Global Occurrence and Emission of Rotaviruses to Surface Waters
Abstract
:1. Introduction
- “All urban wastewater is adequately treated before being reused or discharged to the (aquatic) environment.
- All wastewater is managed in a sustainable way to protect water resources and aquatic ecosystems.” [12].
2. Results
2.1. Literature Review
2.1.1. RV Incidence
| Continent | Country | Duration of Study | # Sample Recruited | Age (years) | (%) Prevalence of RV Diarrheal Cases | Reference |
|---|---|---|---|---|---|---|
| Africa | Kenya | 2009–2011 | 500 | <5 | 38 | [59] |
| Libya | 2007–2008 | 1090 | <5 | 31.5 | [60] | |
| Morocco | 2006–2009 | 1388 | <5 | 41.7 | [61] | |
| Morocco | 2011 | 335 | <5 | 26.6 | [61] | |
| Sierra Leone | 2005 | 128 | <3 | 37.5 | [62] | |
| South Africa | 2003–2006 | 3191 | <5 | 22.8 | [63] | |
| Tunisia | 2007–2010 | 435 | <13 | 27.6 | [64] | |
| Asia | Cambodia | 2005–2007 | 2281 | <5 | 56 | [65] |
| China | 2008–2009 | 766 | <5 | 27.94 | [66] | |
| China | 2011–2012 | 767 | <5 | 34.3 | [67] | |
| India | 2004–2008 | 412 | <3 | 19.2 | [68] | |
| India | 2009–2011 | 1807 | <5 | 35.9 | [69] | |
| India | 2009–2012 | 1191 | <5 | 39 | [70] | |
| India | 2007–2012 | 756 | <5 | 38.4 | [71] | |
| Lao PDR | 2005–2007 | 1158 | <5 | 54 | [72] | |
| Myanmar | 2004–2005 | 2179 | <5 | 56.3 | [73] | |
| South Korea | 2005–2007 | 6057 | <5 | 22 | [74] | |
| South Korea | 2007–2008 | 702 | <1 | 25.2 | [75] | |
| Taiwan | 2005–2007 | 3435 | <5 | 25 | [76] | |
| Europe | Albania | 2007–2010 | 1066 | <5 | 21 | [77] |
| France | 2001–2004 | 457 | <15 | 48.8 | [78] | |
| Spain | 2006–2008 | 2048 | <5 | 40.1 | [79] | |
| Middle East | Iran | 2009–2010 | 163 | <5 | 46.02 | [80] |
| Israel | 2007–2008 | 472 | <5 | 39.1 | [81] | |
| Saudi Arabia | 2002–2003 | 1000 | <6 | 6 | [82] | |
| South America | Argentina | 2004–2007 | 710 | <15 | 19.7 | [83] |
| Venezuela | 2013 | 480 | <5 | 21 | [84] |
2.1.2. Excretion Rate
| Continent | Country | Year of Study | # Samples Collected (n) | # (%) RV Positive | RV Concentration | Reference |
|---|---|---|---|---|---|---|
| Africa | Egypt | 2006–2007 | 72 | 6 (8.3) | NRa | [94] |
| Kenya | 2007–2008 | 13 | 9 (69.2) | NR | [40] | |
| Tunisia | 2003–2007 | 125 | 53(42.4) | NR | [96] | |
| Americas | Argentina | 2009 | 52 | 52 (100) | NR | [97] |
| Brazil | 2004–2005 | 24 | 11(45.8) | NR | [98] | |
| Brazil | 2009–2010 | 24 | 24 (100) | 2.40E+05 genome copies/L b | [99] | |
