Removal of Tramp Elements within 7075 Alloy by Super-Gravity Aided Rheorefining Method
Abstract
:1. Introduction
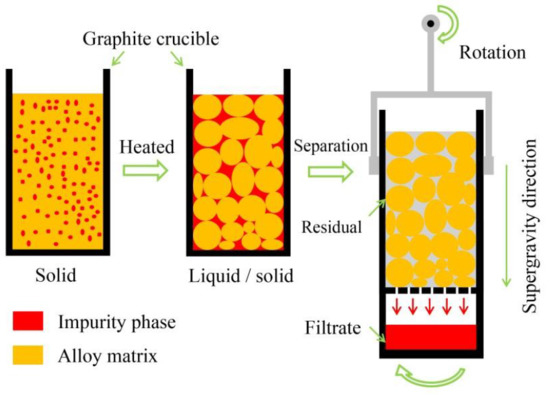
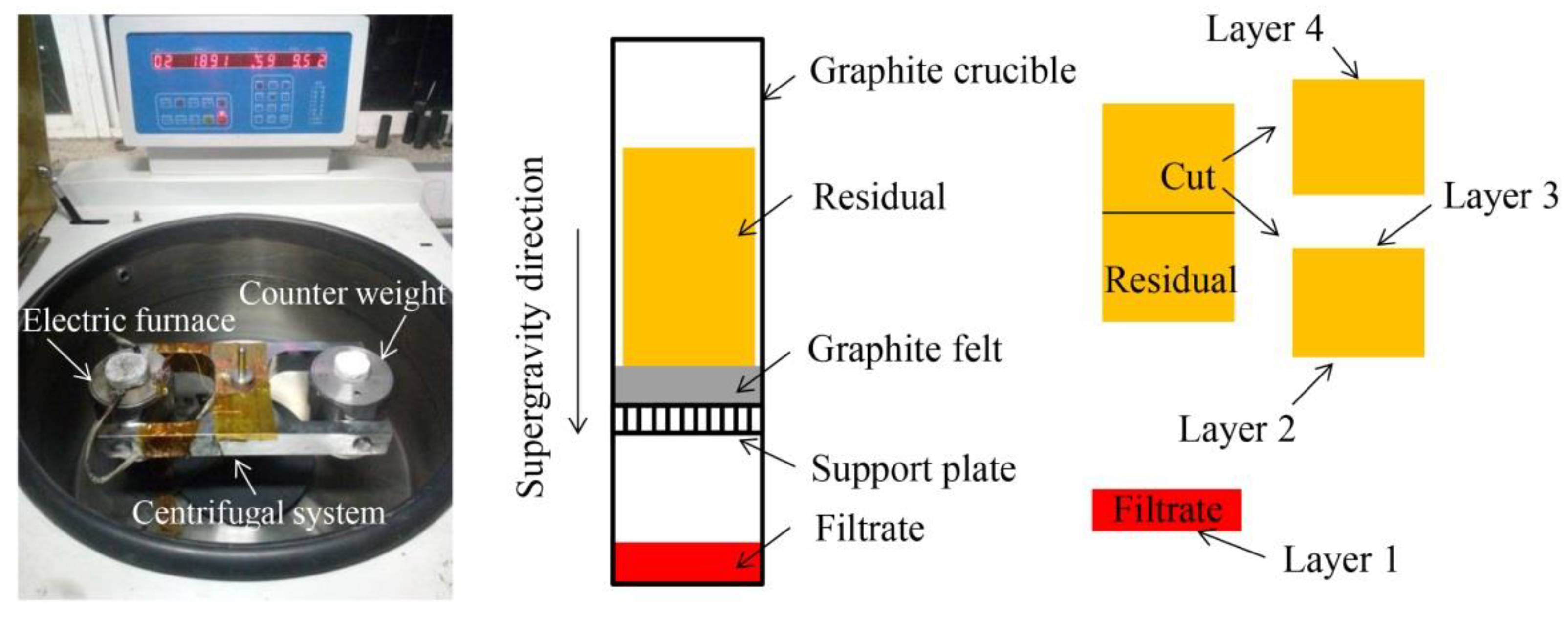
2. Materials and Methods
3. Results
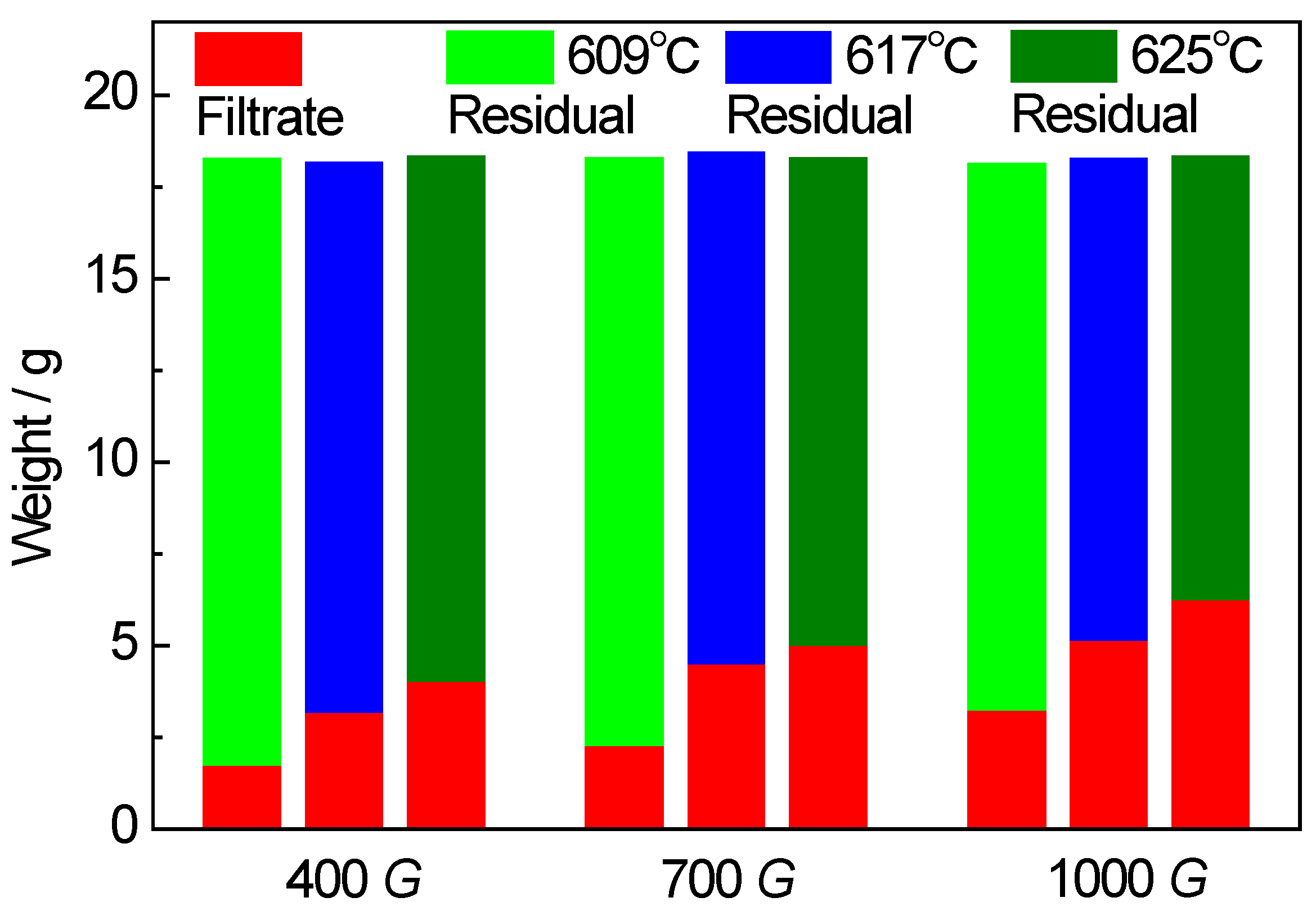
3.1. Separation Effect of Solid/Liquid Phases
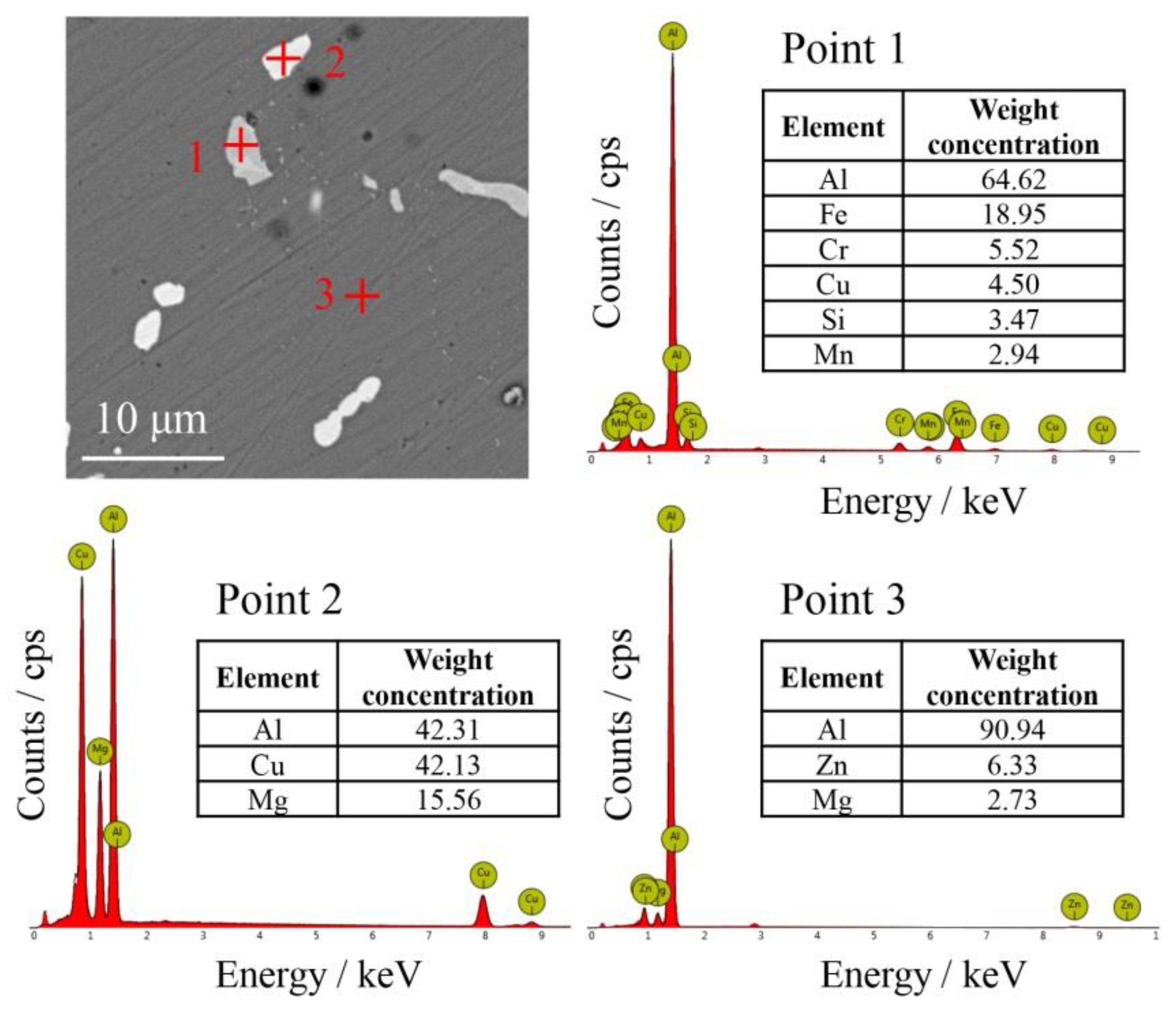
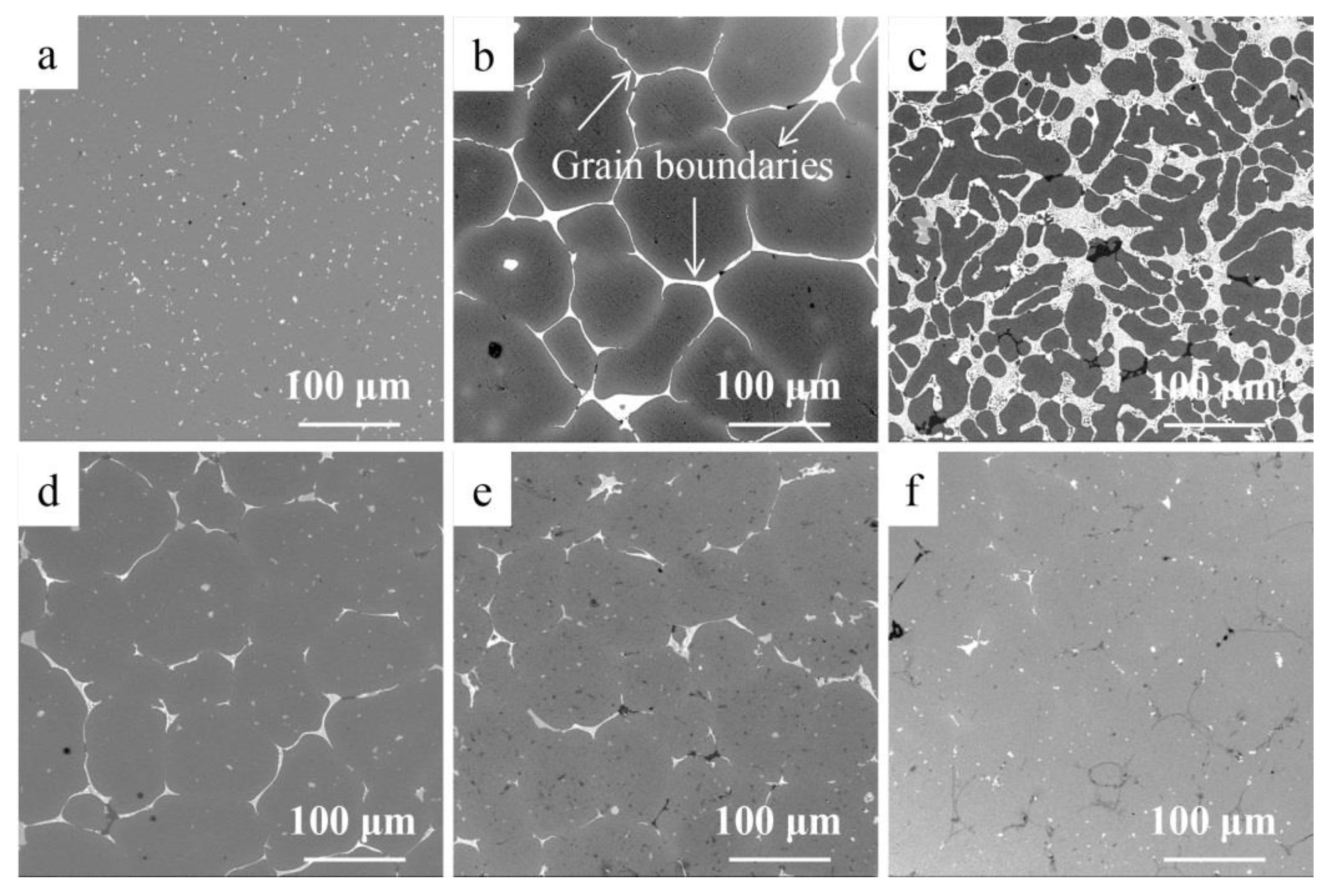
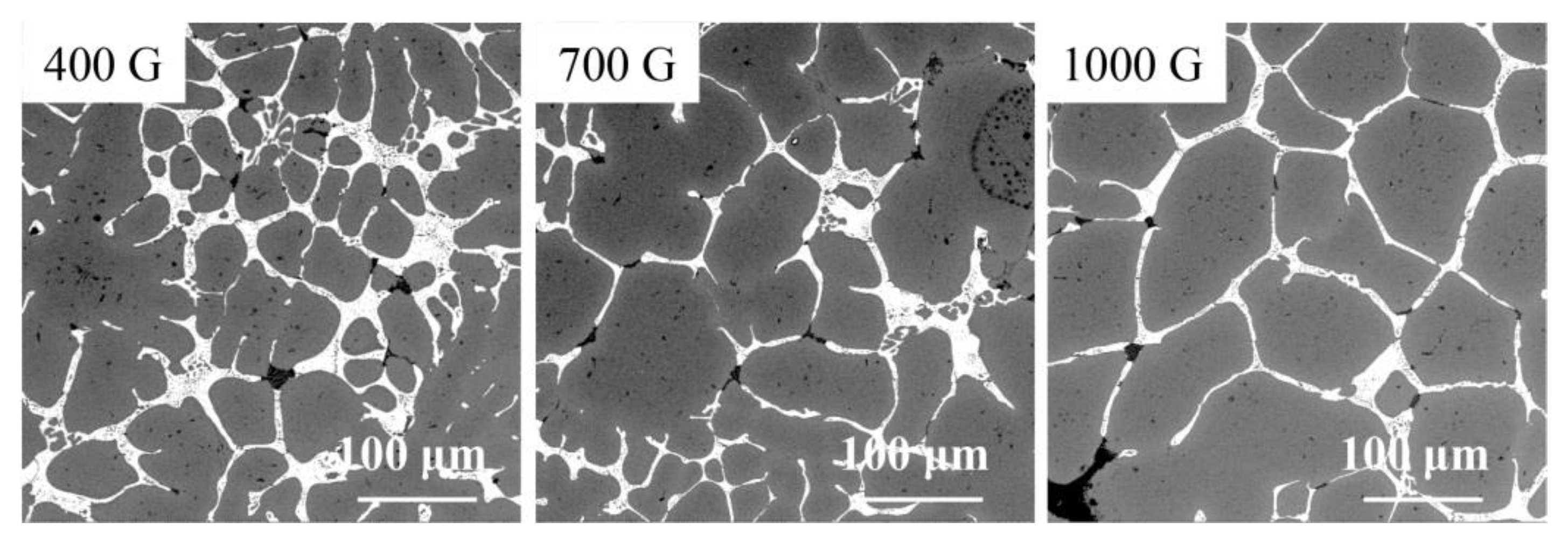
3.2. Morphology in Different Layers
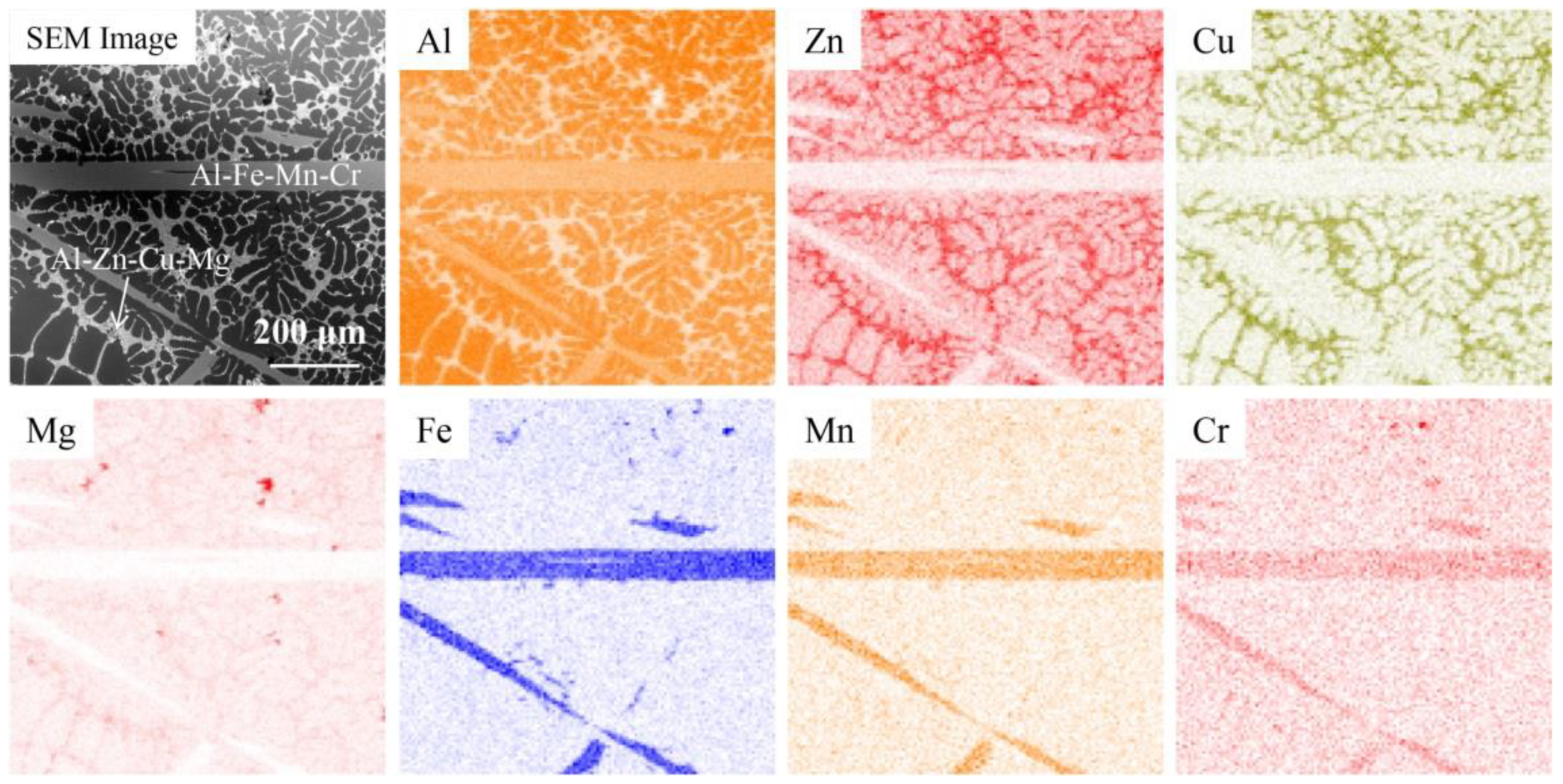
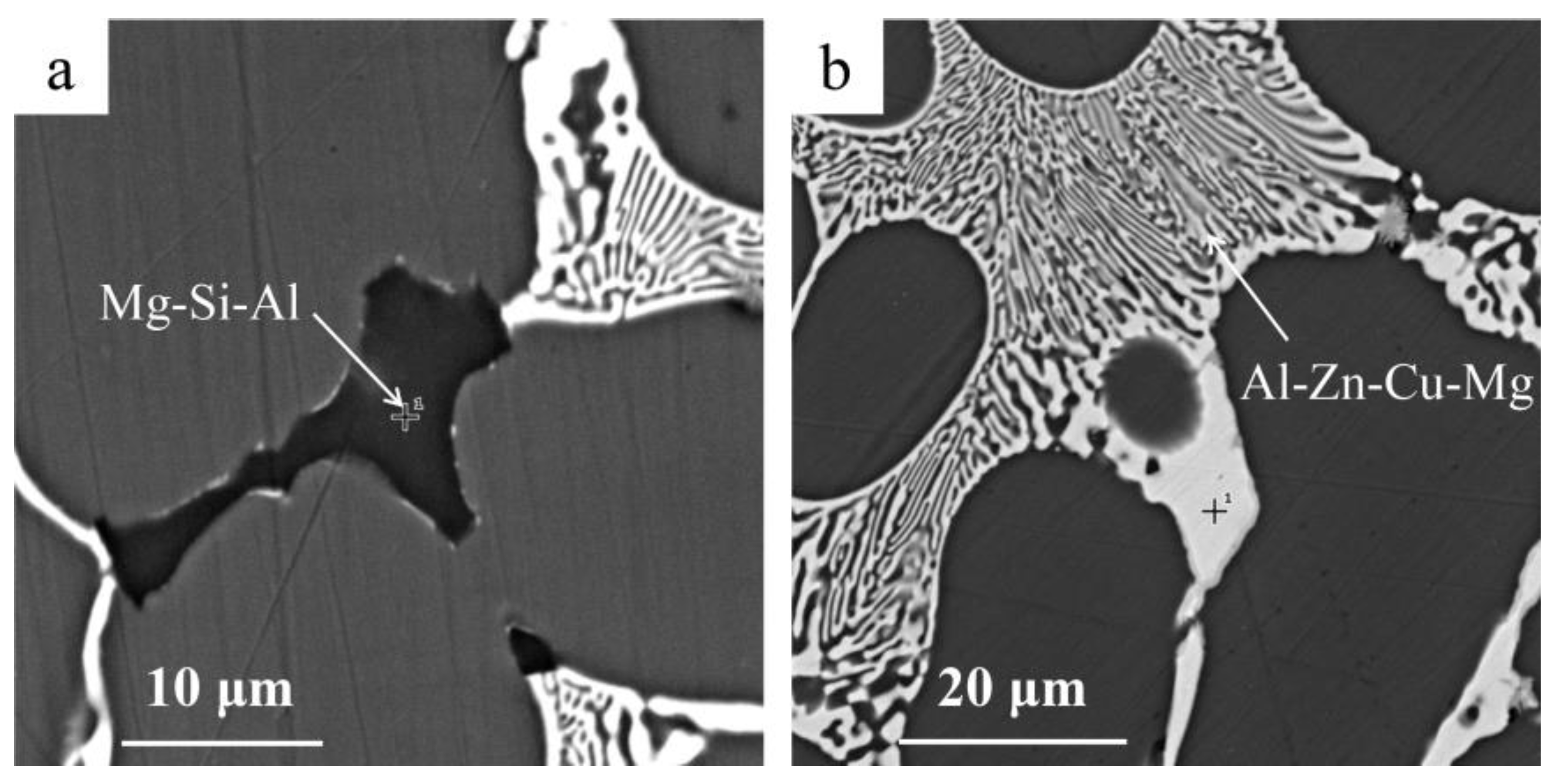
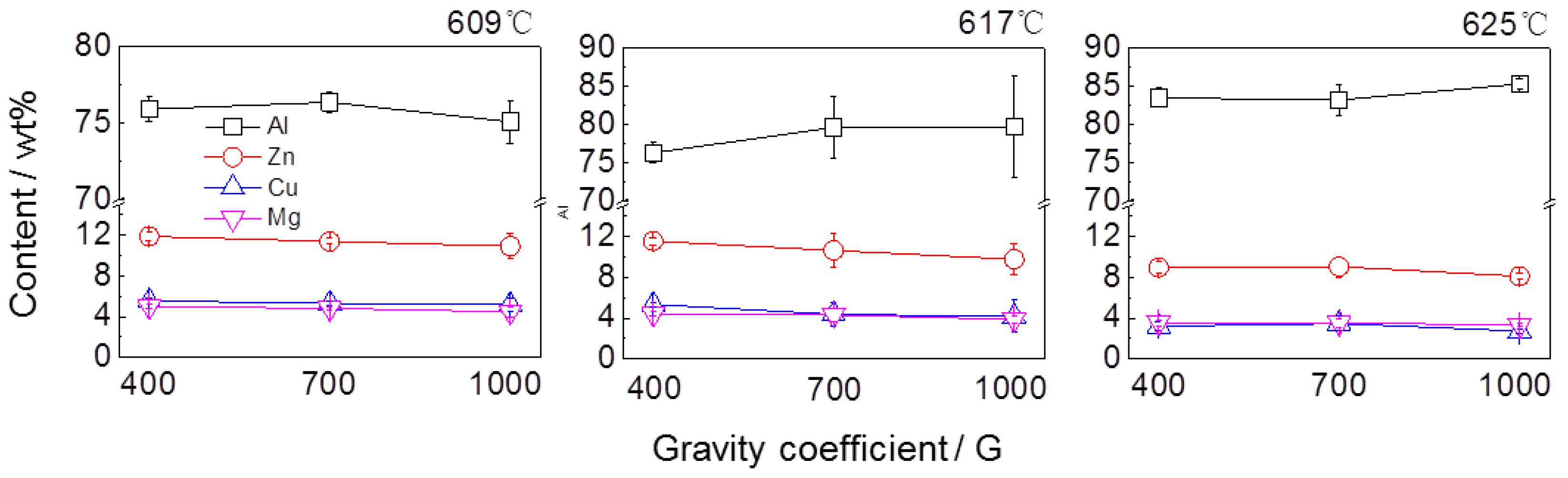
3.3. The Composition and Distribution of Residual and Filtrate
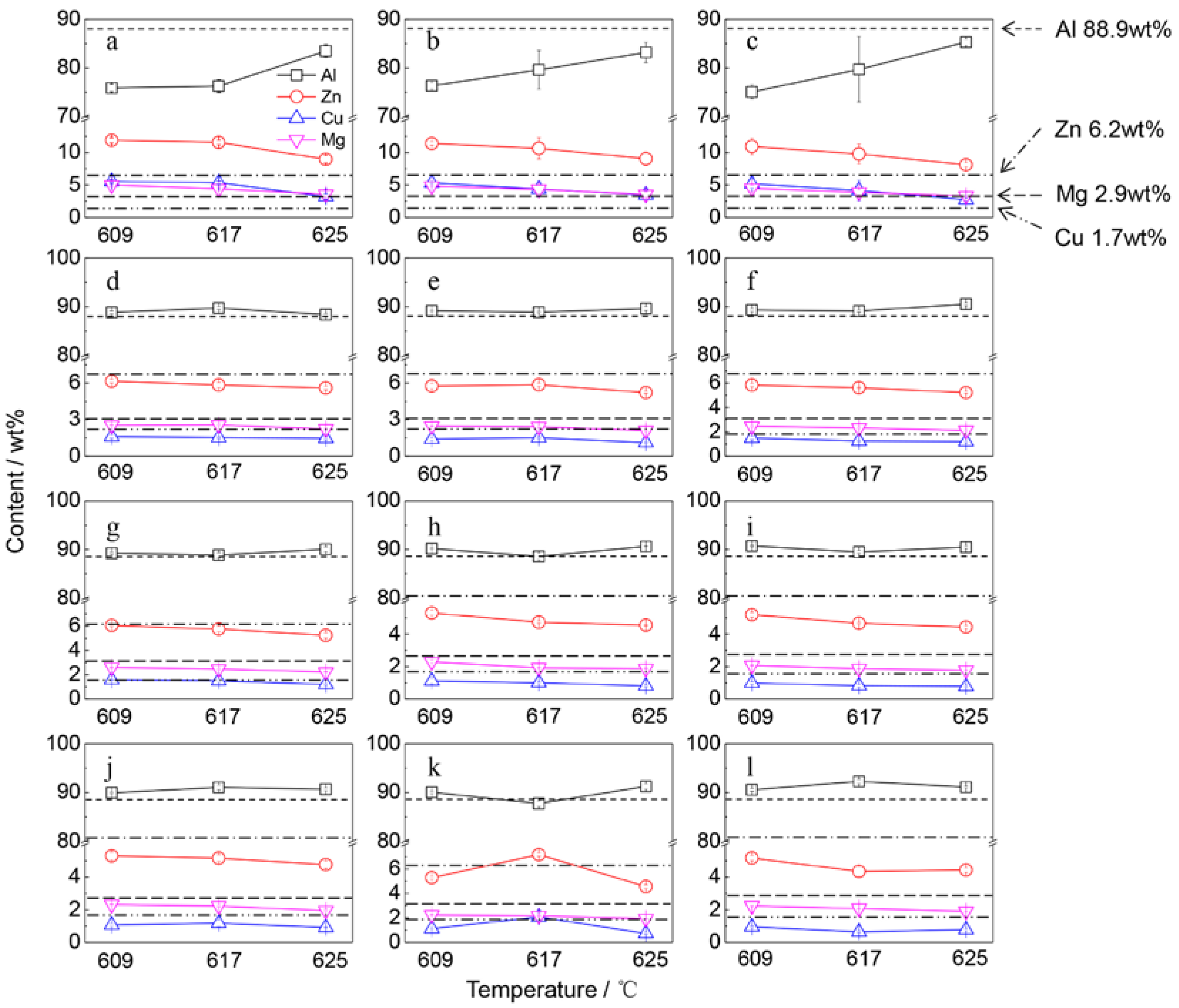
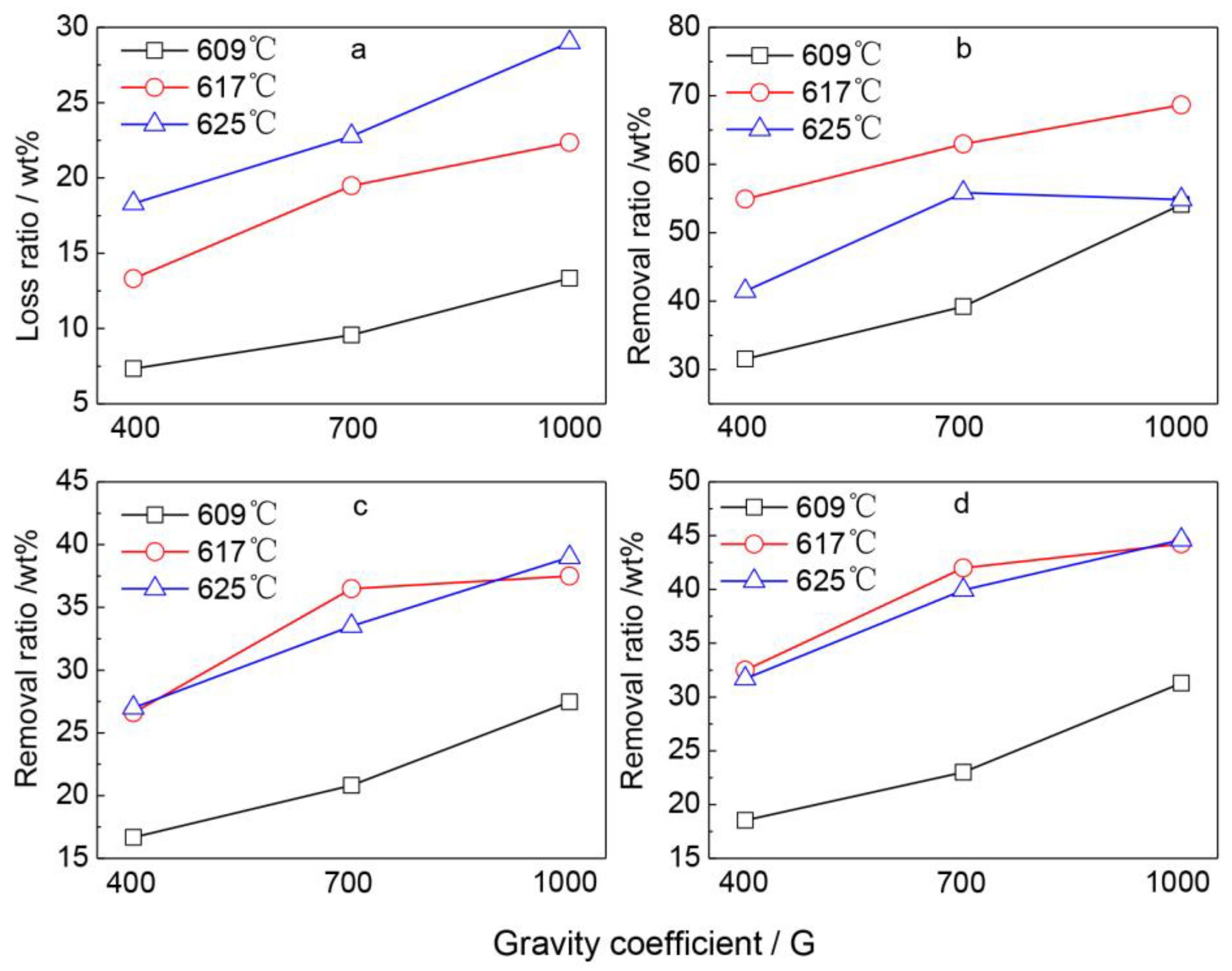
3.4. The Removal Ratio of Tramp Elements
4. Discussion
4.1. Effect of Separation Temperature
4.2. Effect of Gravity Coefficient
4.3. Effect of Separation Time
4.4. Removal Mechanism of Tramp Element
