Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans
Abstract
:1. Introduction
2. Results and Discussion
2.1. MIC Determination
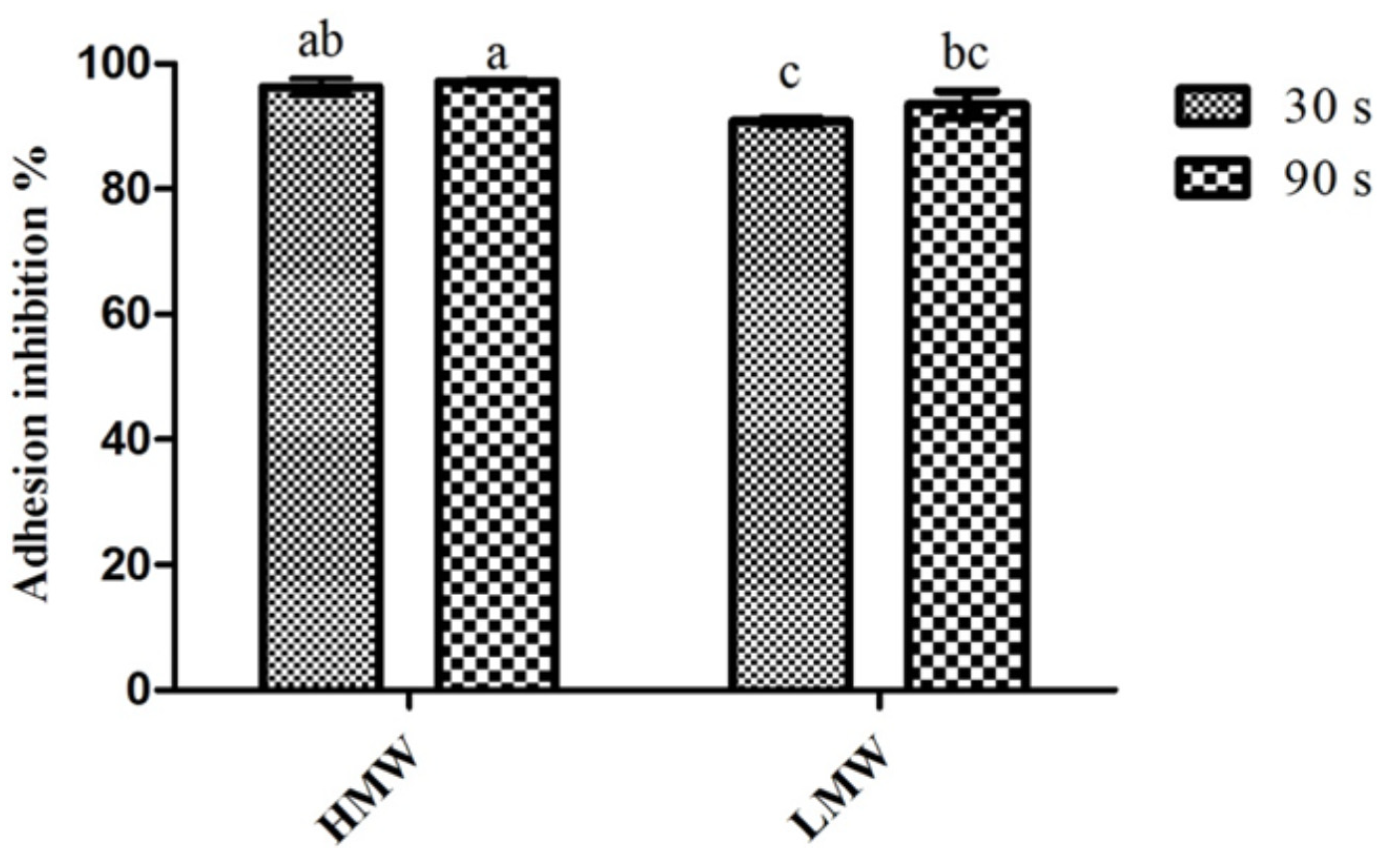
2.2. Adherence to Coated Surfaces
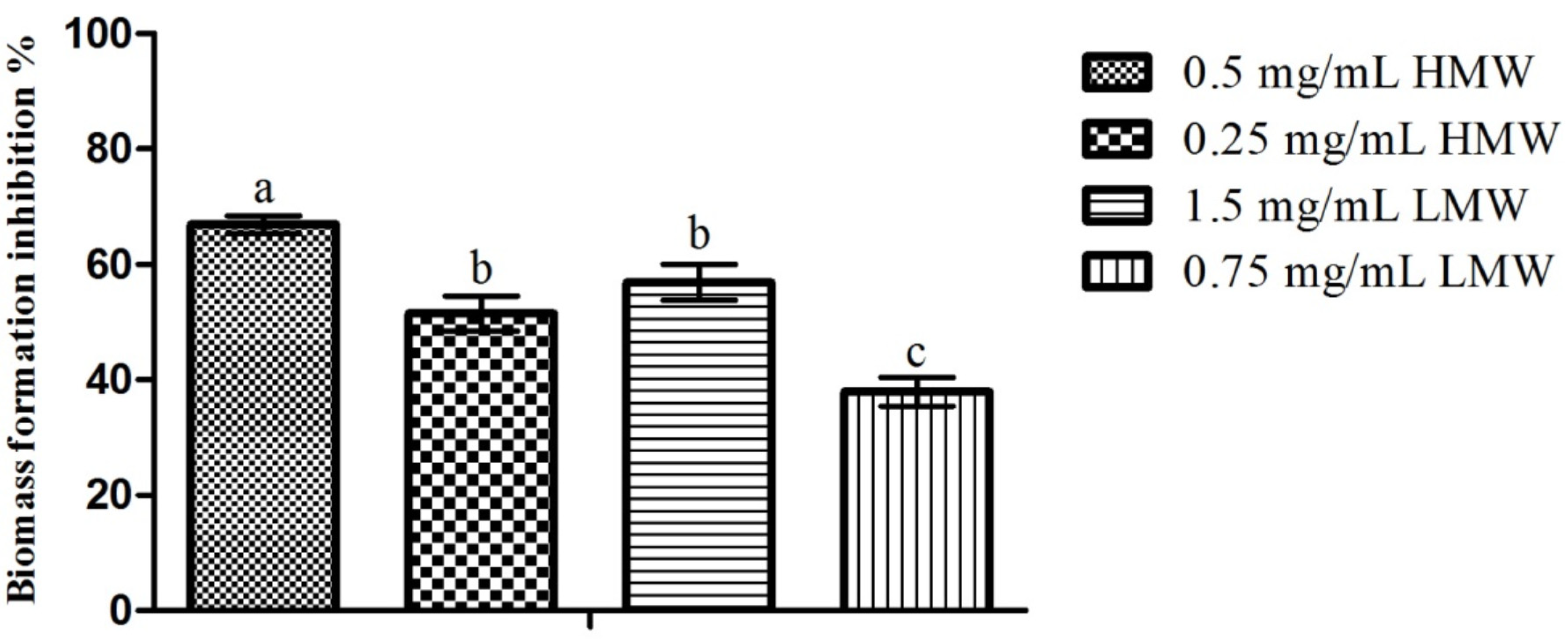
2.3. Microtiter-Plate Test
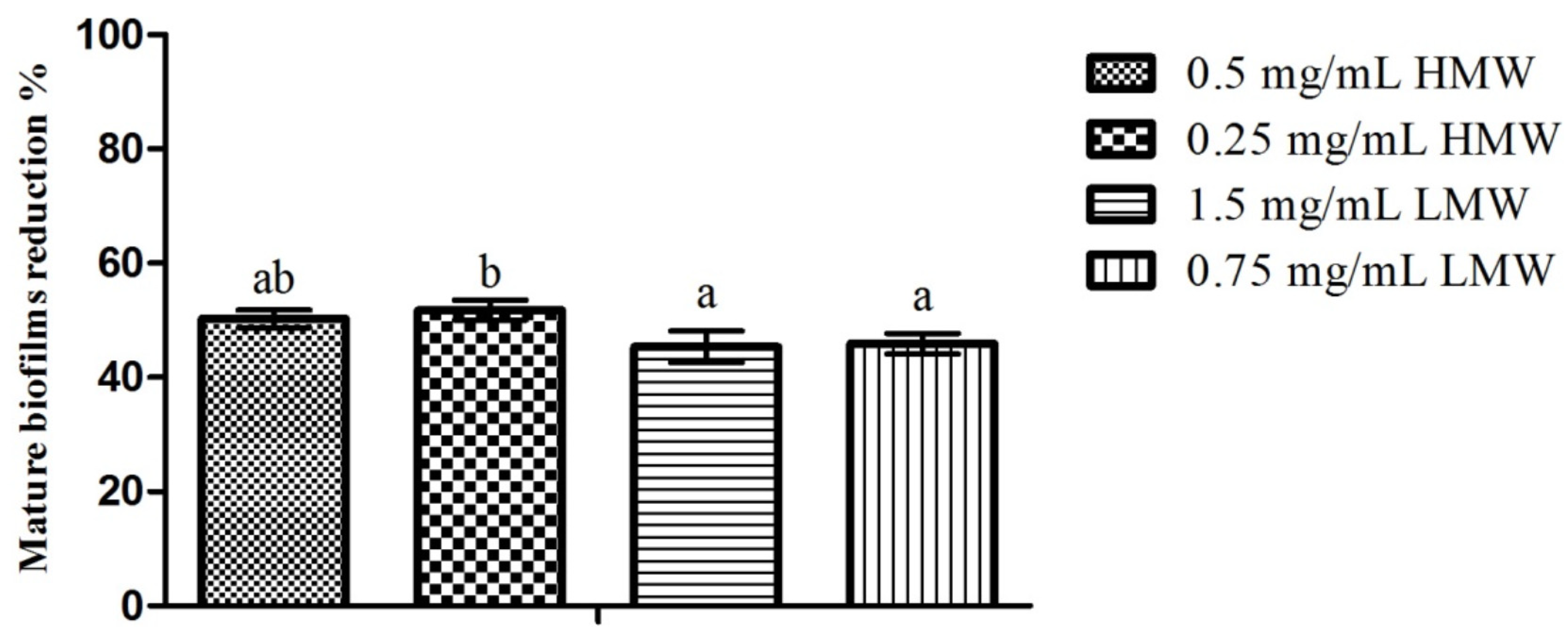
2.4. Mature Biofilms Assays
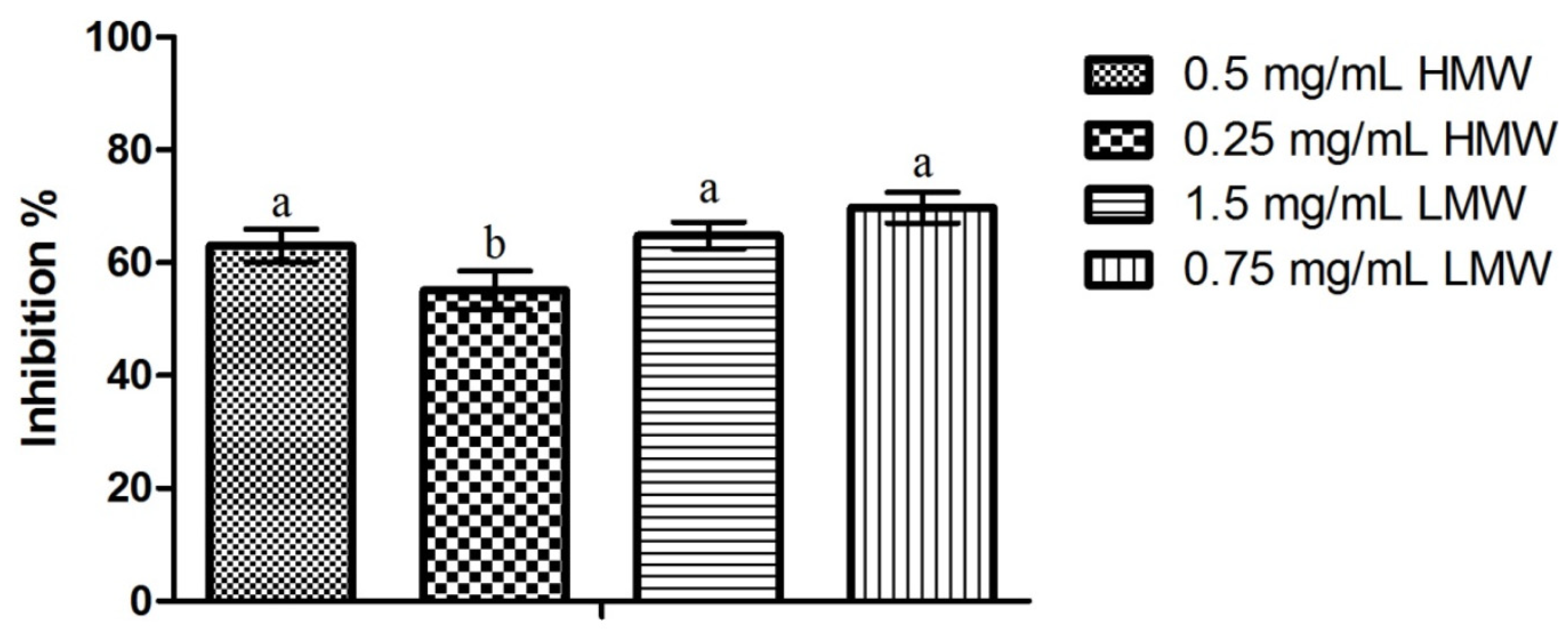
2.5. Dual-Species Biofilms

3. Experimental Section
3.1. Sources of Chitosan and Microorganisms
3.2. Determination of Minimal Inhibitory Concentration
3.3. Adherence
3.4. Microtiter-Plate Test
3.5. Mature Biofilms Assay
3.6. Dual-Species Biofilms
3.7. Statistical Treatment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against candida albicans, candida krusei and candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar]
- Tayel, A.A.; Moussa, S.; El-Tras, W.F.; Knittel, D.; Opwis, K.; Schollmeyer, E. Anticandidal action of fungal chitosan against candida albicans. Int. J. Biol. Macromol. 2010, 47, 454–457. [Google Scholar] [CrossRef]
- Sgherri, C.; Porta, A.; Castellano, S.; Pinzino, C.; Quartacci, M.F.; Calucci, L. Effects of azole treatments on the physical properties of Candida albicans plasma membrane: A spin probe epr study. Biochim. Biophys. Acta (BBA)–Biomembr. 