Improving the Impact of Commercial Paint on Indoor Air Quality by Using Highly Porous Fillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mold Inoculation
2.2.2. Water Vapor Permeability
2.2.3. Moisture Buffering Capacity
2.2.4. Depolluting Capacity
3. Results and Discussion
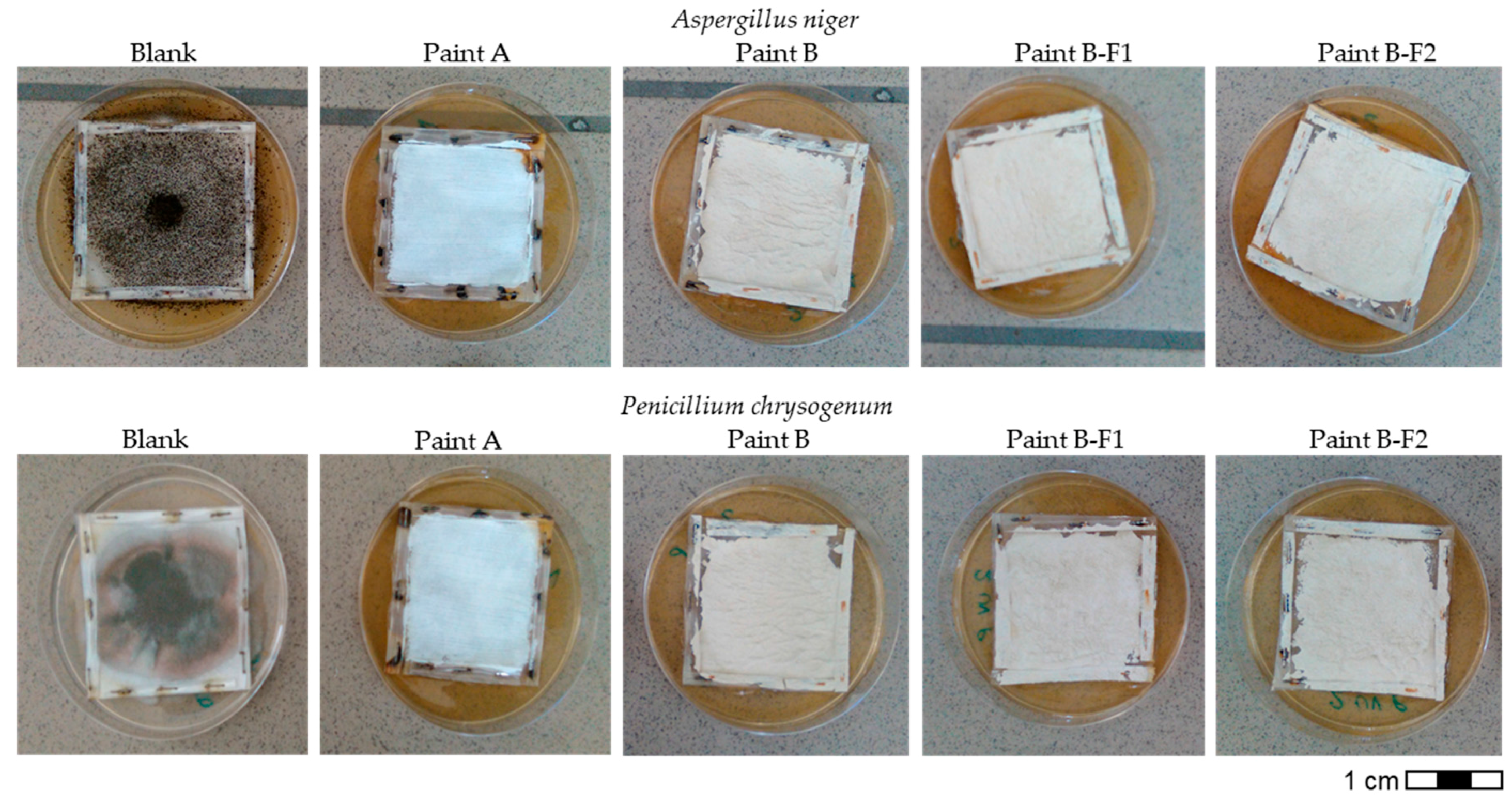
3.1. Mold Growth Inibition
3.2. Water Vapor Permeability
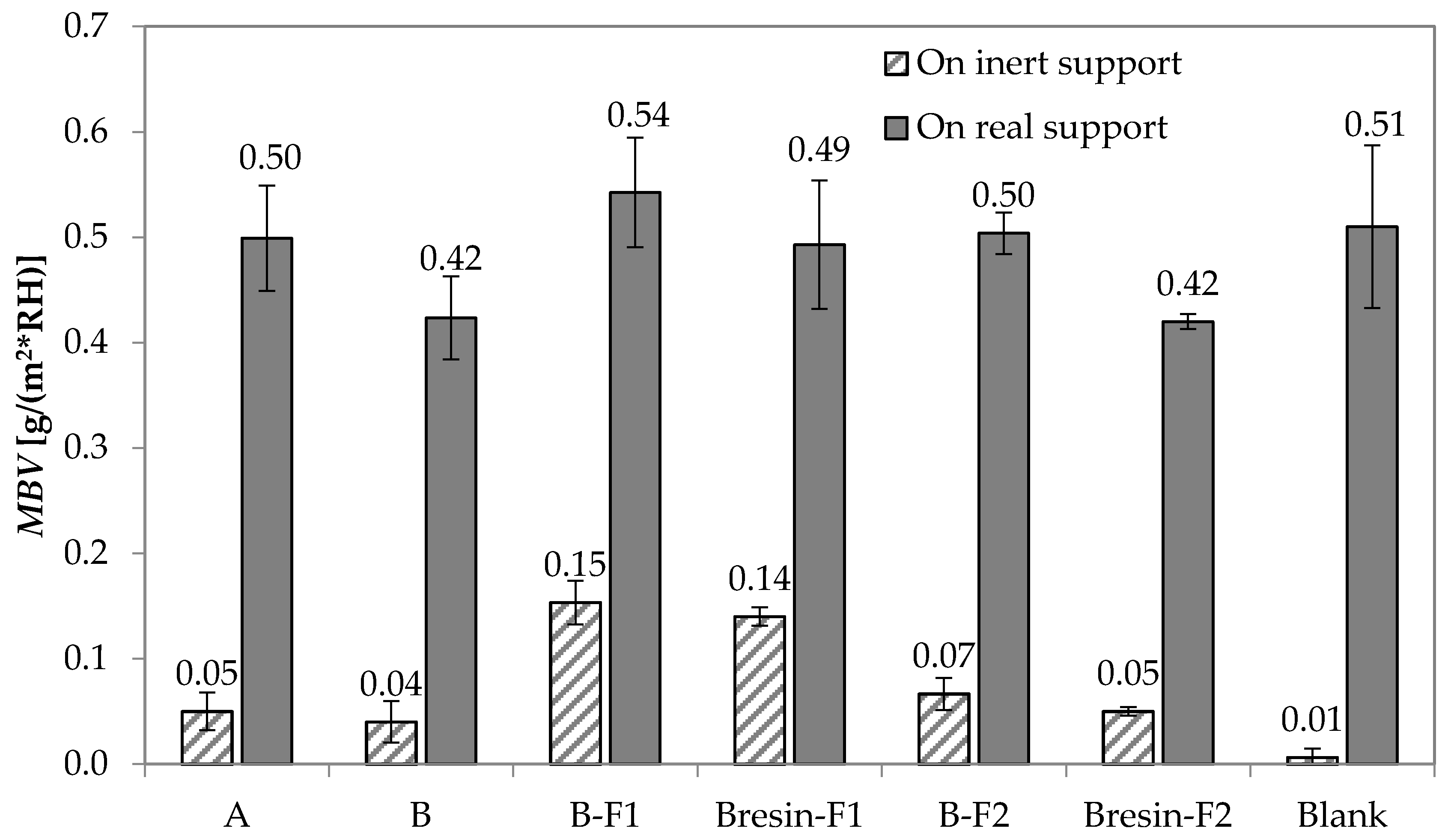
3.3. Moisture Buffering Capacity