| Brazil | 2009 | 7 | 2 (28.6) | NR | [100] | |
| USA | 2011–2012 | 24 | 14 (58.3) | 2.8E+06 genome copies/L c | [101] | |
| Venezuela | 2007–2008 | 12 | 8 (66.7) | NR | [102] | |
| Asia | China | 2006–2007 | 10 | 10 (100) | NR | [103] |
| China | 2006–2007 | 36 | 16 (44.4) | 3.12E+03 genome copies/L b | [104] | |
| India | 2009–2010 | 144 | 111(77) | NR | [105] | |
| Europe | France | 2003–2004 | 29 | 11(37.9) | NR | [106] |
| Italy | 2006–2007 | 16 | 6 (37.5) | NR | [107] | |
| Italy | 2010–2011 | 285 | 172 (60.4) | NR | [108] | |
| Sweden | 2013 | 7 | 7 (100) | NR | [109] | |
| Middle East | Iran | 2010–2011 | 15 | 5 (33.3) | NR | [110] |
2.1.3. Removal during Wastewater Treatment
| Country | Concentration in Sewage (Genome copies/L) | Seasonality in Sewage (m/y) | Wastewater per Capita (L per day) | RV Incidence (Episodes per person per year) | Shedding (Genome Copies per case per day) | References |
|---|---|---|---|---|---|---|
| Brazil | 1.0E+06 | 12 | 144 | 0.24 | 3.1E+10 | [88,99,116] |
| China | 6.8E+03 | 6 | 190 | 0.15 | 2.2E+08 | [88,104,114,115] |
| NL | 4.6E+03 | 6 | 306 | 0.021 | 1.8E+09 | [87,88,90,113] |
| USA | 1.0E+05 | 6 | 265 | 0.0093 | 7.4E+10 | [88,101,111,112] |
| Virus a | Country b | Detection Method c | Treatment process(es) d | n | Removal/Inactivation | Reference | |
|---|---|---|---|---|---|---|---|
| Mean (log10) | Stdev (Log10) | ||||||
| RV | China | ICC-qPCR | Activated sludge | 12 | 2.08 | 0.63 | [117] |
| RV | China | ICC-qPCR | Activated sludge | 12 | 2.83 | 0.49 | [117] |
| RV | China | ICC-qPCR | Activated sludge | 12 | 2 | 1.1 | [117] |
| RV | China | ICC- qPCR | Coagulative precipitation and sand filtration | 12 | 0.72 | 0.08 | [117] |
| PPMV | USA | qPCR | Activated sludge + Cl2 | 12 | 0.76 | 0.53 | [101] |
| PPMV | USA | qPCR | Trickling filter + Cl2 | 12 | 0.99 | 0.12 | [101] |
| AdV | USA | qPCR | Activated sludge + Cl2 | 12 | 0.7 | [101] | |
| AdV | USA | qPCR | Trickling filter + Cl2 | 12 | 1.5 | [101] | |
| JCPyV | USA | qPCR | Activated sludge + Cl2 | 12 | 1.64 | 0.98 | [101] |
| JCPyV | USA | qPCR | Trickling filter + Cl2 | 12 | 2.56 | 0.64 | [101] |
| BKPyV | USA | qPCR | Activated sludge + Cl2 | 12 | 1 | [101] | |
| BKPyV | USA | qPCR | Trickling filter + Cl2 | 12 | 1.5 | [101] | |
| AiV | USA | qPCR | Activated sludge + Cl2 | 12 | 0.94 | 0.33 | [101] |
| AiV | USA | qPCR | Trickling filter + Cl2 | 12 | 0.99 | 0.12 | [101] |
