Influence of σ Phase on the Allotropic Transformation of the Matrix in Co-Re-Cr-Based Alloys with Ni Addition
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
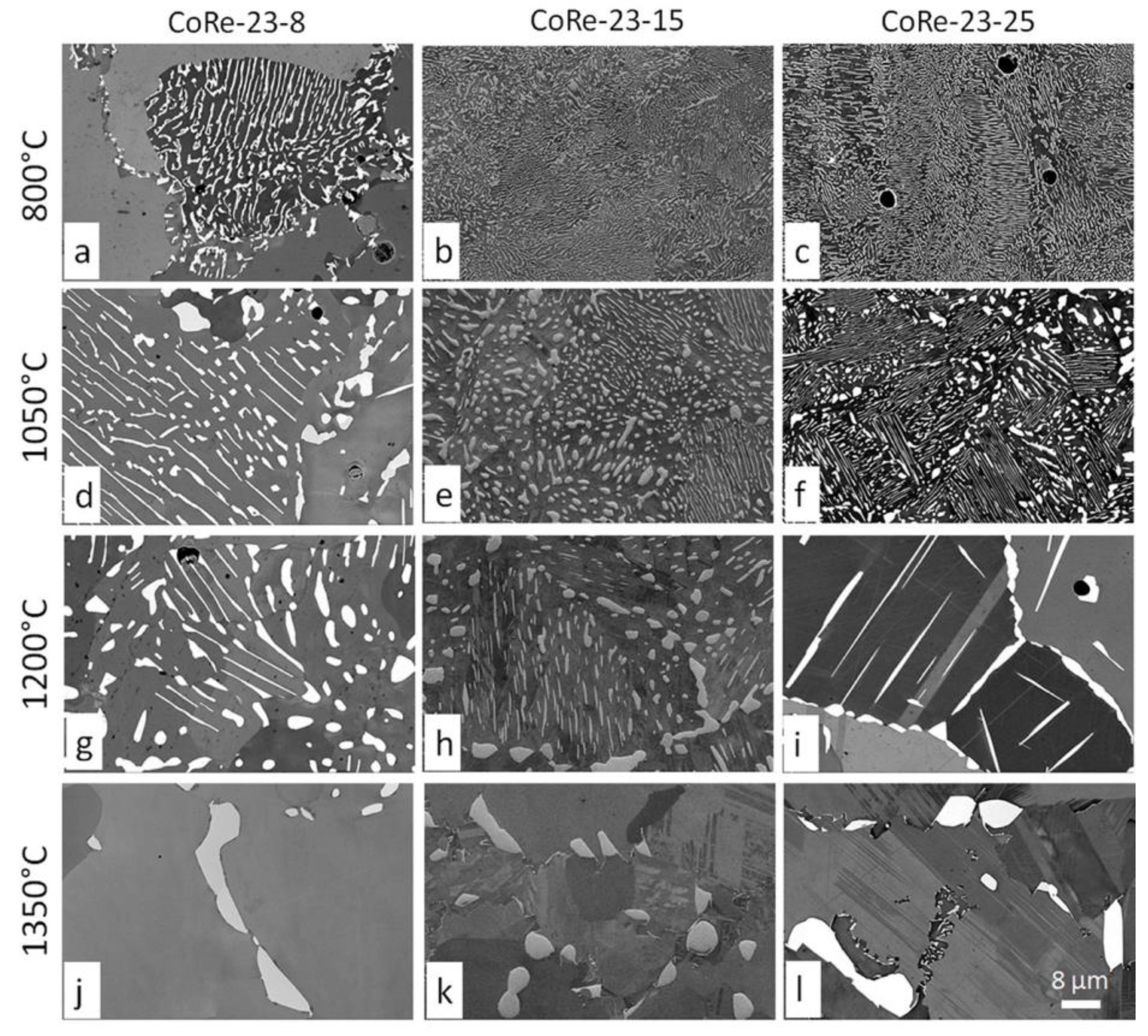
3.1. Microstructure Related to Alloy Composition and Heat Treatment
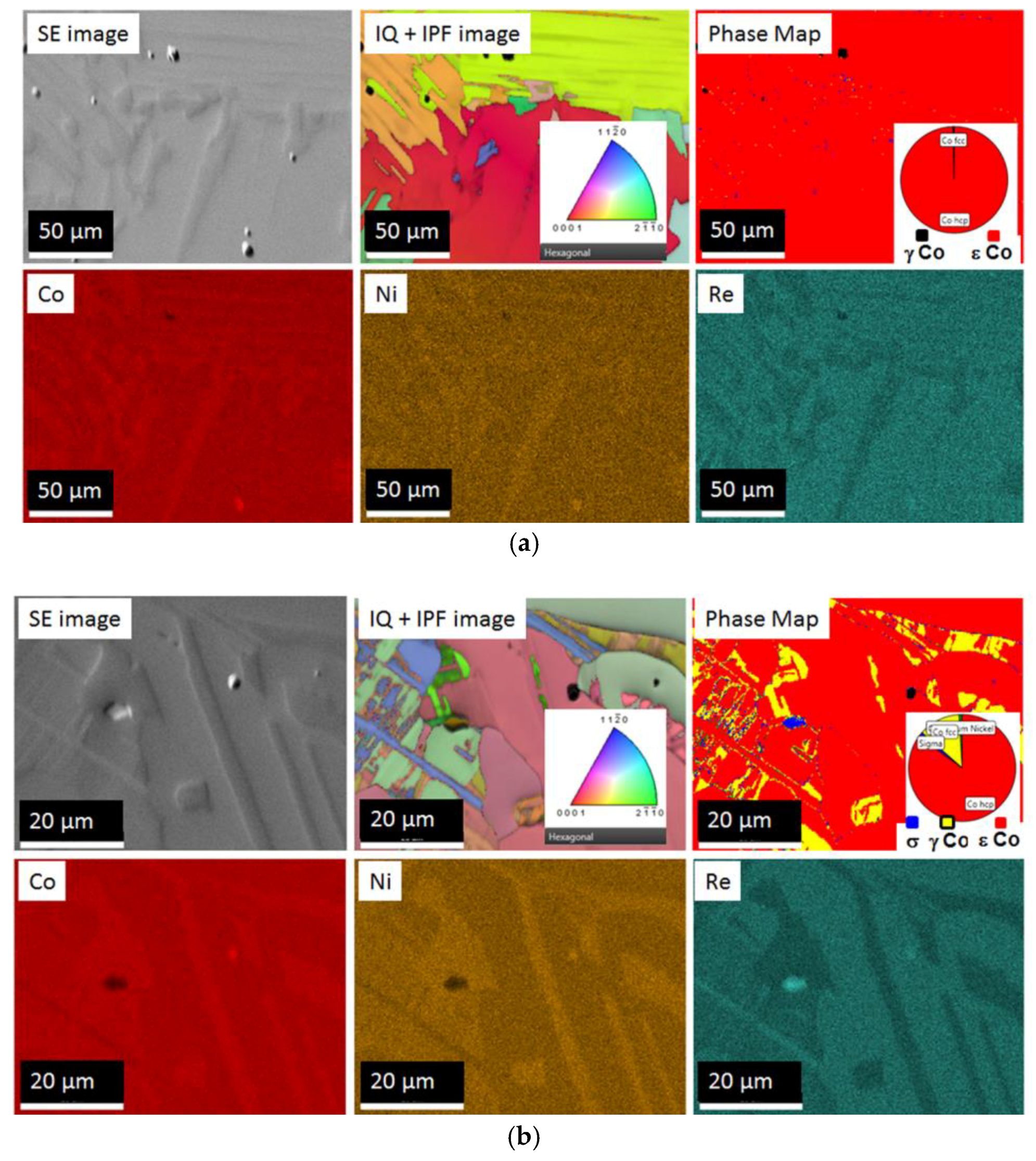
3.2. Combined EBSD and EDS Measurements
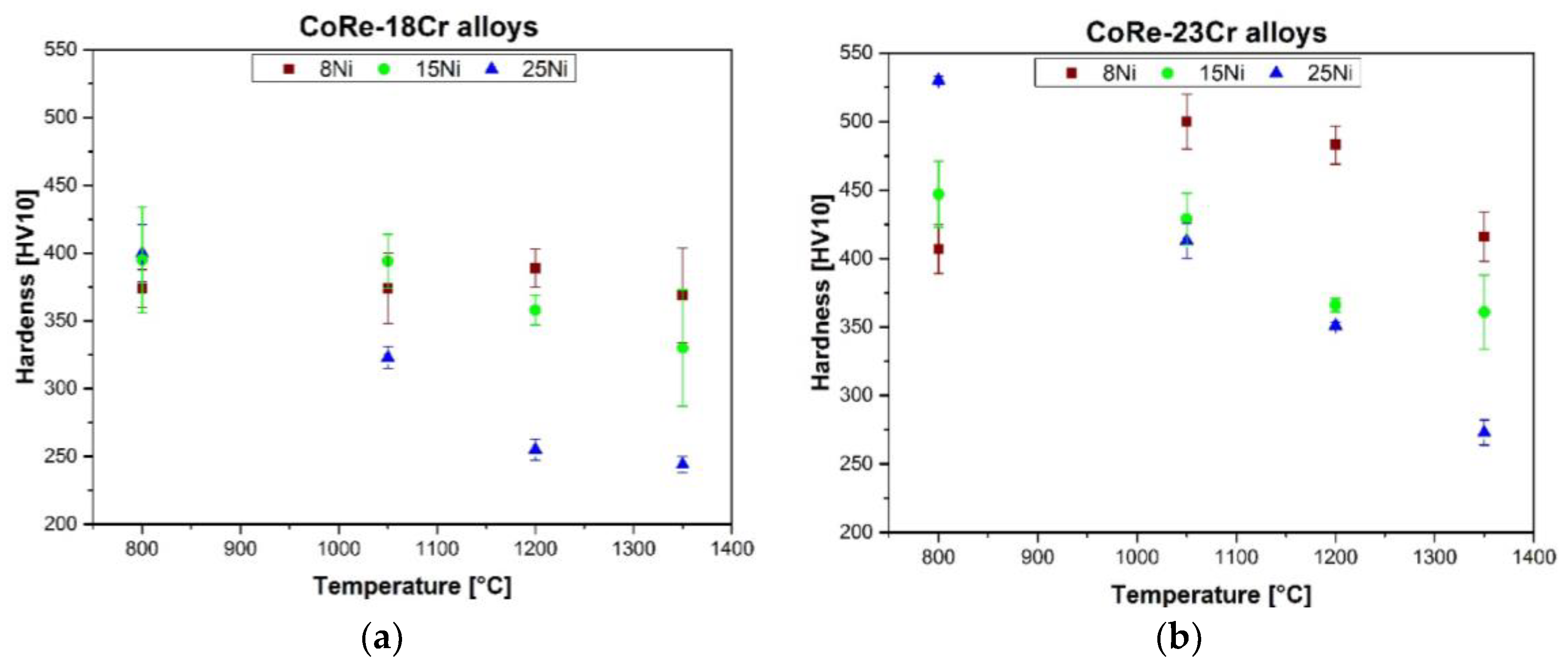
3.3. Hardness
4. Discussion
4.1. Influence of Cr and Ni on the σ Phase
4.2. σ Phase Morphology and Its Influence on the Allotropic Transformation of the Co Matrix
4.3. Matrix Phase Transformation during Cooling
4.4. Influence of the Microstructure on Alloy Hardness
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rösler, J.; Mukherji, D.; Baranski, T. Co-Re-based alloys: A new class of high temperature materials? Adv. Eng. Mater. 2007, 9, 876–881. [Google Scholar] [CrossRef]
- Wang, L.; Gorr, B.; Christ, H.-J.; Mukherji, D.; Rösler, J. The effect of alloyed nickel on the short-term high temperature oxidation behaviour of Co-Re-Cr-based alloys. Corros. Sci. 2015, 93, 19–26. [Google Scholar] [CrossRef]
- Mukherji, D.; Rösler, J.; Fricke, T.; Pieger, S.; Schmitz, F. Materials for Advanced Power Engineering, Energy & Environment; Lecomte-Beckers, J., Contrepois, Q., Beck, T., Kuhn, B., Eds.; Jülich Forschungszentrum: Jülich, Germany, 2010; p. 633. [Google Scholar]
