Iron and Acinetobacter baumannii Biofilm Formation
Abstract
:1. Introduction
2. Results and Discussion
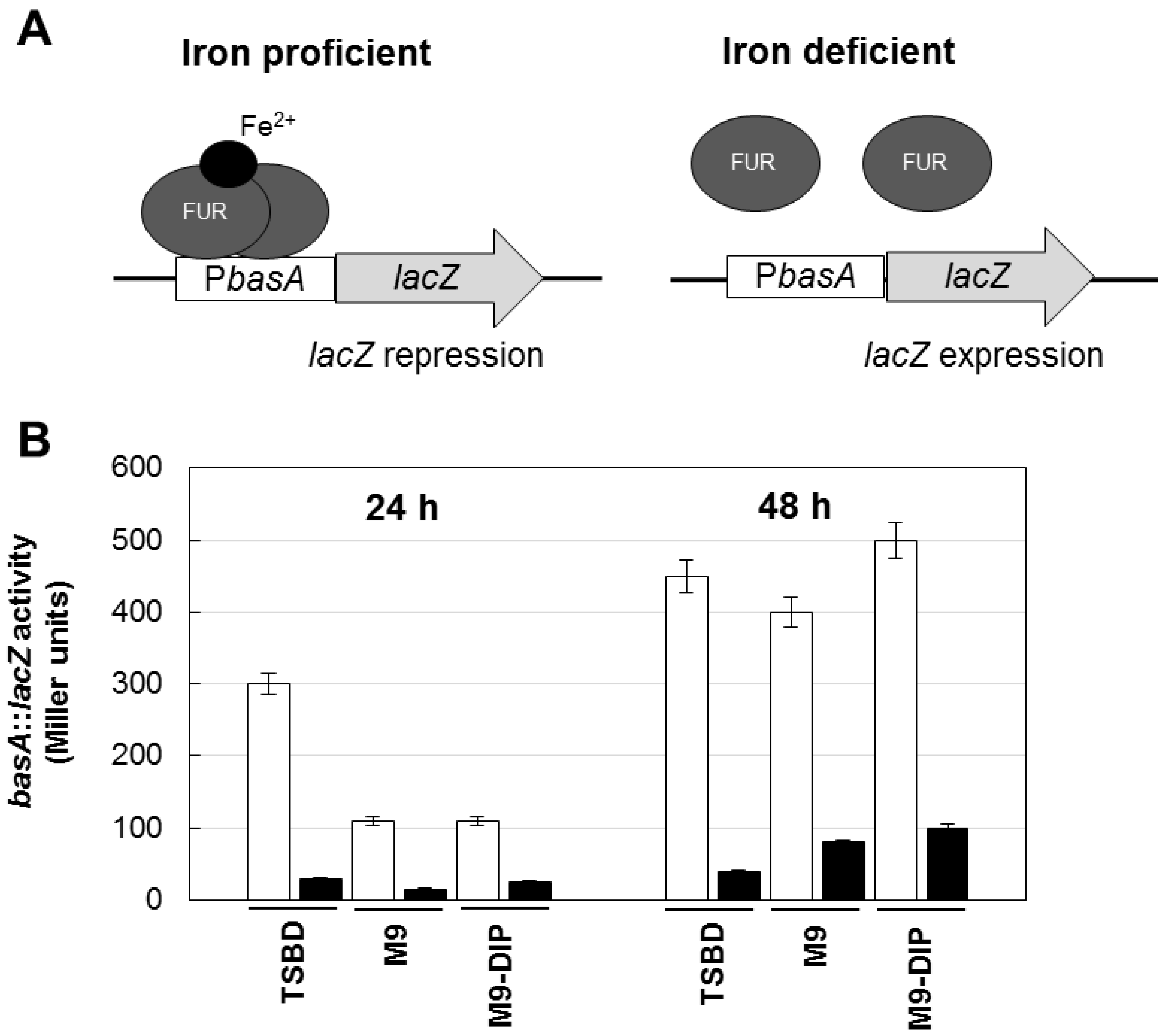
2.1. Definition of Culture Conditions for A. baumannii Biofilm Formation
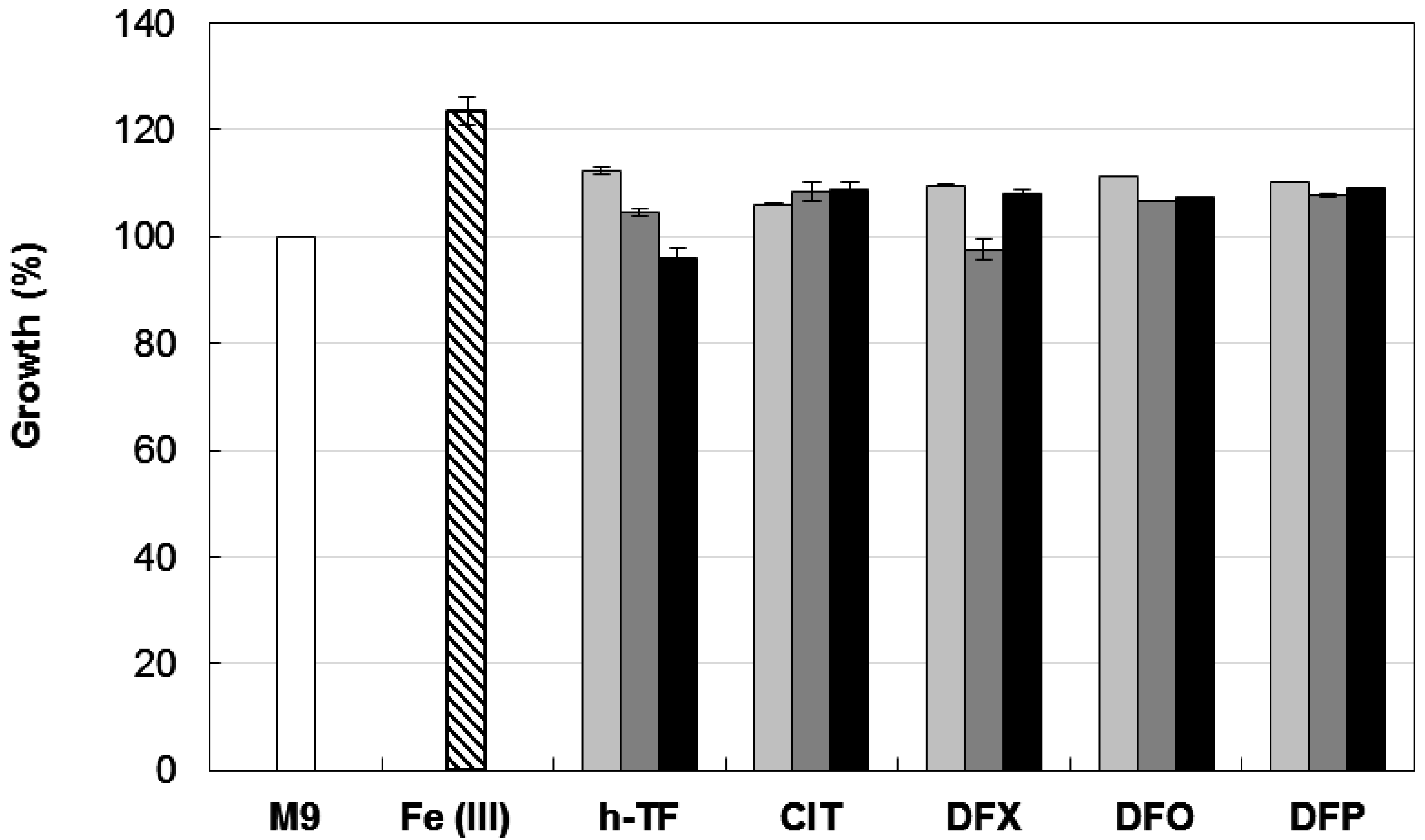

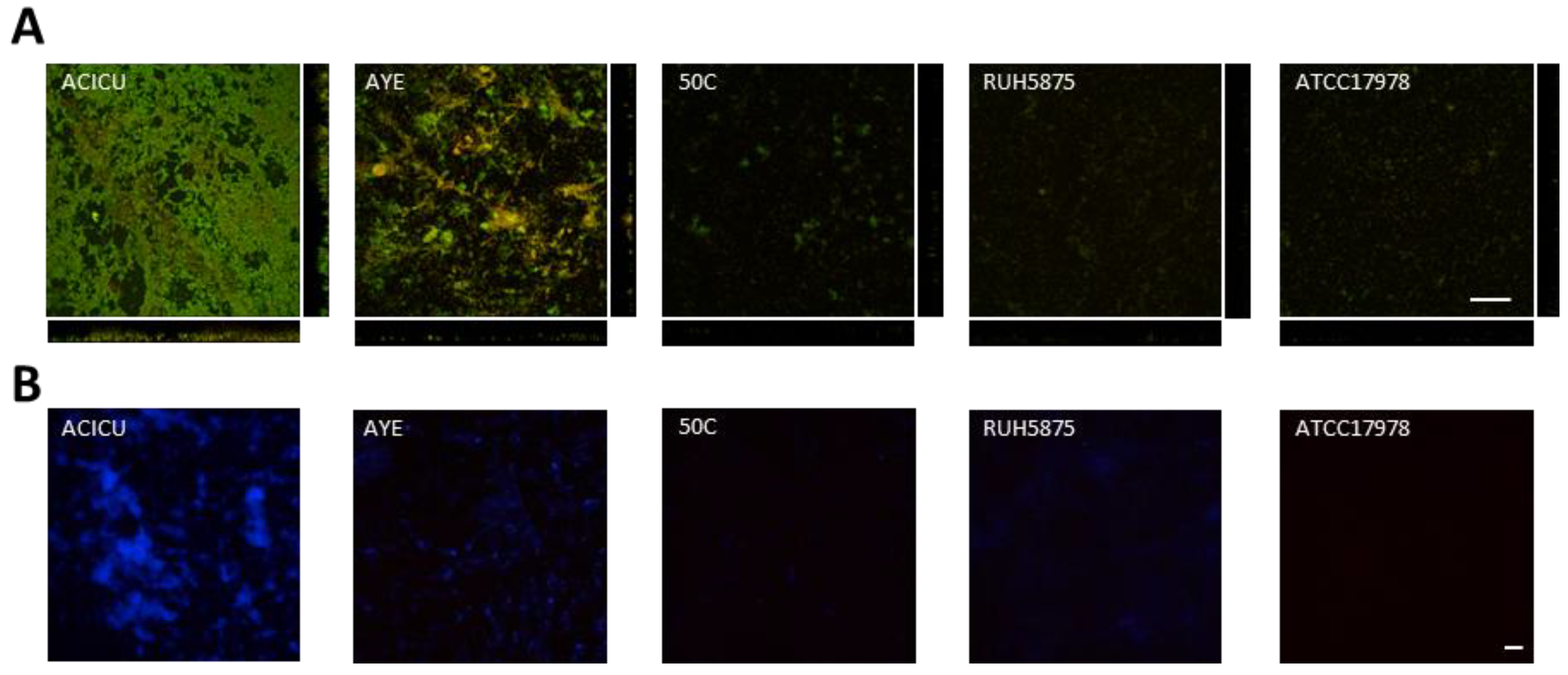
2.2. Effect of Iron Levels on Biofilm Formation by a Collection of Diverse A. baumannii Isolates
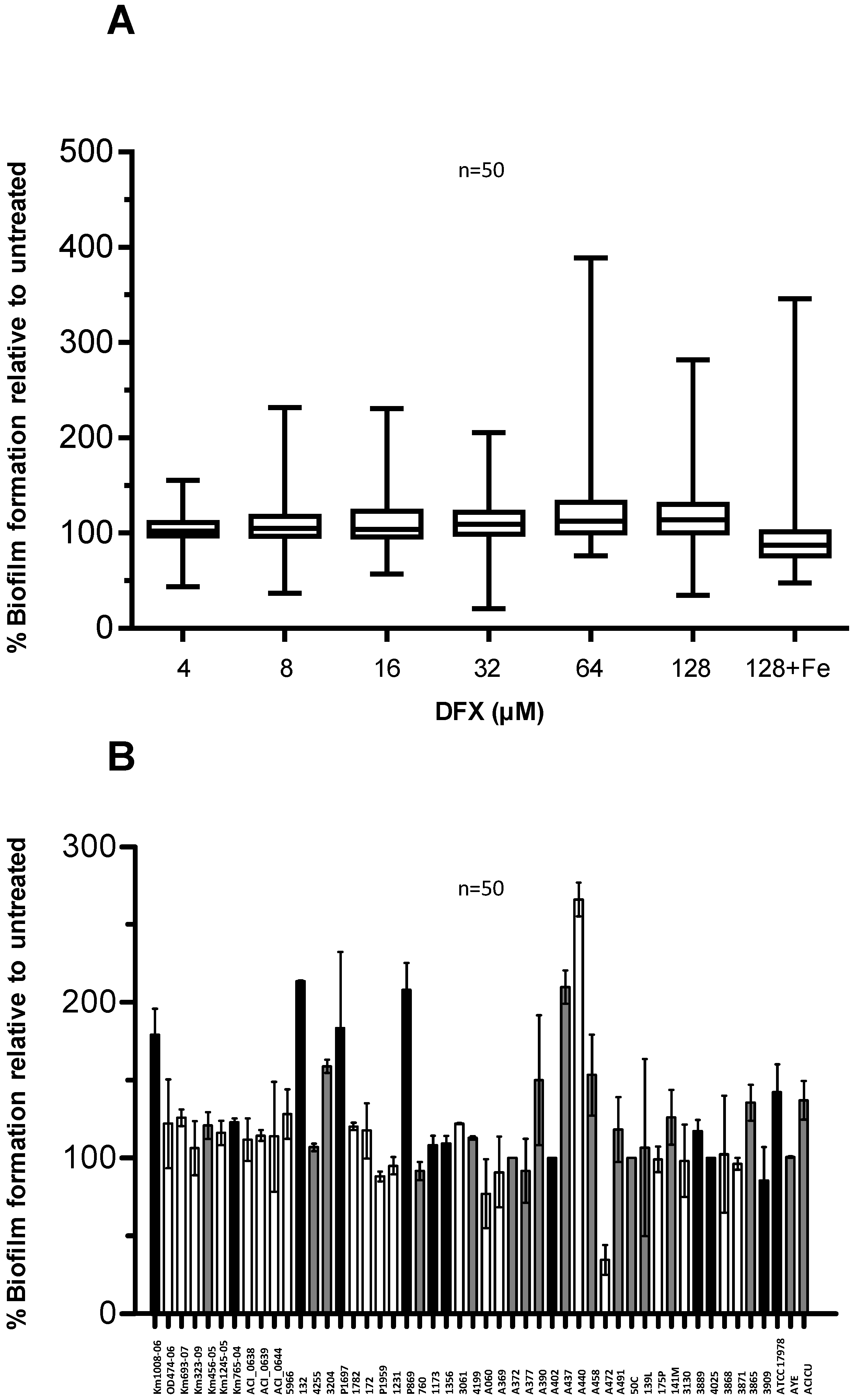
2.3. Effect of Deferasirox on A. baumannii Biofilm Formation

3. Experimental Section
3.1. Bacterial Strains and Growth Media
3.2. Chemicals
3.3. Growth Inhibitory Activity of Iron-Chelators
3.4. Biofilm Formation
3.5. Biofilm Inhibition
3.6. Microscopy Analysis
3.7. β-Galactosidase Activity Assay
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Falagas, M.E.; Bliziotis, I.A.; Siempos, I.I. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: A systematic review of matched cohort and case-control studies. Crit. Care 2006, 10, R48. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Eveillard, M.; Kempf, M.; Belmonte, O.; Pailhoriès, H.; Joly-Guillou, M.L. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int. J. Infect. Dis. 2013, 17, e802–e805. [Google Scholar] [CrossRef] [Green Version]
- Endimiani, A.; Hujer, K.M.; Hujer, A.M.; Bertschy, I.; Rossano, A.; Koch, C.; Gerber, V.; Francey, T.; Bonomo, R.A.; Perreten, V. Acinetobacter baumannii isolates from pets and horses in Switzerland: Molecular characterization and clinical data. J. Antimicrob. Chemother. 