| EV | USA | qPCR | Activated sludge + Cl2 | 12 | 1.5 | [101] | |
| EV | USA | qPCR | Trickling filter + Cl2 | 12 | 2.1 | [101] | |
| EV | NL | CC | Activated sludge | 5 | 1.4 | 0.42 | [90] |
| ReoV | NL | CC | Activated sludge | 5 | 1.2 | 0.22 | [90] |
| HAdV | Spain | qPCR | Tertiary | 1.2 | [119] | ||
| HAdV | Spain | qPCR | Tertiary | 1.9 | [119] | ||
| AdV | USA | qPCR | Membrane Bioreactor | 3.9–5.5 | [120] | ||
| NoVII | USA | qPCR | Membrane Bioreactor | 4.6–5.7 | [120] | ||
2.2. Global Rotavirus Emissions
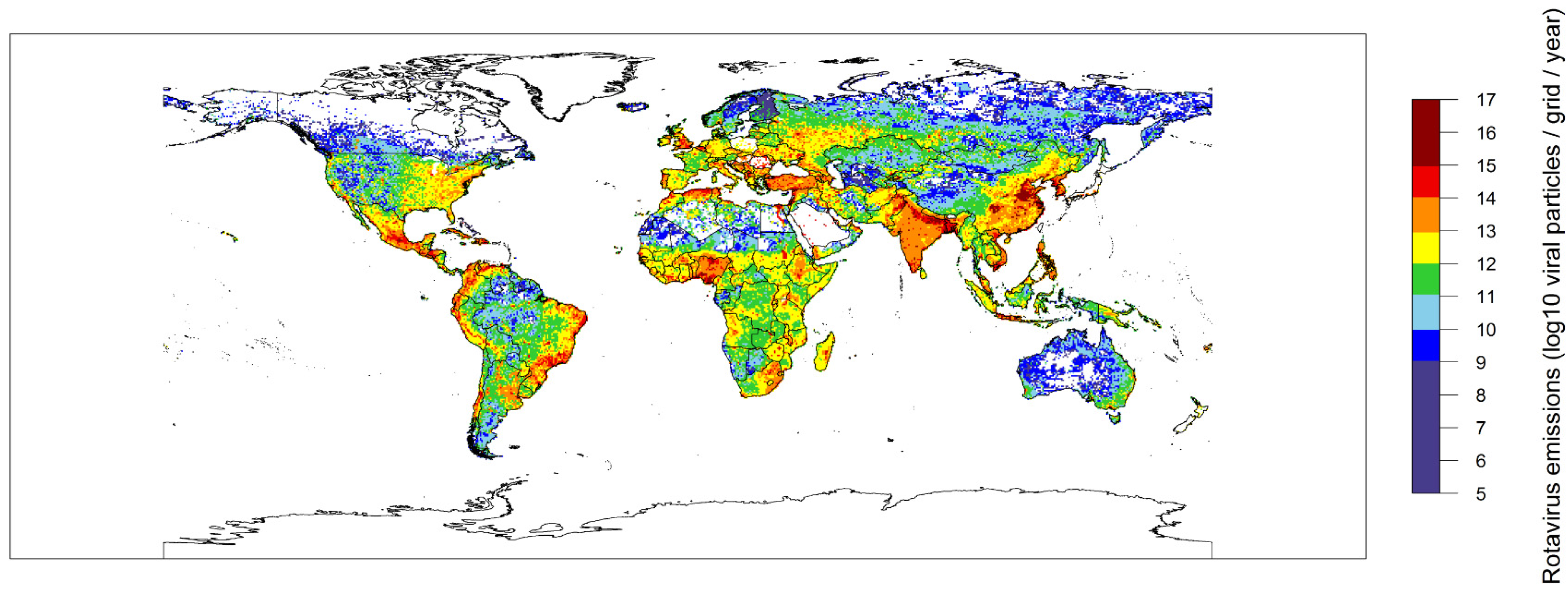
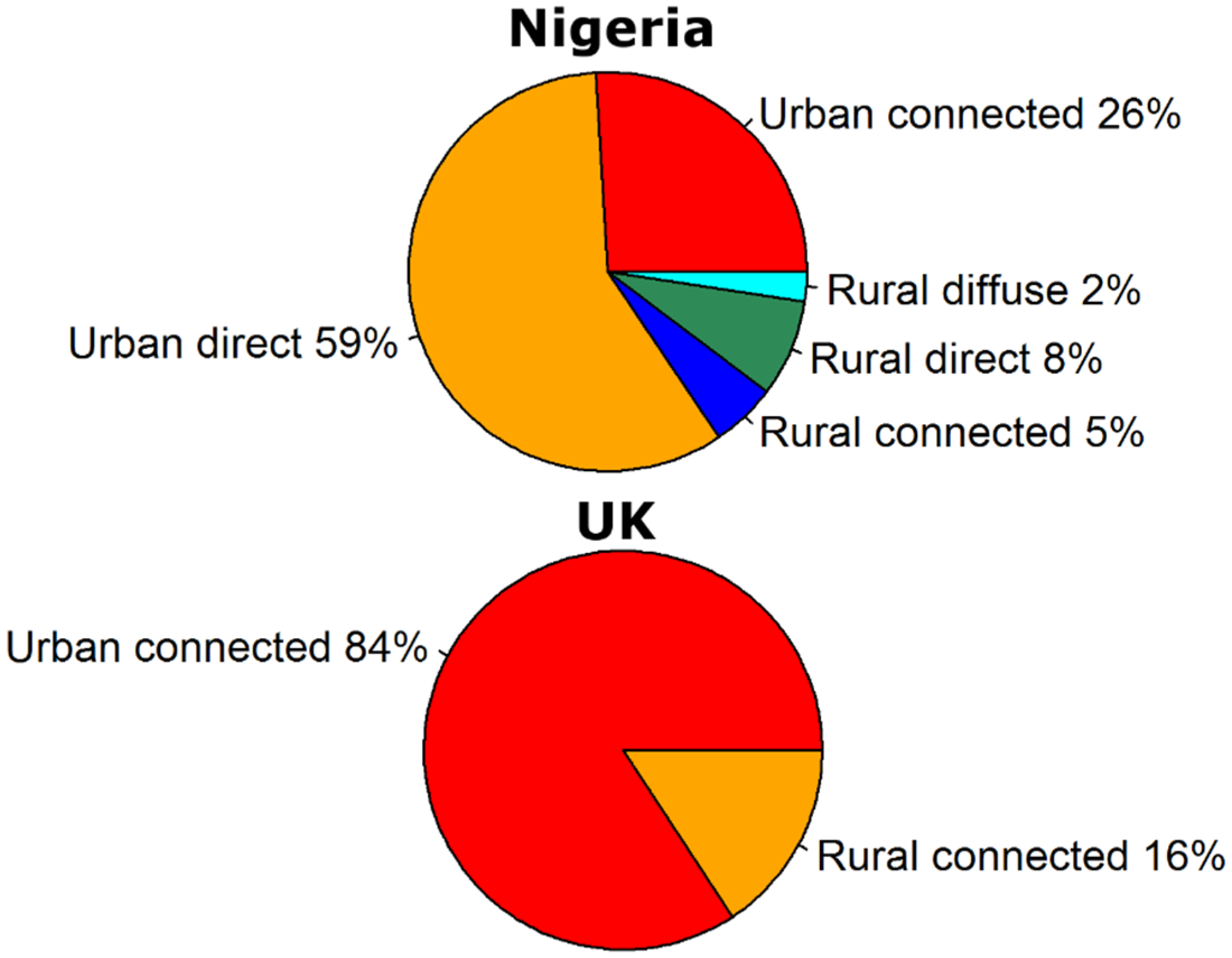
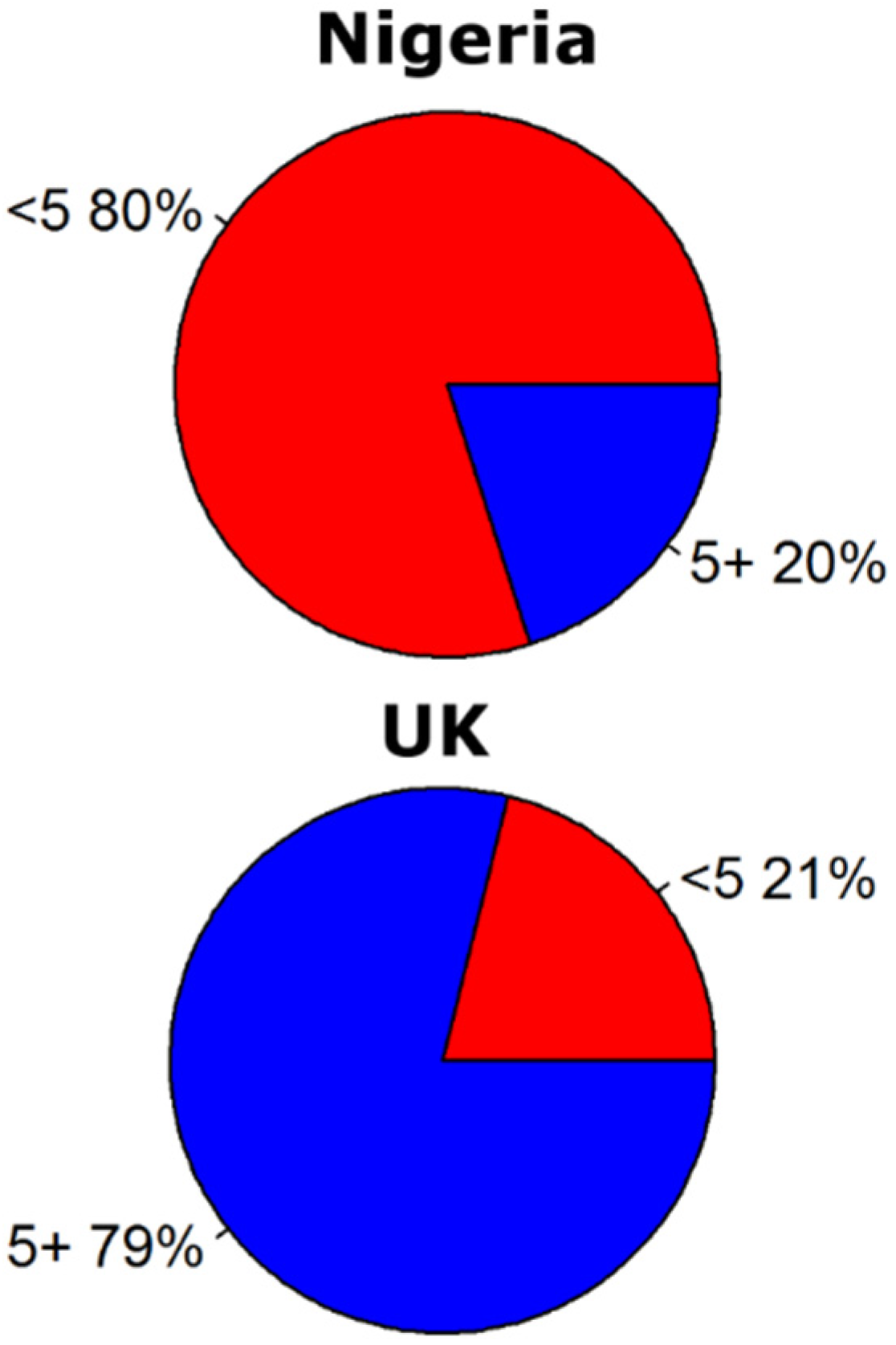
3. Discussion and Conclusions
4. Experimental Section
4.1. Literature Review
4.1.1. Search Strategy
4.1.2. Quality Assessment
4.2. Explanation of the GloWPa-Rota H1 Model
- -
- Pu and Pr are the total urban and rural population of a country, respectively.
- -
- fage the fraction of the population for the age categories (<5, 5–14, 15–25 and 25+)(−).
- -
- fcu and fcr are the fractions of the urban and rural populations using sanitation that is connected to a sewer system (−).
- -
- fdu and fdr are the fractions of the urban and rural populations using sanitation that is a direct source (−). As explained in Vermeulen et al. [124], this includes WHO/UNICEF Joint Monitoring Programme (JMP) sanitation types hanging toilets (for both urban and rural population) and no facility, bush, field, unknown, elsewhere, other unimproved (for urban population only).
- -
- fdifr is the fraction of the rural population that has no sanitation facilities and forms a diffuse source (−). This includes JMP sanitation type no facility, bush, field, unknown, elsewhere, other unimproved Vermeulen et al. [124].
- -
- frun is the fraction of feces transported with runoff from land to surface water (−). frun is assumed to be 0.025, which is the median value for animal manure mobilization estimated in Ferguson et al. [125]. The actual RV mobilization depends on wet weather events, runoff amounts, dry weather duration, manure accumulation on the land and survival in the manure. Such an in-depth analysis is a step too far for this paper, so we assume RV mobilization is comparable to animal manure mobilization. That may be a slight overestimation.
- -
- Vp,age is the average viral particle excretion (viral particles person−1·year−1). Vp differs for the age categories and is calculated as described in Section 2.1.1.