- Mukherji, D.; Strunz, P.; Gilles, R.; Hofmann, M.; Schmitz, F.; Rösler, J. Investigation of phase transformations by in-situ neutron diffraction in a Co-Re-based high temperature alloy. Mater. Lett. 2010, 64, 2608–2611. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, G.E. Atomic number and crystallographic contrast images with the SEM: A review of backscattered electron techniques. Mineral. Mag. 1987, 51, 3–19. [Google Scholar] [CrossRef]
- Beran, P.; Mukherji, D.; Strunz, P.; Gilles, R.; Hölzel, M.; Rösler, J. Coexistence of two cubic-lattice Co matrices at high temperatures in Co-Re-Cr-Ni alloy studied by neutron diffraction. Adv. Mater. Sci. Eng. 2018, 2018, 5410871. [Google Scholar] [CrossRef]
- Christian, J.W. A theory of the transformation in pure cobalt. Proc. R. Soc. A Math. Phys. Eng. Sci. 1951, 206, 51–64. [Google Scholar] [CrossRef]
- Joubert, J.-M. Crystal chemistry and Calphad modeling of the σ phase. Prog. Mater. Sci. 2008, 53, 528–583. [Google Scholar] [CrossRef]
- Kabliman, E.; Ruban, A.V.; Blaha, P.; Peil, O.; Schwarz, K. Ab initio study of lattice site occupancies in binary sigma phases using a single-site mean field model. Appl. Sci. 2012, 2, 654–668. [Google Scholar] [CrossRef]
- Huang, W.; Chang, Y.A. Thermodynamic analysis of the Cr-Re system and prediction of the Cr-Ni-Re system. J. Alloys Compd. 1998, 274, 209–216. [Google Scholar] [CrossRef]
- Kasper, J.S.; Waterstrat, R.M. Ordering of atoms in the σ phase. Acta Cryst. 1956, 9, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.-O.; Fernández Guillermet, A.; Hillert, M.; Jansson, B.; Sundman, B. A compound-energy model of ordering in a phase with sites of different coordination numbers. Acta Metall. 1986, 34, 437–445. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Wu, W. Overview of intermetallic sigma (σ) phase precipitation in stainless steels. ISRN Metall. 2012, 2012, 732471. [Google Scholar] [CrossRef]