2014, 1838, 465–473. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida spp. In the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agent 2014, 43, 78–81. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef]
- Bassetti, M.; Merelli, M.; Righi, E.; Diaz-Martin, A.; Rosello, E.M.; Luzzati, R.; Parra, A.; Trecarichi, E.M.; Sanguinetti, M.; Posteraro, B.; et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J. Clin. Microbiol. 2013, 51, 4167–4172. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodrıguez, N.M.; Rodriguez, M.S.; Heras, A.; Agulló, E. Modified chitosan carrying phosphonic and alkyl groups. Carbohydrate Polymers 2003, 51, 425–429. [Google Scholar] [CrossRef]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Kittur, F.S.; Kumar, A.B.V.; Gowda, L.R.; Tharanathan, R.N. Chitosanolysis by a pectinase isozyme of Aspergillus niger—A non-specific activity. Carbohydr. Polym. 2003, 53, 191–196. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Tarsi, R.; Filippini, O.; Giovanetti, E.; Biagini, G.; Varaldo, P.E. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990, 34, 2019–2023. [Google Scholar] [CrossRef]
- Gil, G.; del Monaco, S.; Cerrutti, P.; Galvagno, M. Selective antimicrobial activity of chitosan on beer spoilage bacteria and brewing yeasts. Biotechnol. Lett. 2004, 26, 569–574. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, R.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. Novel derivatives of chitosan and their antifungal activities in vitro. Carbohydr. Res. 2006, 341, 351–354. [Google Scholar] [CrossRef]
- Zakrzewska, A.; Boorsma, A.; Brul, S.; Hellingwerf, K.J.; Klis, F.M. Transcriptional response of Saccharomyces cerevisiae to the plasma membrane-perturbing compound chitosan. Eukaryot. Cell 2005, 4, 703–715. [Google Scholar] [CrossRef]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Poly. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Schinabeck, M.K.; Long, L.A.; Hossain, M.A.; Chandra, J.; Mukherjee, P.K.; Mohamed, S.; Ghannoum, M.A. Rabbit model of Candida albicans biofilm infection: Liposomal amphotericin B antifungal lock therapy. Antimicrob. Agents Chemother. 2004, 48, 1727–1732. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Demonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter model. J. Infect. Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Şenel, S.; İkinci, G.; Kaş, S.; Yousefi-Rad, A.; Sargon, M.F.; Hıncal, A.A. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm. 2000, 193, 197–203. [Google Scholar] [CrossRef]