- -
- frem is the fraction of viral particles removed by wastewater treatment (−). frem is calculated as described below (Equation (10).
| Variable | Variable Name | Data Source |
|---|---|---|
| P | Population | [127] |
| Pu | Urban population | Urban fraction x P |
| Pr | Rural population | (1 − urban fraction) × P |
| fage | Fraction of the population younger than 5 years of age, from 5 to 14, from 15 to 25 and older than 25. | UN World Population Prospects [128] |
| HDI | Human Development Index | [129] |
| fcu, fcr fdu, fdr fdifr | Fraction connected (urban and rural) Fraction direct (urban and rural) Fraction diffuse (rural only) | WHO/UNICEF JMP data [121], www.wssinfo.org). Year closest to 2010 was taken from JMP country files. When unavailable, fractions were estimated based on the fraction connected used in Van Puijenbroek et al. [130], which were based on WHO/UNICEF [121] supplemented with data from [131,132,133]. When incomplete (mostly missing values only around 0.01–0.02), missing values were added to non-source, or in case non-source was non-existing, to the category with the highest fraction. |
| fp, fs and ft | Fraction primary, primary + secondary and primary + secondary + tertiary treatment | [131,132,133] as explained in Van Puijenbroek et al. [130] |
| Population density in a grid cell | LandScan 2010 data [126] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Onda, K.; LoBuglio, J.; Bartram, J. Global access to safe water: Accounting for water quality and the resulting impact on MDG progress. Int. J. Environ. Res. Public Health 2012, 9, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Moe, C.L.; Rheingans, R.D. Global challenges in water, sanitation and health. J. Water Health 2006, 4, 41. [Google Scholar] [PubMed]
- WHO. Progress on Drinking-Water and Sanitation–2012 Update" Launched on 6 March 2012; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- WHO/UNICEF. Joint Monitoring Programme (JMP) for Water Supply and Sanitation, Progress on Sanitation and Drinking-Water, 2013 Update; WHO/UNICEF: Geneva, Switzerland, 2013. [Google Scholar]
- WHO/UNICEF. Joint Monitoring Programme (JMP) for Water Supply and Sanitation, Progress on Sanitation and Drinking-Water, 2014 Update; WHO/UNICEF: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. Global Health Observatory Data Repository. Mortality and Global Health Estimates: Child Mortality: Causes of Child Death: Number of Deaths by Cause: By Region: World: Diarrhoeal Diseases; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Walker, C.L.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global burden of childhood pneumonia and diarrhea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Inter-Agency Group for Child Mortality Estimation. Levels & Trends in Child Mortality, Report 2013. New York: United Nations Children’s Fund. Available online: http://www.childinfo.org/files/Child_Mortality_Report_2013.pdf (accessed on 16 March 2015).
- WHO/UNICEF. First Consultation on post-2015 Monitoring of Drinking-Water and Sanitation; WHO/UNICEF: Geneva, Switzerland, 2011. [Google Scholar]
- Baum, R.; Luh, J.; Bartram, J. Sanitation: A global estimate of sewerage connections without treatment and the resulting impact on MDG progress. Environ. Sci. Technol. 2013, 47, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Open Working Group Proposal for Sustainable Development Goals. Available online: https://sustainabledevelopment.un.org/sdgsproposal (accessed on 10 March 2015).
- La Rosa, G.; Fratini, M.; della Libera, S.; Iaconelli, M.; Muscillo, M. Emerging and potentially emerging viruses in water environments. Ann. Ist. Super. Sanita 2012, 48, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.F.; Davidson, G.P.; Holmes, I.H.; Ruck, B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet 1973, 2, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.; Kapikian, A. Rotaviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Wolters Kluwer/Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2007; Volume 2, pp. 1917–1974. [Google Scholar]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef] [PubMed]
- QMRAwiki. Available online: Http://qmrawiki.Canr.Msu.Edu/index.Php/rotavirus:_dose_response_models (accessed on 16 March 2015).
- O’Ryan, M.L.; Lucero, Y.; Vidal, R. Enteric viruses in wastewaters: An interesting approach to evaluate the potential impact of rotavirus vaccination on viral circulation. Expert Rev. Vaccines 2012, 11, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.F. Natural history of human rotavirus infection. Arch. Virol. Suppl. 1996, 12, 119–128. [Google Scholar] [PubMed]
- Ward, R.L.; Bernstein, D.I.; Young, E.C.; Sherwood, J.R.; Knowlton, D.R.; Schiff, G.M. Human rotavirus studies in volunteers: Determination of infectious dose and serological response to infection. J. Infect. Dis. 1986, 154, 871–880. [Google Scholar] [CrossRef] [PubMed]
- WHO. Generic portocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children: Field test version. 2002. [Google Scholar]
- Caceres, V.M.; Kim, D.K.; Bresee, J.S.; Horan, J.; Noel, J.S.; Ando, T.; Steed, C.J.; Weems, J.J.; Monroe, S.S.; Gibson, J.J. A viral gastroenteritis outbreak associated with person-to-person spread among hospital staff. Infect. Control Hosp. Epidemiol. 1998, 19, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wikswo, M.E.; Hall, A.J. Outbreaks of acute gastroenteritis transmitted by person-to-person contact--United States, 2009–2010. MMWR Surveill. Summ. 2012, 61, 1–12. [Google Scholar] [PubMed]
- CDC. Foodborne outbreak of group A rotavirus gastroenteritis among college students--District of Columbia, March-April 2000. MMWR Morb. Mortal. Wkly. Rep. 2000, 49, 1131–1133. [Google Scholar]
- Mwenda, J.M.; Ntoto, K.M.; Abebe, A.; Enweronu-Laryea, C.; Amina, I.; McHomvu, J.; Kisakye, A.; Mpabalwani, E.M.; Pazvakavambwa, I.; Armah, G.E.; et al. Burden and epidemiology of rotavirus diarrhea in selected African countries: Preliminary results from the African Rotavirus Surveillance Network. J. Infect. Dis. 2010, 202 (Suppl.), S5–S11. [Google Scholar] [CrossRef]
- Lee, R.M.; Lessler, J.; Lee, R.A.; Rudolph, K.E.; Reich, N.G.; Perl, T.M.; Cummings, D.A. Incubation periods of viral gastroenteritis: A systematic review. BMC Infect. Dis. 2013, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Burton, A.; Lanata, C.; Boschi-Pinto, C.; Shibuya, K.; Steele, D.; Birmingham, M.; Glass, R.I. Global mortality associated with rotavirus disease among children in 2004. J. Infect. Dis. 2009, 200 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Peter, R.; Aramugam, R.; Ismail, N.; Veeraraghavan, B.; Kang, G. Group A rotavirus gastroenteritis in older children and adults at a hospital in Southern India. Vaccine 2014, 32 (Suppl. 1), A33–A35. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Weber, S.G. Rotavirus infection in adults. Lancet Infect. Dis. 2004, 4, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gastanaduy, P.A.; Curns, A.T.; Parashar, U.D.; Lopman, B.A. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA 2013, 310, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nakagomi, T.; Kamisawa, T.; Sakaki, N.; Muramoto, K.; Mikami, T.; Nara, H.; Nakagomi, O. Winter seasonality and rotavirus diarrhea in adults. Lancet 2001, 357, 1950. [Google Scholar] [CrossRef] [PubMed]