- Mukherji, D.; Rösler, J. Co-Re-based alloys for high temperature applications: Design considerations and strengthening mechanisms. J. Phys. Conf. Ser. 2010, 240, 012066. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Z. Martensitic Transformation, 1st ed; Academic press: New York, NY, USA, 1978; p. 480. ISBN 9780323148818. [Google Scholar]
- Ross, E.W. René 100: A sigma-free turbine blade alloy. JOM 1967, 19, 12–14. [Google Scholar] [CrossRef]





| Alloy Designation | Co | Re | Cr | Ni |
|---|---|---|---|---|
| CoRe-18-8 | Rest | 17 | 18 | 8 |
| CoRe-18-15 | Rest | 17 | 18 | 15 |
| CoRe-18-25 | Rest | 17 | 18 | 25 |
| CoRe-23-8 | Rest | 17 | 23 | 8 |
| CoRe-23-15 | Rest | 17 | 23 | 15 |
| CoRe-23-25 | Rest | 17 | 23 | 25 |
| Alloy | CoRe-23-15 | CoRe-23-25 |
|---|---|---|
| Lattice parameter (nm) | Measured by neutron diffraction | |
| σ phase Tetragonal: Pearson symbol tP30; [space group P42/mnm, sp.gp # 136] | ||
| a | 0.9010 | 0.9062 |
| c | 0.4672 | 0.4691 |
| ε phase Hexagonal: Pearson symbol hP2; [space group P63/mmc, sp.gp # 194] | ||
| a | 0.2557 | 0.2559 |
| c | 0.4145 | 0.41498 |
| γ phase Cubic: Pearson symbol cF4; [space group Fm-3m, sp.gp # 225] | ||
| a | 0.3605 | 0.36117 |
| Alloy Composition (atomic %) | Volume Fraction of Phase (Primary + Secondary) | ||||
|---|---|---|---|---|---|
| Cr | Ni | Heat Treatment Temperature | |||
| 800 °C | 1050 °C | 1200 °C | 1350 °C | ||