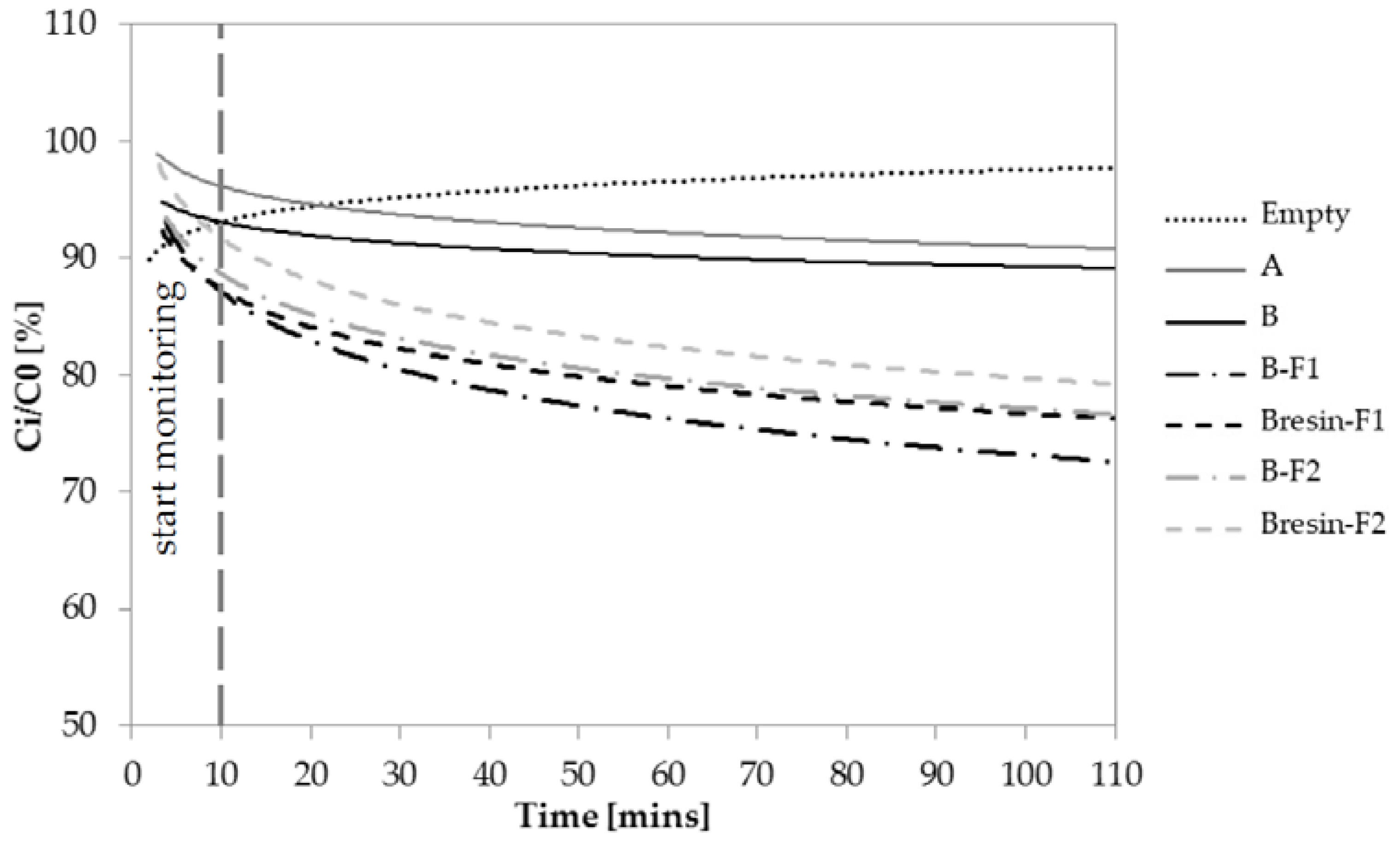
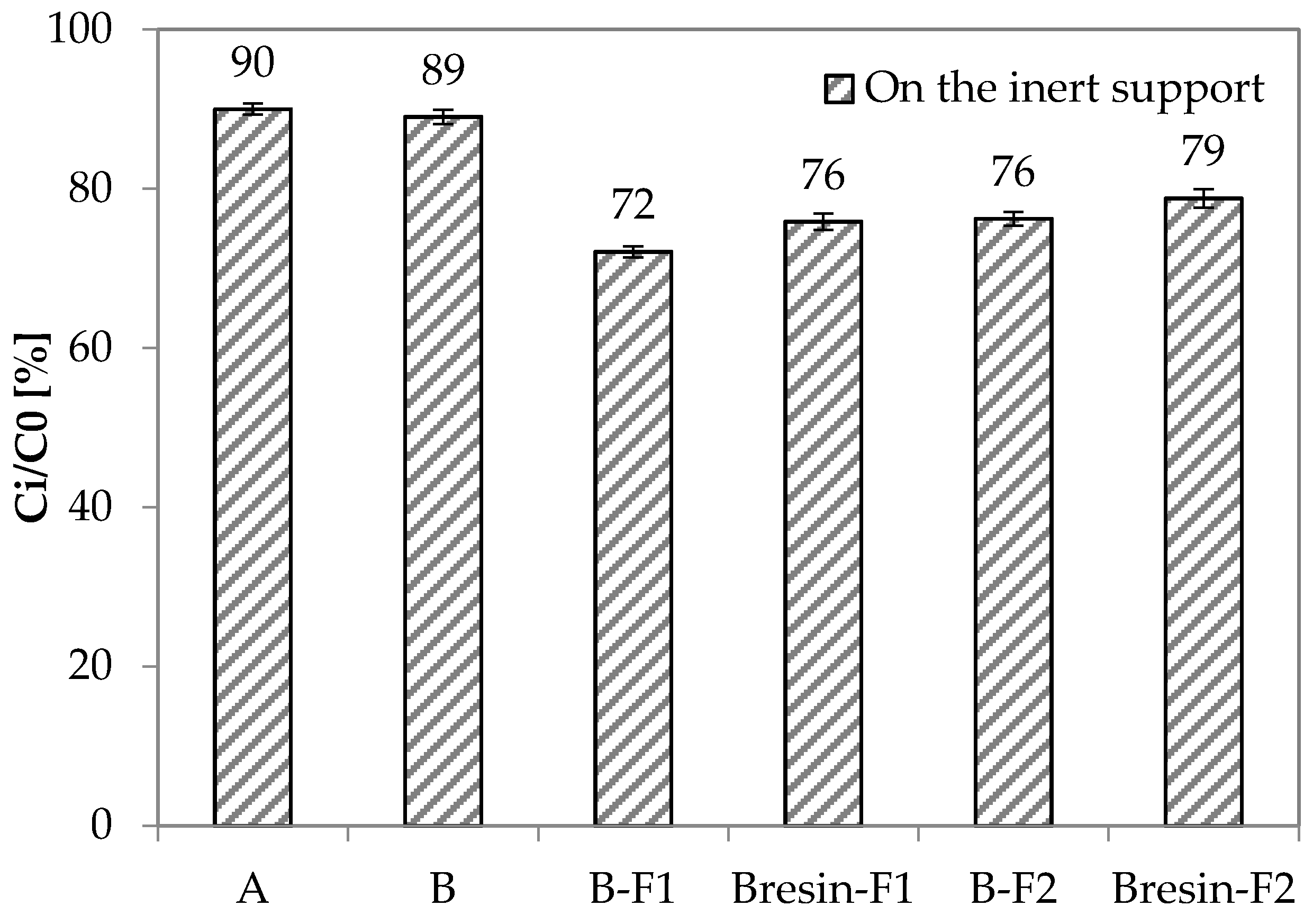
3.4. Depolluting Capacity
4. Conclusions
- The use of unconventional fillers, especially F1, slightly reduces the fluidity of the paint only if added in the formulation. However, this fact does not affect the applicability of the paint.
- The use of unconventional fillers, both as addition or as total replacement of the siliceous filler, does not affect the ability of paint to inhibit the growth of molds.
- Paint A is more breathable than Paint B. The application of Paint A on the mortar substrate does not substantially change the substrate breathability. The application of Paint B halves the breathability of the substrate. However, the use of unconventional fillers counteracts this decrease up to 75%, if added, and up to 25% as a substitute for the original filler, especially when F1 is used.
- Paint A has a slightly higher moisture buffering capacity than Paint B. The application of Paint A on the mortar substrate does not substantially change the substrate moisture buffering capacity. The application of Paint B decreases the moisture buffering capacity of 18%. However, the use of unconventional fillers counteracts this decrease up to 29%, if added, and up to 17% as a substitute of the original filler, especially when F1 is used.