- WHO. Rotavirus vaccines. WHO position paper—January 2013. Wkly. Epidemiol. Rec. 2013, 88, 49–64. [Google Scholar]
- Grabow, W.O.K. Overview of health- related water virology. Hum. Viruses Water 2007, 17, 1–25. [Google Scholar]
- Mellou, K.; Katsioulis, A.; Potamiti-Komi, M.; Pournaras, S.; Kyritsi, M.; Katsiaflaka, A.; Kallimani, A.; Kokkinos, P.; Petinaki, E.; Sideroglou, T.; et al. A large waterborne gastroenteritis outbreak in Central Greece, March 2012: Challenges for the investigation and management. Epidemiol. Infect. 2014, 142, 40–50. [Google Scholar]
- Gallay, A.; De Valk, H.; Cournot, M.; Ladeuil, B.; Hemery, C.; Castor, C.; Bon, F.; Megraud, F.; Le Cann, P.; Desenclos, J. A large multi-pathogen waterborne community outbreak linked to fecal contamination of a groundwater system, France, 2000. Clin. Microbiol. Infect. 2006, 12, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, M.A.; Haas, N.L.; Hunt, R.J. Vulnerability of drinking-water wells in La Crosse, Wisconsin, to enteric-virus contamination from surface water contributions. Appl. Environ. Microbiol. 2004, 70, 5937–5946. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, M.; Yakupogullari, Y.; Otlu, B.; Ozturk, S.; Ozden, M.; Ozer, A.; Sener, K.; Durmaz, R. A waterborne outbreak of epidemic diarrhea due to group A rotavirus in Malatya, Turkey. Microbiol. Q. J. Microbiol. Sci. 2011, 34, 17. [Google Scholar]
- Villena, C.; Gabrieli, R.; Pinto, R.; Guix, S.; Donia, D.; Buonomo, E.; Palombi, L.; Cenko, F.; Bino, S.; Bosch, A. A large infantile gastroenteritis outbreak in Albania caused by multiple emerging rotavirus genotypes. Epidemiol. Infect. 2003, 131, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Prato, R.; Chironna, M.; Sallustio, A.; Caputi, G.; Conversano, M.; Ciofi Degli Atti, M.; DLAncona, F.; Germinario, C.; Quarto, M. Large outbreak of viral gastroenteritis caused by contaminated drinking water in Apulia, Italy, May-October 2006. Euro Surveill. 2007, 12, E070419. [Google Scholar] [PubMed]
- Kiulia, N.M.; Netshikweta, R.; Page, N.A.; Van Zyl, W.B.; Kiraithe, M.M.; Nyachieo, A.; Mwenda, J.M.; Taylor, M.B. The detection of enteric viruses in selected urban and rural river water and sewage in Kenya, with special reference to rotaviruses. J. Appl. Microbiol. 2010, 109, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, W.B.; Page, N.; Grabow, W.; Steele, A.D.; Taylor, M.B. Molecular epidemiology of group A rotaviruses in water sources and selected raw vegetables in Southern Africa. Appl. Environ. Microbiol. 2006, 72, 4554–4560. [Google Scholar] [CrossRef] [PubMed]
- Gratacap-Cavallier, B.; Genoulaz, O.; Brengel-Pesce, K.; Soule, H.; Innocenti-Francillard, P.; Bost, M.; Gofti, L.; Zmirou, D.; Seigneurin, J. Detection of human and animal rotavirus sequences in drinking water. Appl. Environ. Microbiol. 2000, 66, 2690–2692. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.; Lodder, W.; Van Leeuwen, A.D.; de Roda Husman, A. Detection of infectious rotavirus in naturally contaminated source waters for drinking water production. J. Appl. Microbiol. 2009, 107, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.; Jones, E.; Gerba, C. Viruses in recreational water-borne disease outbreaks: A review. J. Appl. Microbiol. 2009, 107, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.M.; Fiore, L. Advances in understanding of rotaviruses as food- and waterborne pathogens and progress with vaccine developmen. In Viruses in Food and Water: Risks, Surveillance and Control; Cook, N., Ed.; Woodhead Publ Ltd: Cambridge, UK, 2013; pp. 362–400. [Google Scholar]
- Glass, R.I.; Parashar, U.D.; Bresee, J.S.; Turcios, R.; Fischer, T.K.; Widdowson, M.A.; Jiang, B.; Gentsch, J.R. Rotavirus vaccines: Current prospects and future challenges. Lancet 2006, 368, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.E.; Patel, M.M.; Steele, A.D.; Gentsch, J.R.; Payne, D.C.; Cortese, M.M.; Nakagomi, O.; Cunliffe, N.A.; Jiang, B.; Neuzil, K.M.; et al. Global impact of rotavirus vaccines. Expert Rev. Vaccines 2010, 9, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Linhares, A.C.; Justino, M.C. Rotavirus vaccination in Brazil: Effectiveness and health impact seven years post-introduction. Expert Rev. Vaccines 2014, 13, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Msimang, V.M.; Page, N.; Groome, M.J.; Moyes, J.; Cortese, M.M.; Seheri, M.; Kahn, K.; Chagan, M.; Madhi, S.A.; Cohen, C. Impact of rotavirus vaccine on childhood diarrheal hospitalization after introduction into the South African public immunization program. Pediatr. Infect. Dis. J. 2013, 32, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Kollaritsch, H.; Kundi, M.; Giaquinto, C.; Paulke-Korinek, M. Rotavirus vaccines: A story of success. Clin. Microbiol. Infect. 2015. [Google Scholar] [CrossRef]
- Rha, B.; Tate, J.E.; Payne, D.C.; Cortese, M.M.; Lopman, B.A.; Curns, A.T.; Parashar, U.D. Effectiveness and impact of rotavirus vaccines in the United States - 2006–2012. Expert Rev. Vaccines 2014, 13, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, C.; Dominiak-Felden, G.; Van Damme, P.; Myint, T.T.; Maldonado, Y.A.; Spoulou, V.; Mast, T.C.; Staat, M.A. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: A systematic review of the experience in industrialized countries. Hum. Vaccines 2011, 7, 734–748. [Google Scholar] [CrossRef]
- Karafillakis, E.; Hassounah, S.; Atchison, C. Effectiveness and impact of rotavirus vaccines in Europe, 2006–2014. Vaccine 2015, 33, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Than, V.T.; Jeong, S.; Kim, W. A systematic review of genetic diversity of human rotavirus circulating in South Korea. Infect. Genet. Evol. 2014, 28, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Degiuseppe, J.I.; Parra, G.I.; Stupka, J.A. Genetic diversity of G3 rotavirus strains circulating in Argentina during 1998–2012 assessed by full genome analyses. PLoS ONE 2014, 9, e110341. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.T. Rotavirus diversity and evolution in the post-vaccine world. Discov. Med. 2012, 13, 85–97. [Google Scholar] [PubMed]
- Gerba, C.P.; Rose, J.B.; Haas, C.N.; Crabtree, K.D. Waterborne rotavirus: A risk assessment. Water Res. 1996, 30, 2929–2940. [Google Scholar] [CrossRef]
- Hofstra, N.; Bouwman, A.; Beusen, A.; Medema, G. Exploring global cryptosporidium emissions to surface water. Sci. Total Environ. 2013, 442, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kiulia, N.M.; Nyaga, M.M.; Seheri, M.L.; Wolfaardt, M.; van Zyl, W.B.; Esona, M.D.; Irimu, G.; Inoti, M.; Gatinu, B.W.; Njenga, P.K.; et al. Rotavirus G and P types circulating in the Eastern region of Kenya: Predominance of G9 and emergence of G12 genotypes. Pediatr. Infect. Dis. J. 2014, 33, S85–S88. [Google Scholar] [CrossRef] [PubMed]