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moore, J.J. Recycling of non-ferrous metals. Int. Met. Rev. 1978, 23, 241–264. [Google Scholar] [CrossRef]
- Gao, J.W.; Shu, D.; Wang, J.; Sun, B.D. Study on iron purification from aluminium melt by Na2B4O7 flux. Mater. Sci. Technol. 2009, 25, 619–624. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Shu, D.; Zhang, S.; Sun, B.D. Removal of non-metallic inclusions from aluminum by electroslag refining. Mater. Trans. 2011, 52, 2266–2269. [Google Scholar] [CrossRef]
- Ichikawa, K.; Katoh, M.; Asuke, F.; Nakazawa, Y. High efficient recovery of pure aluminum from Al-Sn and Al-Ni alloys by rheorefining process. Mater. Trans. 1997, 38, 622–629. [Google Scholar] [CrossRef]
- Cho, T.T.; Sugiyama, S.; Yanagimoto, J. Effect of process parameters on purification of aluminium alloys by backward extrusion process under a semisolid condition. Mater. Trans. 2016, 57, 404–409. [Google Scholar] [CrossRef]
- Kim, S.W.; Im, U.H.; Cha, H.C.; Kim, S.H.; Jang, J.E.; Kim, K.Y. Removal of primary iron rich phase from aluminum-silicon melt by centrifugal separation. China Foundry 2013, 10, 112–117. [Google Scholar] [CrossRef]
- Shu, D.; Li, T.X.; Sun, B.D.; Zhou, Y.H.; Wang, J.; Xu, Z.M. Numerical calculation of the electromagnetic expulsive force upon nonmetallic inclusions in an aluminum melt: Part II. Cylindrical particles. Metall. Mater. Trans. B 2000, 31, 1535–1540. [Google Scholar] [CrossRef]
- Shu, D.; Li, T.X.; Sun, B.D.; Zhou, Y.H.; Wang, J.; Xu, Z.M. Numerical calculation of the electromagnetic expulsive force upon nonmetallic inclusions in an aluminum melt: Part I. Spherical particles. Mater. Trans. B 2000, 31, 1527–1533. [Google Scholar] [CrossRef]
- Hashimoto, E.; Ueda, Y. Zone refining of high-purity aluminum. Mater. Tran. 1994, 35, 262–265. [Google Scholar] [CrossRef]
- Mehrabian, R.; Geiger, D.R.; Flemings, G.A. Refining by partial solidification. Mater. Trans. 1974, 5, 785–787. [Google Scholar] [CrossRef]