- Paint A and Paint B do not have depolluting properties in terms of VOCs adsorption capacity. However, Paint B becomes adsorbent when unconventional adsorbent fillers are used. Under the current test conditions, the fillers F1 and F2 give 20% and 16% higher adsorption capacity to Paint B, respectively, when they are added to Paint B as it is.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.; Yan, C.; Xiao, F. Quantitative energy performance assessment methods for existing buildings. Energy Build. 2012, 55, 873–888. [Google Scholar] [CrossRef]
- Orazio, M.D.; Palladini, M.; Aquilanti, L.; Clementi, F. Experimental evaluation of the growth rate of mould on finishes for indoor housing environments: Effects of the 2002/91/EC directive. Build. Environ. 2009, 44, 1668–1674. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Giosuè, C.; Ruello, M.L.; Fava, G. Appraisal of a hybrid air cleaning process. Environ. Sci. Pollut. Res. 2017, 24, 12638–12645. [Google Scholar] [CrossRef] [PubMed]
- Wells, E.M.; Berges, M.; Metcalf, M.; Kinsella, A.; Foreman, K.; Dearborn, D.G.; Greenberg, S. Indoor air quality and occupant comfort in homes with deep versus conventional energy efficiency renovations. Build. Environ. 2015, 93, 331–338. [Google Scholar] [CrossRef]
- Aguado, S.; Polo, A.C.; Bernal, M.P.; Coronas, J.; Santamaría, J. Removal of pollutants from indoor air using zeolite membranes. J. Membr. Sci. 2004, 240, 159–166. [Google Scholar] [CrossRef]
- Ozga, I.; Ghedini, N.; Giosuè, C.; Sabbioni, C.; Tittarelli, F.; Bonazza, A. Assessment of air pollutant sources in the deposit on monuments by multivariate analysis. Sci. Total Environ. 2014, 490. [Google Scholar] [CrossRef] [PubMed]
- Vilcekova, S.; Meciarova, L.; Burdove, E.K.; Katunska, J.; Kosicanova, D.; Doroudiani, S. Indoor environmental quality of classrooms and occupants’ comfort in a special education school in Slovak Republic. Build. Environ. 2017, 120, 29–40. [Google Scholar] [CrossRef]
- Lorencik, S.; Yu, Q.L.; Brouwers, H.J.H. Design and performance evaluation of the functional coating for air purification under indoor conditions. Appl. Catal. B Environ. 2015, 168–169, 77–86. [Google Scholar] [CrossRef]
- Meininghaus, R.; Gunnarsen, L.; Knudsen, H.N. Diffusion and Sorption of Volatile Organic Compounds in Building Materials—Impact on Indoor Air Quality. Environ. Sci. Technol. 2000, 34, 3101–3108. [Google Scholar] [CrossRef]
- Vieira, J.; Senff, L.; Gonçalves, H.; Silva, L.; Ferreira, V.M.; Labrincha, J.A. Functionalization of mortars for controlling the indoor ambient of buildings. Energy Build. 2014, 70, 224–236. [Google Scholar] [CrossRef]
- Di Giuseppe, E.; D’Orazio, M. Moisture buffering “active” devices for indoor humidity control: Preliminary experimental evaluations. Energy Procedia 2014, 62, 42–51. [Google Scholar] [CrossRef]
- Aïssa, A.H.; Puzenat, E.; Plassais, A.; Herrmann, J.-M.; Haehnel, C.; Guillard, C. Characterization and photocatalytic performance in air of cementitious materials containing TiO2. Case study of formaldehyde removal. Appl. Catal. B Environ. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Darling, E.; Morrison, G.C.; Corsi, R.L. Passive removal materials for indoor ozone control. Build. Environ. 2016, 106, 33–44. [Google Scholar] [CrossRef]
- Dashtizadeh, A.; Abdouss, M.; Mahdavi, H.; Khorassani, M. Acrylic coatings exhibiting improved hardness, solvent resistance and glossiness by using silica nano-composites. Appl. Surf. Sci. 2011, 257, 2118–2125. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shimizu, M.; Sato, H. The contaminant removal efficiency of an air cleaner using the adsorption/desorption effect. Build. Environ. 2009, 44, 1371–1377. [Google Scholar] [CrossRef]
- George, C.; Beeldens, A.; Barmpas, F.; Doussin, J.F.; Manganelli, G.; Herrmann, H.; Kleffmann, J.; Mellouki, A. Impact of photocatalytic remediation of pollutants on urban air quality. Front. Environ. Sci. Eng. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Tryba, B.; Wrobel, R.J.; Homa, P.; Morawski, A.W. Improvement of photocatalytic activity of silicate paints by removal of K2SO4. Atmos. Environ. 2015, 115, 47–52. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO2-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, M.V.; Del Curto, B.; Ormellese, M.; Pedeferri, M.P. Photocatalytic and self-cleaning activity of colored mortars containing TiO2. Constr. Build. Mater. 2013, 46, 167–174. [Google Scholar] [CrossRef]