- Abugalia, M.; Cuevas, L.; Kirby, A.; Dove, W.; Nakagomi, O.; Nakagomi, T.; Kara, M.; Gweder, R.; Smeo, M.; Cunliffe, N. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J. Med. Virol. 2011, 83, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- El Qazoui, M.; Oumzil, H.; Baassi, L.; El Omari, N.; Sadki, K.; Amzazi, S.; Benhafid, M.; El Aouad, R. Rotavirus and norovirus infections among acute gastroenteritis children in Morocco. BMC Infect. Dis. 2014, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Jere, K.C.; Sawyerr, T.; Seheri, L.M.; Peenze, I.; Page, N.A.; Geyer, A.; Steele, A.D. A first report on the characterization of rotavirus strains in Sierra Leone. J. Med. Virol. 2011, 83, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Seheri, L.M.; Page, N.; Dewar, J.B.; Geyer, A.; Nemarude, A.L.; Bos, P.; Esona, M.; Steele, A.D. Characterization and molecular epidemiology of rotavirus strains recovered in Northern Pretoria, South Africa during 2003–2006. J. Infect. Dis. 2010, 202, S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Hassine-Zaafrane, M.; Sdiri-Loulizi, K.; Ben Salem, I.; Kaplon, J.; Ayouni, S.; Ambert-Balay, K.; Sakly, N.; Pothier, P.; Aouni, M. The molecular epidemiology of circulating rotaviruses: Three-year surveillance in the region of Monastir, Tunisia. BMC Infect. Dis. 2011, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Nyambat, B.; Meng, C.Y.; Vansith, K.; Vuthy, U.; Rin, E.; Kirkwood, C.; Bogdanovic-Sakran, N.; Kilgore, P.E. Hospital-based surveillance for rotavirus diarrhea in Phnom Penh, Cambodia, March 2005 through February 2007. Vaccine 2009, 27, F81–F84. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Ma, H.; Jin, M.; Wang, X.; Wang, J.; Xu, L.; Lin, S.; Shen, Z.; Chen, Z.; Qiu, Z.; et al. Etiology and epidemiology of viral diarrhea in children under the age of five hospitalized in Tianjin, China. Arch. Virol. 2012, 157, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Sai, L.; Sun, J.; Shao, L.; Chen, S.; Liu, H.; Ma, L. Epidemiology and clinical features of rotavirus and norovirus infection among children in Ji’nan, China. Virol. J. 2013, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Awasthi, S.; Nag, V.; Tandon, R. Genomic diversity of group A rotavirus strains in patients aged 1–36 months admitted for acute watery diarrhea in Northern India: A hospital-based study. Clin. Microbiol. Infect. 2010, 16, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Paulose, A.; Chitralekha, S.; Nair, M.; Kang, G.; Kilgore, P. Prevalence of rotavirus diarrhea among hospitalized under-five children. Indian Pediatr. 2014, 51, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Babji, S.; Arumugam, R.; Sarvanabhavan, A.; Moses, P.D.; Simon, A.; Aggarwal, I.; Mathew, A.; Sr, A.; Kang, G. Multi-center surveillance of rotavirus diarrhea in hospitalized children <5 years of age in India, 2009–2012. Vaccine 2014, 32 (Suppl. 1), A10–A12. [Google Scholar]
- Tiku, V.R.; Sharma, S.; Verma, A.; Kumar, P.; Raghavendhar, S.; Aneja, S.; Paul, V.K.; Bhan, M.K.; Ray, P. Rotavirus diversity among diarrheal children in Delhi, India during 2007–2012. Vaccine 2014, 32 (Suppl. 1), A62–A67. [Google Scholar] [CrossRef] [PubMed]
- Aloun, D.S.; Nyambat, B.; Phetsouvanh, R.; Douangboupha, V.; Keonakhone, P.; Xoumphonhphakdy, B.; Vongsouvath, M.; Kirkwood, C.; Bogdanovic-Sakran, N.; Kilgore, P.E. Rotavirus diarrhea among children aged less than 5 years at Mahosot Hospital, Vientiane, LAO PDR. Vaccine 2009, 27 (Suppl. 5), F85–F88. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.; Thu, H.M.; Oo, W.M.; Aye, K.M.; Shwe, T.T.; Mar, W.; Kirkwood, C.D. Genotyping of rotavirus isolates collected from children less than 5 years of age admitted for diarrhea at the Yangon Children’s Hospital, Myanmar. Vaccine 2009, 27, F89–F92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hong, S.K.; Lee, S.G.; Suh, C.I.; Park, S.W.; Lee, J.H.; Kim, J.H.; Kim, D.S.; Kim, H.M.; Jang, Y.T.; et al. Human rotavirus genotypes in hospitalized children, South Korea, April 2005 to March 2007. Vaccine 2009, 27 (Suppl. 5), F97–F101. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.O.; Son, D.W.; Shim, S.Y.; Ryoo, E.; Kim, W.; Jung, Y.C. Clinical characteristics and genotypes of rotaviruses in a neonatal intensive care unit. Pediatr. Neonatol. 2012, 53, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.T.; Liang, S.Y.; Tsao, K.C.; Huang, C.G.; Lin, C.Y.; Lin, J.S.; Su, C.Y.; Eng, H.L.; Yang, J.Y.; Chen, P.J.; et al. Hospital-based surveillance and molecular epidemiology of rotavirus infection in Taiwan, 2005–2007. Vaccine 2009, 27 (Suppl. 5), F50–F54. [Google Scholar] [CrossRef] [PubMed]
- Kota, M.; Bino, S.; Delogu, R.; Simaku, A.; Neza, B.; Ruggeri, F.M.; Fiore, L. Epidemiology of rotavirus diarrhea in Albania. Arch. Virol. 2014, 159, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Lorrot, M.; Bon, F.; El Hajje, M.J.; Aho, S.; Wolfer, M.; Giraudon, H.; Kaplon, J.; Marc, E.; Raymond, J.; Lebon, P.; et al. Epidemiology and clinical features of gastroenteritis in hospitalized children: Prospective survey during a 2-year period in a Parisian Hospital, France. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Fauquier, A.; Montero, V.; Colomina, J.; Gonzalez-Galan, V.; Aznar, J.; Aisa, M.L.; Gutierrez, C.; Sainz de Baranda, C.; Wilhelmi, I. Global study of viral diarrhea in hospitalized children in Spain: Results of structural surveillance of viral gastroenteritis network (VIGESS-net) 2006–2008. J. Clin. Virol. 2011, 52, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kargar, M.; Akbarizadeh, A.R. Prevalence and molecular genotyping of group A rotaviruses in Iranian children. Indian J. Virol. 2012, 23, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, K.; Shulman, L.; Rubinstein, U.; Kasem, E.; Kremer, A.; Goren, S.; Zilberstein, I.; Chodick, G.; Ephros, M.; Cohen, D.; et al. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of Israeli children <5 years of age, 2007–2008. J. Infect. Dis. 2009, 200 (Suppl. 1), S254–S263. [Google Scholar]