- de Moraes, H.L.; de Oliveira, J.R.; Espinosa, D.C.R.; Tenório, J.A.S. Removal of iron from molten recycled aluminum through intermediate phase flitration. Mater. Trans. 2006, 47, 1731–1736. [Google Scholar] [CrossRef]
- Kennedy, M.W.; Akhtar, S.; Bakken, J.A.; Aune, R.E. Electromagnetically modified filtration of aluminum melts-part I: Electromagnetic theory and 30 PPI ceramic foam filter experimental results. Metall. Mater. Trans. B 2013, 44, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoon, E.P. Elimination of Fe element in A380 aluminum alloy scrap by electromagnetic force. J. Mater. Sci. Lett. 2000, 19, 253–255. [Google Scholar] [CrossRef]
- Xu, Z.M.; Li, T.X.; Zhou, Y.H. Elimination of Fe in Al-Si cast alloy scrap by electromagnetic filtration. J. Mater. Sci. 2003, 38, 4557–4565. [Google Scholar] [CrossRef]
- Lee, G.C.; Kim, M.G.; Park, J.P.; Lim, J.H.; Jung, J.H.; Baek, E.R. Iron Removal in aluminum melts containing scrap by electromagnetic stirring. Mater. Sci. Forum 2010, 638–642, 267–272. [Google Scholar] [CrossRef]