- Lucas, S.S.; Ferreira, V.M.; Aguiar, J.L.B. De Incorporation of titanium dioxide nanoparticles in mortars—Influence of microstructure in the hardened state properties and photocatalytic activity. Cem. Concr. Res. 2013, 43, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Gandolfo, A.; Bartolomei, V.; Gomez Alvarez, E.; Tlili, S.; Gligorovski, S.; Kleffmann, J.; Wortham, H. The effectiveness of indoor photocatalytic paints on NOx and HONO levels. Appl. Catal. B Environ. 2015, 166–167, 84–90. [Google Scholar] [CrossRef]
- Hochmannova, L.; Vytrasova, J. Photocatalytic and antimicrobial effects of interior paints. Prog. Org. Coat. 2010, 67, 1–5. [Google Scholar] [CrossRef]
- Tittarelli, F.; Shah, S.P. Effect of low dosages of waste GRP dust on fresh and hardened properties of mortars: Part 1. Constr. Build. Mater. 2013, 47, 1532–1538. [Google Scholar] [CrossRef]
- Tittarelli, F. Effect of low dosages of waste GRP dust on fresh and hardened properties of mortars: Part 2. Constr. Build. Mater. 2013, 47, 1539–1543. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Hu, P.; Liu, L.; Chu, P.K. Reutilization of Industrial Ultrafine Carbon Ash (PM2.5) as Rubber Reinforcement Filler. Environ. Prog. Sustain. Energy 2016, 35, 1132–1138. [Google Scholar] [CrossRef]
- Malaki, M.; Hashemzadeh, Y.; Karevan, M. Effect of nano-silica on the mechanical properties of acrylic polyurethane coatings. Prog. Org. Coat. 2016, 101, 477–485. [Google Scholar] [CrossRef]
- Dianatdar, A.; Jamshidi, M. Investigation of inter-relationship between tensile behavior and photocatalytic activity of an acrylic-based composite upon UVA exposure. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Stazi, F.; Nacci, A.; Tittarelli, F.; Pasqualini, E.; Munafò, P. An experimental study on earth plasters for earthen building protection: The effects of different admixtures and surface treatments. J. Cult. Herit. 2016, 17, 27–41. [Google Scholar] [CrossRef]
- Azadi, M.; Bahrololoom, M.E.; Heidari, F. Enhancing the mechanical properties of an epoxy coating with rice husk ash, a green product. J. Coat. Technol. Res. 2011, 8, 117–123. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizao, C.; Farinha, J.P.S. Functional films from silica/polymer nanoparticles. Materials (Basel) 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Al-Kattan, A.; Wichser, A.; Vonbank, R.; Brunner, S.; Ulrich, A.; Zuin, S.; Arroyo, Y.; Golanski, L.; Nowack, B. Characterization of materials released into water from paint containing nano-SiO2. Chemosphere 2015, 119, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.; Vieira, R.; Mendes, A.M.; Magalhães, F.D. Nanocomposite acrylic paint with self-cleaning action. J. Coat. Technol. Res. 2012, 9, 687–693. [Google Scholar] [CrossRef]
- Moura, A.; Flores-colen, I.; de Brito, J. Study of the effect of three anti-graffiti products on the physical properties of different substrates. Constr. Build. Mater. 2016, 107, 157–164. [Google Scholar] [CrossRef]
- Zuin, S.; Massari, A.; Ferrari, A.; Golanski, L. Formulation effects on the release of silica dioxide nanoparticles from paint debris to water. Sci. Total Environ. 2014, 476–477, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Olad, A.; Nofouzi, K. A self-cleaning coating based on commercial grade polyacrylic latex modified by TiO2/Ag-exchanged-zeolite-A nanocompositeoto. Appl. Surf. Sci. 2015, 346, 543–553. [Google Scholar] [CrossRef]
- Gandolfo, A.; Rouyer, L.; Wortham, H.; Gligorovski, S. The influence of wall temperature on NO2 removal and HONO levels released by indoor photocatalytic paints. Appl. Catal. B Environ. 2017, 209, 429–436. [Google Scholar] [CrossRef]
- Fang, L.; Clausen, G.; Fanger, P.O. Impact of Temperature and Humidity on the Perception of Indoor Air Quality. Indoor Air 1998, 8, 80–90. [Google Scholar] [CrossRef]