- Tayeb, H.T.; Dela Cruz, D.M.; Al-Qahtani, A.; Al-Ahdal, M.N.; Carter, M.J. Enteric viruses in pediatric diarrhea in Saudi Arabia. J. Med. Virol. 2008, 80, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Esteban, L.E.; Rota, R.P.; Gentsch, J.R.; Jiang, B.; Esona, M.; Glass, R.I.; Glikmann, G.; Castello, A.A. Molecular epidemiology of group A rotavirus in Buenos Aires, Argentina 2004–2007: Reemergence of G2P[4] and emergence of G9P[8] strains. J. Med. Virol. 2010, 82, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.G.; Liprandi, F.; Ludert, J.E. Molecular epidemiology of enteric viruses in children with sporadic gastroenteritis in Valencia, Venezuela. J. Med. Virol. 2011, 83, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.L.; Perin, J.; Aryee, M.J.; Boschi-Pinto, C.; Black, R.E. Diarrhea incidence in low-and middle-income countries in 1990 and 2010: A systematic review. BMC Public Health 2012, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and etiology of diarrheal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.; Koopmans, M.; Kortbeek, L.; Wannet, W.; Vinje, J.; Van Leusden, F.; Bartelds, A.; Van Duynhoven, Y. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: Incidence and etiology. Am. J. Epidemiol. 2001, 154, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Sarkar, R.; Menon, V.K.; Babji, S.; Paul, A.; Rajendran, P.; Sowmyanarayanan, T.V.; Moses, P.D.; Iturriza-Gomara, M.; Gray, J.J. Rotavirus shedding in symptomatic and asymptomatic children using reverse transcription-quantitative PCR. J. Med. Virol. 2013, 85, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Gajardo, R.; Bouchriti, N.; Pinto, R.M.; Bosch, A. Genotyping of rotaviruses isolated from sewage. Appl. Environ. Microbiol. 1995, 61, 3460–3462. [Google Scholar] [PubMed]
- Lodder, W.J.; de Roda Husman, A.M. Presence of noroviruses and other enteric viruses in sewage and surface waters in the Netherlands. Appl. Environ. Microbiol. 2005, 71, 1453–1461. [Google Scholar] [CrossRef]
- Lodder, W.J.; Rutjes, S.A.; Takumi, K.; de Roda Husman, A.M. Aichi virus in sewage and surface water, the Netherlands. Emerg. Infect. Dis. 2013, 19, 1222–1230. [Google Scholar] [CrossRef]
- Lodder, W.J.; Wuite, M.; de Roda Husman, A.M.; Rutjes, S.A. Environmental surveillance of human parechoviruses in sewage in the Netherlands. Appl. Environ. Microbiol. 2013, 79, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Prado, T.; Silva, D.M.; Guilayn, W.C.; Rose, T.L.; Gaspar, A.M.; Miagostovich, M.P. Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res. 2011, 45, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Ali, M.A.; El-Nady, H.G.; Aho, S.; Pothier, P.; Belliot, G. Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. J. Appl. Microbiol. 2010, 108, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.A.; Ziros, P.G.; Mpalasopoulou, A.; Galanis, A.; Vantarakis, A. Molecular detection of multiple viral targets in untreated urban sewage from Greece. Virol. J. 2011, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Sdiri-Loulizi, K.; Hassine, M.; Aouni, Z.; Gharbi-Khelifi, H.; Chouchane, S.; Sakly, N.; Neji-Guediche, M.; Pothier, P.; Aouni, M.; Ambert-Balay, K. Detection and molecular characterization of enteric viruses in environmental samples in Monastir, Tunisia between January 2003 and April 2007. J. Appl. Microbiol. 2010, 109, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Barril, P.A.; Giordano, M.O.; Isa, M.B.; Masachessi, G.; Ferreyra, L.J.; Castello, A.A.; Glikmann, G.; Nates, S.V. Correlation between rotavirus A genotypes detected in hospitalized children and sewage samples in 2006, Cordoba, Argentina. J. Med. Virol. 2010, 82, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.F.; Guimaraes, F.R.; Fumian, T.M.; Victoria, M.; Vieira, C.B.; Luz, S.; Shubo, T.; Leite, J.P.; Miagostovich, M.P. Environmental dissemination of group A rotavirus: P-type, G-type and subgroup characterization. Water Sci. Technol. 2009, 60, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Fumian, T.M.; Leite, J.P.; Rose, T.L.; Prado, T.; Miagostovich, M.P. One year environmental surveillance of rotavirus specie A (RVA) genotypes in circulation after the introduction of the rotarix® vaccine in Rio de Janeiro, Brazil. Water Res. 2011, 45, 5755–5763. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, A.D.; Fleck, J.D.; Kluge, M.; Comerlato, J.; Bergamaschi, B.; Luz, R.B.; Arantes, T.S.; Silva, J.V.; Thewes, M.R.; Spilki, F.R. Assessment of enteric viruses in a sewage treatment plant located in Porto Alegre, Southern Brazil. Braz. J. Biol. 2012, 72, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Iker, B.C.; Pepper, I.L.; Gerba, C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes--identification of potential viral indicators. Sci. Total Environ. 2014, 488℃489, 290–296. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, J.; Querales, L.; Caraballo, L.; Vizzi, E.; Liprandi, F.; Takiff, H.; Betancourt, W.Q. Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage-polluted river waters in Caracas, Venezuela. Appl. Environ. Microbiol. 2009, 75, 387–394. [Google Scholar] [CrossRef] [PubMed]
- He, X.Q.; Cheng, L.; Li, W.; Xie, X.M.; Ma, M.; Wang, Z.J. Detection and distribution of rotavirus in municipal sewage treatment plants (STPs) and surface water in Beijing. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2008, 43, 424–429. [Google Scholar] [CrossRef] [PubMed]
- He, X.Q.; Cheng, L.; Zhang, D.Y.; Xie, X.M.; Wang, D.H.; Wang, Z. One-year monthly survey of rotavirus, astrovirus and norovirus in three sewage treatment plants (STPs) in Beijing, China and associated health risk assessment. Water Sci. Technol. 2011, 64, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Vivek, R.; Zachariah, U.G.; Ramachandran, J.; Eapen, C.E.; Rajan, D.P.; Kang, G. Characterization of hepatitis E virus from sporadic hepatitis cases and sewage samples from Vellore, South India. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Arraj, A.; Bohatier, J.; Arraj, A.; Aumeran, C.; Bailly, J.L.; Laveran, H.; Traoré, O. An epidemiological study of enteric viruses in sewage with molecular characterization by RT-PCR and sequence analysis. J. Water Health 2008, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Grassi, T.; Bagordo, F.; Idolo, A.; Lugoli, F.; Gabutti, G.; De Donno, A. Rotavirus detection in environmental water samples by tangential flow ultrafiltration and RT-nested PCR. Environ. Monit. Assess. 2010, 164, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.M.; Bonomo, P.; Ianiro, G.; Battistone, A.; Delogu, R.; Germinario, C.; Chironna, M.; Triassi, M.; Campagnuolo, R.; Cicala, A.; et al. Rotavirus genotypes in sewage treatment plants and in children hospitalized with acute diarrhea in Italy, 2010–2011. Appl. Environ. Microbiol. 2015, 81, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Hellmer, M.; Paxeus, N.; Magnius, L.; Enache, L.; Arnholm, B.; Johansson, A.; Bergstrom, T.; Norder, H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014, 80, 6771–6781. [Google Scholar] [CrossRef] [PubMed]
- Kargar, M.; Javdani, N.; Najafi, A.; Tahamtan, Y. First molecular detection of group A rotavirus in urban and hospital sewage systems by nested RT-PCR in Shiraz, Iran. J. Environ. Health Sci. Eng. 2013, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.C.; Wikswo, M.; Parashar, U.D. Rotavirus. Pediatr. Infect. Dis. J. 2011, 30, S54–S55. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.G. Chemistry of wastewater. Encyclopedia of Life Support System (EOLSS). Available online: http://www.eolss.net/eolsssamplechapters/c06/e6-13-04-05/E6-13-04-05-TXT-05.aspx (accessed on 8 March 2015).