- He, Y.J.; Li, Q.L.; Liu, W. Separating effect of a novel combined magnetic field on inclusions in molten aluminum alloy. Metall. Mater. Trans. B 2012, 43, 1149–1155. [Google Scholar] [CrossRef]
- He, Y.J.; Li, Q.L.; Liu, W. Effect of combined magnetic field on the eliminating inclusions from liquid aluminum alloy. Mater. Lett. 2011, 65, 1226–1228. [Google Scholar] [CrossRef]
- Zhao, L.X.; Guo, Z.C.; Wang, Z.; Wang, M.Y. Removal of low-content impurities from Al by super-gravity. Metall. Mater. Trans. B 2010, 41, 505–508. [Google Scholar] [CrossRef]
- Cho, T.T.; Sugiyama, S.; Yanagimoto, J. Effect of process parameters of backward extrusion by servo press on purification of A7075 alloy under the semisolid condition. Mater. Trans. 2016, 57, 1351–1356. [Google Scholar] [CrossRef]
- Sugiyama, S.; Meng, Y.; Yanagimoto, J. Refining and recycling of metal scraps by semisolid processing. Solid State Phenom. 2012, 192–193, 494–499. [Google Scholar] [CrossRef]
- Song, G.Y.; Song, B.; Yang, Z.B.; Yang, Y.H.; Zhang, J. Removal of inclusions from molten aluminum by supergravity filtration. Metall. Mater. Trans. B 2016, 47, 3435–3445. [Google Scholar] [CrossRef]
- Song, G.Y.; Song, B.; Yang, Z.B.; Yang, Y.H.; Xin, W.B. Separating behavior of nonmetallic inclusions in molten aluminum under super-gravity field. Metall. Mater. Trans. B 2015, 46, 2190–2197. [Google Scholar] [CrossRef]
- Li, C.; Gao, J.T.; Guo, Z.C. Isothermal enrichment of P-concentrating phase from CaO–SiO2–FeO–MgO–P2O5 melt with super gravity. ISIJ Int. 2016, 56, 759–764. [Google Scholar] [CrossRef]
- Li, C.; Gao, J.T.; Wang, F.Q.; Guo, Z.C. Enriching Fe-bearing and P-bearing phases from steelmaking slag melt by super gravity. Ironmak. Steelmak. 2016, 45, 1–6. [Google Scholar] [CrossRef]
- Li, J.C.; Guo, Z.C.; Gao, J.T. Laboratory assessment of isothermal separation of V containing spinel phase from vanadium slag by centrifugal casting. Ironmak. Steelmak. 2014, 41, 710–714. [Google Scholar] [CrossRef]
