase the gastric pH. After a 5 min pause piglets received 10 g feed supplemented with 5 ml of a PBS suspension including either 1010 CFU S. suis serotype 2, 1010 CFU S. suis serotype 9 or no added bacteria (for each n = 9 piglets). The health of the piglets was closely monitored including measurement of body temperature, evaluation of feeding, breathing and movement of animals as well as fecal consistency every 8 h. A body temperature above 40.0 °C was considered elevated. On days 1, 5, 8, 11 and 15 with regard to S. suis application blood samples were taken from all living piglets for hematological screening. White blood cells were counted using a hemocytometer chamber. Leukocytes were differentiated by Wright stained blood smears.
Two piglets per group (serotype 2, serotype 9 and control) were sacrificed on days 5, 8 and 15 post
S. suis application. On day 11 three piglets per group were killed. Necropsy was conducted with every animal including comprehensive sampling for bacteriological and histological examinations as described [
34,
35]. In addition to various samples of internal organs taken in all our infection experiments numerous
Lnn. jejunales,
Lnn. ileocaecales and
Lnn. colici were collected and investigated bacteriologically and histologically. For bacteriological analysis the lymph nodes were cut in many sections. The cut surfaces were slightly pressed against Columbia blood and StaphStrep selective agar plates. The plates were streaked for single colonies. All α-hemolytic streptococci detected in internal organs or mesenterial intestinal lymph nodes were screened in the MP PCR for profiling of virulence-associated factors.
4.7. Statistics
Statistical analysis was performed by SAS 9.3 for Windows using PROC MEANS or the Wilcoxon test for differences between time points and student’s t-test (PROC GLM) for those between groups or bacteria. Values are expressed as mean ± standard deviation (SD). Probabilities lower than 0.05 were considered significant.