| 18 | 8 | 0.4 | 0 | 0 | 0 |
| 15 | 0.3 | 0 * | 0 | 0 | |
| 25 | 13.6 | 10.3 | 0 * | 0 | |
| 23 | 8 | 1.2 | 14.0 | 15.0 | 2.6 |
| 15 | 8.2 | 26.0 | 15.0 | 1.2 | |
| 25 | 35.2 | 27.3 | 14.9 | 1.2 | |
| Heat Treatment Temperature | CoRe-18 | CoRe-23 | ||||
|---|---|---|---|---|---|---|
| 8 Ni | 15 Ni | 25 Ni | 8 Ni | 15 Ni | 25 Ni | |
| 800 °C | ε | ε | γ | ε | ε | γ |
| 1050 °C | ε + γ | ε + γ | ||||
| 1200 °C | ε + γ | |||||
| 1350 °C | ε + γ | |||||
| Heat Treatment Temperature | CoRe-18 | CoRe-23 | ||||
|---|---|---|---|---|---|---|
| 8 Ni | 15 Ni | 25 Ni | 8 Ni | 15 Ni | 25 Ni | |
| 800 °C | ε | ε + γ | γ | ε | ε + γ | γ |
| 1050 °C | γ | |||||
| 1200 °C | ε + γ | γ | ||||
| 1350 °C | γ | γ | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörries, K.; Mukherji, D.; Rösler, J.; Esleben, K.; Gorr, B.; Christ, H.-J. Influence of σ Phase on the Allotropic Transformation of the Matrix in Co-Re-Cr-Based Alloys with Ni Addition. Metals 2018, 8, 706. https://doi.org/10.3390/met8090706
Dörries K, Mukherji D, Rösler J, Esleben K, Gorr B, Christ H-J. Influence of σ Phase on the Allotropic Transformation of the Matrix in Co-Re-Cr-Based Alloys with Ni Addition. Metals. 2018; 8(9):706. https://doi.org/10.3390/met8090706
Chicago/Turabian StyleDörries, Kai, Debashis Mukherji, Joachim Rösler, Katharina Esleben, Bronislava Gorr, and Hans-Juergen Christ. 2018. "Influence of σ Phase on the Allotropic Transformation of the Matrix in Co-Re-Cr-Based Alloys with Ni Addition" Metals 8, no. 9: 706. https://doi.org/10.3390/met8090706