- Cerca, N.; Martins, S.; Pier, G.B.; Oliveira, R.; Azeredo, J. The relationship between inhibition of bacterial adhesion to a solid surface by sub-mics of antibiotics and subsequent development of a biofilm. Res. Microbiol. 2005, 156, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Cobrado, L.; Azevedo, M.M.; Silva-Dias, A.; Ramos, J.P.; Pina-Vaz, C.; Rodrigues, A.G. Cerium, chitosan and hamamelitannin as novel biofilm inhibitors? J. Antimicrob. Chemother. 2012, 67, 1159–1162. [Google Scholar] [CrossRef]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Pina-Vaz, C.; Rodrigues, A.G. In vivo antibiofilm effect of cerium, chitosan and hamamelitannin against usual agents of catheter-related bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 126–130. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Vega-González, A.; Mendoza-Novelo, B.; López-Romero, E.; Ruiz-Baca, E.; Quintanar-Escorza, M.A.; Villagómez-Castro, J.C. The effect of biomaterials and antifungals on biofilm formation by Candida species: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2513–2527. [Google Scholar] [CrossRef]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Study of the effects of chitosan upon streptococcus mutans adherence and biofilm formation. Anaerobe 2013, 20, 27–31. [Google Scholar] [CrossRef]
- Azcurra, A.I.; Barembaum, S.R.; Bojanich, M.A.; Calamari, S.E.; Aguilar, J.; Battellino, L.J.; Dorronsoro, S.T. Effect of the high molecular weight chitosan and sodium alginate on Candida albicans hydrophobicity and adhesion to cells. Med. Oral Patol. Oral Cir. Bucal 2006, 11, E120–E125. [Google Scholar]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg–Camb. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Meth. 2000, 40, 175–179. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, E.; Silva, S.; Tavaria, F.; Pintado, M. Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans. Pathogens 2014, 3, 908-919. https://doi.org/10.3390/pathogens3040908
Costa E, Silva S, Tavaria F, Pintado M. Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans. Pathogens. 2014; 3(4):908-919. https://doi.org/10.3390/pathogens3040908
Chicago/Turabian StyleCosta, Eduardo, Sara Silva, Freni Tavaria, and Manuela Pintado. 2014. "Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans" Pathogens 3, no. 4: 908-919. https://doi.org/10.3390/pathogens3040908