2011, 66, 2248–2254. [Google Scholar] [CrossRef]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Zarrilli, R. Global spread of drug-resistant Acinetobacter baumannii: Molecular epidemiology and management of antimicrobial resistance. Future Microbiol. 2011, 6, 407–422. [Google Scholar]
- Neonakis, I.K.; Spandidos, D.A.; Petinaki, E. Confronting multidrug-resistant Acinetobacter baumannii: A review. Int. J. Antimicrob. Agents 2011, 37, 102–109. [Google Scholar] [CrossRef] [PubMed]
- García-Quintanilla, M.; Pulido, M.R.; López-Rojas, R.; Pachón, J.; McConnell, M.J. Emerging therapies for multidrug resistant Acinetobacter baumannii. Trends Microbiol. 2013, 21, 157–163. [Google Scholar]
- Jawad, A.; Seifert, H.; Snelling, A.M.; Heritage, J.; Hawkey, P.M. Survival of Acinetobacter baumannii on dry surfaces: Comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 1998, 36, 1938–1941. [Google Scholar] [PubMed]
- Antunes, L.C.; Imperi, F.; Carattoli, A.; Visca, P. Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS One 2011, 6, e22674. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, M.; Antunes, L.C.; Marchetti, V.; Triassi, M.; Visca, P.; Zarrilli, R. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect. Dis. 2013, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Actis, L.A. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009, 4, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar] [PubMed]