- Lelievre, D.; Colinart, T.; Glouannec, P. Hygrothermal behavior of bio-based building materials including hysteresis effects: Experimental and numerical analyses. Energy Build. 2014, 84, 617–627. [Google Scholar] [CrossRef]
- Mensah-Attipoe, J.; Reponen, T.; Salmela, A.; Veijalainen, A.M.; Pasanen, P. Susceptibility of green and conventional building materials to microbial growth. Indoor Air 2015, 25, 273–284. [Google Scholar] [CrossRef] [PubMed]
- WHO. Development of WHO Guidelines for Indoor Air Quality. In Proceedings of the Working Group Meeting, Bonn, Germany, 23–24 October 2006; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2006; pp. 1–27. [Google Scholar]
- Latif, E.; Lawrence, M.; Shea, A.; Walker, P. Moisture buffer potential of experimental wall assemblies incorporating formulated hemp-lime. Build. Environ. 2015, 93, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Lelièvre, D.; Colinart, T.; Glouannec, P. Modeling the moisture buffering behavior of a coated biobased building material by including hysteresis. Energy Procedia 2015, 78, 255–260. [Google Scholar] [CrossRef]
- Ramos, N.M.M.; Delgado, J.M.P.Q.; De Freitas, V.P. Influence of finishing coatings on hygroscopic moisture buffering in building elements. Constr. Build. Mater. 2010, 24, 2590–2597. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Vacher, S.; Hernandez, C.; Bartschi, C.; Pousserau, N. Impact of paint and wall-paper on mould growth on plasterboards and aluminum. Build. Environ. 2010, 45, 916–921. [Google Scholar] [CrossRef]
- Giosuè, C.; Pierpaoli, M.; Mobili, A.; Ruello, M.L.; Tittarelli, F. Influence of Binders and Lightweight Aggregates on the Properties of Cementitious Mortars: From Traditional Requirements to Indoor Air. Materials 2017, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Tittarelli, F.; Giosuè, C.; Mobili, A.; Ruello, M.L. Influence of binders and aggregates on VOCs adsorption and moisture buffering activity of mortars for indoor applications. Cem. Concr. Compos. 2015, 57. [Google Scholar] [CrossRef]
- Giosuè, C.; Mobili, A.; Toscano, G.; Ruello, M.L.; Tittarelli, F. Effect of Biomass Waste Materials as Unconventional Aggregates in Multifunctional Mortars for Indoor Application. Procedia Eng. 2016, 161, 655–659. [Google Scholar] [CrossRef]
- Liguori, B.; Cassese, A.; Colella, C. Entrapping noxious cations in ceramic matrices. J. Porous Mater. 2007, 14. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Citterio, B.; Biavasco, F.; Stazi, F.; Barcelli, S.; Munafò, P. Nanotechnology on wood: The effect of photocatalytic nanocoatings against Aspergillus niger. J. Cult. Herit. 2017, 27, 125–136. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Dhir, R.K. Performance of cementitious renderings and masonry mortars containing recycled aggregates from construction and demolition wastes. Constr. Build. Mater. 2016, 105, 400–415. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Bellezze, T.; Tittarelli, F. Metakaolin and fly ash alkali-activated mortars compared with cementitious mortars at the same strength class. Cem. Concr. Res. 2016, 88. [Google Scholar] [CrossRef]
- Mobili, A.; Giosuè, C.; Bitetti, M.; Tittarelli, F. Cement mortars and geopolymers with the same strength class. Proc. Inst. Civ. Eng. Constr. Mater. 2016, 169, 3–12. [Google Scholar] [CrossRef]
- Slanina, P.; Šilarová, Š. Moisture transport through perforated vapour retarders. Build. Environ. 2009, 44, 1617–1626. [Google Scholar] [CrossRef]
- Rode, C.; Peuhkuri, R.; Mortensen, L.H.; Hansen, K.K.; Time, B.; Gustavsen, A.; Ojanen, T.; Ahonen, J.; Svennberg, K.; Arfvidsson, J. Moisture Buffering of Building Materials, BYG DTU Report; Technical University of Denmark: Kongens Lyngby, Denmark, 2005; ISBN 8778771951. [Google Scholar]
- Frey, S.E.; Destaillats, H.; Cohn, S.; Ahrentzen, S.; Fraser, M.P. The effects of an energy efficiency retrofit on indoor air quality. Indoor Air 2015, 25, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Methyl-Ethyl-Ketone (2-Butanone)—Hazard Summary, EPA U.S. Environmental Protection Agency. 2016. Available online: https://www.epa.gov/sites/production/files/2016-09/documents/methyl-ethyl-ketone.pdf (accessed on 19 August 2017).