- Yang, H.J.; Chu, G.W.; Zhang, J.W.; Shen, Z.G.; Chen, J.F. Micromixing efficiency in a rotating packed bed: experiments and simulation. Ind. Eng. Chem. Res. 2005, 44, 7730–7737. [Google Scholar] [CrossRef]
- Ahmad, A.H.; Naher, S.; Brabazon, D. Thermal profiles and fraction solid of aluminium 7075 at different cooling rate conditions. Key Eng. Mater. 2013, 554–557, 582–595. [Google Scholar] [CrossRef]
- Li, J.C.; Guo, Z.C.; Gao, J.T. Isothermal enriching perovskite phase from CaO-TiO2-SiO2-Al2O3-MgO melt by super gravity. ISIJ Int. 2014, 54, 743–749. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. The solidification characteristics of Fe-rich intermetallics in Al-11.5Si-0.4Mg cast alloys. Metall. Mater. Trans. A 2004, 35, 1425–1435. [Google Scholar] [CrossRef]


| Element | Si | Fe | Cu | Mg | Mn | Cr | Zn | Al |
|---|---|---|---|---|---|---|---|---|
| Content/wt % | <0.1 | 0.15 | 1.7 | 2.9 | 0.9 | 0.18 | 6.2 | Bal. |
| Trials | Temperature (°C) | Gravity Coefficient | Holding Time (min) | Separation Time (min) |
|---|---|---|---|---|
| 1 | 609 | 400 | 20 | 5 |
| 2 | 609 | 700 | 20 | 5 |
| 3 | 609 | 1000 | 20 | 5 |
| 4 | 617 | 400 | 20 | 5 |
| 5 | 617 | 700 | 20 | 5 |
| 6 | 617 | 1000 | 20 | 5 |
| 7 | 625 | 400 | 20 | 5 |
| 8 | 625 | 700 | 20 | 5 |
| 9 | 625 | 1000 | 20 | 5 |
| 10 | 625 | 700 | 20 | 1 |
| 11 | 617 | 1 | 25 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Wen, X.; Bao, Q.; Guo, Z. Removal of Tramp Elements within 7075 Alloy by Super-Gravity Aided Rheorefining Method. Metals 2018, 8, 701. https://doi.org/10.3390/met8090701
Guo L, Wen X, Bao Q, Guo Z. Removal of Tramp Elements within 7075 Alloy by Super-Gravity Aided Rheorefining Method. Metals. 2018; 8(9):701. https://doi.org/10.3390/met8090701
Chicago/Turabian StyleGuo, Lei, Xiaochun Wen, Qipeng Bao, and Zhancheng Guo. 2018. "Removal of Tramp Elements within 7075 Alloy by Super-Gravity Aided Rheorefining Method" Metals 8, no. 9: 701. https://doi.org/10.3390/met8090701