- Rodríguez-Baño, J.; Martí, S.; Soto, S.; Fernández-Cuenca, F.; Cisneros, J.M.; Pachón, J.; Pascual, A.; Martínez-Martínez, L.; McQueary, C.; Actis, L.A.; et al. Spanish Group for the Study of Nosocomial Infections (GEIH). Biofilm formation in Acinetobacter baumannii: Associated features and clinical implications. Clin. Microbiol. Infect. 2008, 14, 276–278. [Google Scholar]
- Lee, H.W.; Koh, Y.M.; Kim, J.; Lee, J.C.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, J. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin. Microbiol. Infect. 2008, 14, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, M.M.; Sawicka-Grzelak, A.; Marchel, H.; Luczak, M.; Sivan, A. Biofilm production by clinical strains of Acinetobacter baumannii isolated from patients hospitalized in two tertiary care hospitals. FEMS Immunol. Med. Microbiol. 2008, 53, 140–144. [Google Scholar] [CrossRef] [PubMed]
- McQueary, C.N.; Actis, L.A. Acinetobacter baumannii biofilms: Variations among strains and correlations with other cell properties. J. Microbiol. 2011, 49, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Iron availability and infection. Biochim. Biophys. Acta 2009, 1790, 600–605. [Google Scholar] [CrossRef]
- Antunes, L.C.; Imperi, F.; Towner, K.J.; Visca, P. Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res. Microbiol. 2011, 162, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zimbler, D.L.; Penwell, W.F.; Gaddy, J.A.; Menke, S.M.; Tomaras, A.P.; Connerly, P.L.; Actis, L.A. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 2009, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Arivett, B.A.; McConnell, M.J.; López-Rojas, R.; Pachón, J.; Actis, L.A. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect. Immun. 2012, 80, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, B.L.; Skaar, E.P. The contribution of nutrient metal acquisition and metabolism to Acinetobacter baumannii survival within the host. Front. Cell. Infect. Microbiol. 2013, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- De Léséleuc, L.; Harris, G.; KuoLee, R.; Chen, W. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5397–5400. [Google Scholar]
- Antunes, L.C.; Imperi, F.; Minandri, F.; Visca, P. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5961–5970. [Google Scholar] [CrossRef] [PubMed]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Nucleo, E.; Steffanoni, L.; Fugazza, G.; Migliavacca, R.; Giacobone, E.; Navarra, A.; Pagani, L.; Landini, P. Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC Microbiol. 2009, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.G.; Gianoulis, T.A.; Pukatzki, S.; Mekalanos, J.J.; Ornston, L.N.; Gerstein, M.; Snyder, M. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007, 21, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Guterman, S.K. Colicin B: Mode of action and inhibition by enterochelin. J. Bacteriol. 1973, 114, 1217–1224. [Google Scholar] [PubMed]
- Fournier, P.E.; Vallenet, D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C.; et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006, 2, e7. [Google Scholar] [CrossRef] [PubMed]
- Iacono, M.; Villa, L.; Fortini, D.; Bordoni, R.; Imperi, F.; Bonnal, R.J.; Sicheritz-Ponten, T.; de Bellis, G.; Visca, P.; Cassone, A.; et al. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 2008, 52, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- D’Arezzo, S.; Principe, L.; Capone, A.; Petrosillo, N.; Petrucca, A.; Visca, P. Changing carbapenemase gene pattern in an epidemic multidrug-resistant Acinetobacter baumannii lineage causing multiple outbreaks in central Italy. J. Antimicrob. Chemother. 2011, 66, 54–61. [Google Scholar]
- Van Dessel, H.; Dijkshoorn, L.; van der Reijden, T.; Bakker, N.; Paauw, A.; van den Broek, P.; Verhoef, J.; Brisse, S. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 2004, 155, 105–112. [Google Scholar]
- Ohman, D.; Sadoff, J.; Iglewski, B. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: Isolation and characterization. Infect. Immun. 1980, 28, 899–908. [Google Scholar] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar]
- Jurcisek, J.A.; Dickson, A.C.; Bruggeman, M.E.; Bakaletz, L.O. In vitro biofilm formation in an 8-well chamber slide. J. Vis. Exp. 2011, 47. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Available online: www.sigmaaldrich.com (accessed on 14 August 2014).