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chen, W. Effect of paint composition, nano-metal types and substrate on the improvement of biological resistance on paint finished building material. Build. Environ. 2017, 117, 49–59. [Google Scholar] [CrossRef]
- Rahim, M.; Douzane, O.; Tran Le, A.D.; Promis, G.; Laidoudi, B.; Crigny, A.; Dupre, B.; Langlet, T. Characterization of flax lime and hemp lime concretes: Hygric properties and moisture buffer capacity. Energy Build. 2015, 88, 91–99. [Google Scholar] [CrossRef]
- Topçuoğlu, Ö.; Altinkaya, S.A.; Balköse, D. Characterization of waterborne acrylic based paint films and measurement of their water vapor permeabilities. Prog. Org. Coat. 2006, 56, 269–278. [Google Scholar] [CrossRef]
- Bonazza, A.; Vidorni, G.; Natali, I.; Ciantelli, C.; Giosuè, C.; Tittarelli, F. Durability assessment to environmental impact of nano-structured consolidants on Carrara marble by field exposure tests. Sci. Total Environ. 2017, 575, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gong, G.; Yu, C.W.; Huang, Z. Proposing ultimate moisture buffering value (UMBV) for characterization of composite porous mortars. Constr. Build. Mater. 2015, 82, 81–88. [Google Scholar] [CrossRef]




| Aggregate | Code | Specific Surface (m2/g) | Density ssd * (kg/m3) | Water Absorption after 24 h (%) |
|---|---|---|---|---|
| Filler 1 | F1 | 750 | 1310 | 86 |
| Filler 2 | F2 | 600 | 1600 | 22 |
| Symbol | Observed Growth on Specimens |
|---|---|
| - | None |
| + | Contaminated around the inoculum |
| ++ | Widespread contamination |
| Week | Blank | Paint A | Paint B | Paint B-F1 | Paint B-F2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A 1 | P 2 | A 1 | P 2 | A 1 | P 2 | A 1 | P 2 | A 1 | P 2 | |||||||||||
| Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | Test 1 | Test 2 | |
| 1 | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | ++ | ++ | ++ | ++ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 4 | ++ | ++ | ++ | ++ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giosuè, C.; Belli, A.; Mobili, A.; Citterio, B.; Biavasco, F.; Ruello, M.L.; Tittarelli, F. Improving the Impact of Commercial Paint on Indoor Air Quality by Using Highly Porous Fillers. Buildings 2017, 7, 110. https://doi.org/10.3390/buildings7040110
Giosuè C, Belli A, Mobili A, Citterio B, Biavasco F, Ruello ML, Tittarelli F. Improving the Impact of Commercial Paint on Indoor Air Quality by Using Highly Porous Fillers. Buildings. 2017; 7(4):110. https://doi.org/10.3390/buildings7040110
Chicago/Turabian StyleGiosuè, Chiara, Alberto Belli, Alessandra Mobili, Barbara Citterio, Francesca Biavasco, Maria Letizia Ruello, and Francesca Tittarelli. 2017. "Improving the Impact of Commercial Paint on Indoor Air Quality by Using Highly Porous Fillers" Buildings 7, no. 4: 110. https://doi.org/10.3390/buildings7040110