- StatLine. Available online: http://statline.cbs.nl/StatWeb/publication/?VW=T&DM=SLnl&PA=71476ned&LA=nl (accessed on 16 March 2015).
- Wang, X.-Y.; Xu, Z.-Y.; von Seidlein, L.; Zhang, Y.-L.; Zhao, S.-J.; Hao, Z.-Y.; Han, O.P.; Kilgore, P.; Xing, Z.-C.; Han, C.-Q. Incidence of diarrhea caused by rotavirus infections in rural Zhengding, China: Prospective, population-based surveillance. J. Infect. Dis. 2005, 192, S100–S105. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. National Statistics Database. Available online: http://data.stats.gov.cn/workspace/index?m=fsnd (accessed on 8 March 2015).
- Sartori, A.M.C.; Valentim, J.; Soárez, P.C.; Novaes, H.M.D. Rotavirus morbidity and mortality in children in Brazil. Rev. Panam. Salud Publica 2008, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gu, A.Z.; Zeng, S.Y.; Yang, W.; He, M.; Shi, H.C. Monitoring and evaluation of infectious rotaviruses in various wastewater effluents and receiving waters revealed correlation and seasonal pattern of occurrences. J. Appl. Microbiol. 2011, 110, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Verbyla, M.E.; Mihelcic, J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015, 71, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manzano, J.; Alonso, J.; Ferrús, M.; Moreno, Y.; Amorós, I.; Calgua, B.; Hundesa, A.; Guerrero-Latorre, L.; Carratala, A.; Rusiñol, M. Standard and new fecal indicators and pathogens in sewage treatment plants, microbiological parameters for improving the control of reclaimed water. Water Sci. Technol. 2012, 66, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.M.; Nelson, K.L.; Drewes, J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015, 49, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. Progress on Sanitation and Drinking-Water. 2012 Update; WHO/UNICEF: Geneva, Switzerland, 2013. [Google Scholar]
- Feachem, R.; Mara, D.D.; Bradley, D.J. Sanitation and Disease; John Wiley & Sons: Washington DC, USA, 1983. [Google Scholar]
- Global Water Pathogen Project (GWPP). Available online: www.waterpathogens.org (accessed on 8 March 2015).
- Vermeulen, L.C.; De Kraker, J.; Hofstra, N.; Kroeze, C.; Medema, G.J. Modelling the impact of sanitation, population growth and urbanization on human emissions of cryptosporidium to surface waters—A case study for Bangladesh and India. Environ. Res. Lett. 2015. submitted. [Google Scholar]
- Ferguson, C.M.; Croke, B.F.W.; Beatson, P.J.; Ashbolt, N.J.; Deere, D.A. Development of a process-based model to predict pathogen budgets for the Sydney drinking water catchment. J. Water Health 2007, 5, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Bright, E.A.; Coleman, P.R.; Rose, A.N.; Urban, M.L. Landscan 2010 High Resolution Global Population Data Set, 2010 ed.; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011. [Google Scholar]
- SSP Database. Available online: Https://secure.Iiasa.Ac.At/web-apps/ene/sspdb/dsd?Action=htmlpage&page=about (accessed on 16 March 2015).
- UN World Population Prospects. Available online: Http://esa.Un.Org/wpp/excel-data/population.Htm (accessed on 8 March 2015).
- UNDP. Human Development Report 2010, 20th Anniversary edition. The Real Wealth of Nations: Pathways to Human Development; United Nations Development Programme (UNDP): New York, NY, USA, 2010. [Google Scholar]
- Van Puijenbroek, P.J.T.M.; Bouwman, A.F.; Beusen, A.H.W.; Lucas, P.L. Global implementation of two shared socioeconomic pathways for future sanitation and wastewater flows. Water Sci. Technol. 2015, 71, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Van Drecht, G.; Bouwman, A.F.; Harrison, J.; Knoop, J.M. Global nitrogen and phosphate in urban wastewater for the period 1970 to 2050. Glob. Biogeochem. Cycles 2009, 23, GB0A03. [Google Scholar] [CrossRef]
- Miller, M.; Parker, C. Sanitation Status of African Cities. Available online: http://www.iwawaterwiki.org/xwiki/bin/view/Articles/AfricanCitiesSanitationStatus (accessed on 8 April 2015).
- Eurostat. Eurostat Statistics. Available online: Http://ec.Europa.Eu/eurostat/web/environment/water/main-tables (accessed on 8 April 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiulia, N.M.; Hofstra, N.; Vermeulen, L.C.; Obara, M.A.; Medema, G.; Rose, J.B. Global Occurrence and Emission of Rotaviruses to Surface Waters. Pathogens 2015, 4, 229-255. https://doi.org/10.3390/pathogens4020229
Kiulia NM, Hofstra N, Vermeulen LC, Obara MA, Medema G, Rose JB. Global Occurrence and Emission of Rotaviruses to Surface Waters. Pathogens. 2015; 4(2):229-255. https://doi.org/10.3390/pathogens4020229
Chicago/Turabian StyleKiulia, Nicholas M., Nynke Hofstra, Lucie C. Vermeulen, Maureen A. Obara, Gertjan Medema, and Joan B. Rose. 2015. "Global Occurrence and Emission of Rotaviruses to Surface Waters" Pathogens 4, no. 2: 229-255. https://doi.org/10.3390/pathogens4020229