- Hider, R.; Xiaole, K.; Lucker, T.; Conlon, K.; Harland, R. SPD602 is a selective iron chelator which is able to mobilise the non-transferrin-bound iron pool. Blood 2013, 122, 1673. [Google Scholar]
- Luo, G.; Spellberg, B.; Gebremariam, T.; Lee, H.; Xiong, Y.Q.; French, S.W.; Bayer, A.; Ibrahim, A.S. Combination therapy with iron chelation and vancomycin in treating murine staphylococcemia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Pongas, G.N.; Albert, N.; Ben-Ami, R.; Walsh, T.J.; Kontoyiannis, D.P. Activity of deferasirox in Mucorales: Influences of species and exogenous iron. Antimicrob. Agents. Chemother. 2011, 55, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Nisbet-Brown, E.; Olivieri, N.F.; Giardina, P.J.; Grady, R.W.; Neufeld, E.J.; Séchaud, R.; Krebs-Brown, A.J.; Anderson, J.R.; Alberti, D.; Sizer, K.C.; et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: A randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2003, 361, 1597–1602. [Google Scholar]
- Vallenet, D.; Nordmann, P.; Barbe, V.; Poirel, L.; Mangenot, S.; Bataille, E.; Dossat, C.; Gas, S.; Kreimeyer, A.; Lenoble, P.; et al. Comparative analysis of Acinetobacters: Three genomes for three lifestyles. PLoS One 2008, 19, e1805. [Google Scholar] [CrossRef]
- D’Andrea, M.M.; Giani, T.; D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P.; Luzzaro, F.; Rossolini, G.M. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 3528–3533. [Google Scholar]
- Vaneechoutte, M.; Devriese, L.A.; Dijkshoorn, L.; Lamote, B.; Deprez, P.; Verschraegen, G.; Haesebrouck, F. Acinetobacter baumannii-infected vascular catheters collected from horses in an equine clinic. J. Clin. Microbiol. 2000, 38, 4280–4281. [Google Scholar] [PubMed]
- Zordan, S.; Prenger-Berninghoff, E.; Weiss, R.; van der Reijden, T.; van den Broek, P.; Baljer, G.; Dijkshoorn, L. Multidrug-resistant Acinetobacter baumannii in veterinary clinics, Germany. Emerg. Infect. Dis. 2011, 17, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, F.M.; Struelens, M.J.; Towner, K.J.; Gould, I.M.; ARPAC Steering Group; ARPAC Consensus Conference Participants. Report of the Consensus Conference on Antibiotic Resistance; Prevention and Control (ARPAC). Clin. Microbiol. Infect. 2005, 11, 938–954. [Google Scholar]
- Towner, K.J.; Levi, K.; Vlassiadi, M.; on behalf of the ARPAC Steering Group. Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin. Microbiol. Infect. 2008, 14, 161–167. [Google Scholar]
- ImageJ. Available online: http://rsbweb.nih.gov/ij/ (accessed on 14 August 2014).
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972; pp. 252–255. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gentile, V.; Frangipani, E.; Bonchi, C.; Minandri, F.; Runci, F.; Visca, P. Iron and Acinetobacter baumannii Biofilm Formation. Pathogens 2014, 3, 704-719. https://doi.org/10.3390/pathogens3030704
Gentile V, Frangipani E, Bonchi C, Minandri F, Runci F, Visca P. Iron and Acinetobacter baumannii Biofilm Formation. Pathogens. 2014; 3(3):704-719. https://doi.org/10.3390/pathogens3030704
Chicago/Turabian StyleGentile, Valentina, Emanuela Frangipani, Carlo Bonchi, Fabrizia Minandri, Federica Runci, and Paolo Visca. 2014. "Iron and Acinetobacter baumannii Biofilm Formation" Pathogens 3, no. 3: 704-719. https://doi.org/10.3390/pathogens3030704




