Time Evolution Characterization of Atmospheric-Pressure Plasma Jet (APPJ)-Synthesized Pt-SnOx Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pt-SnOx CEs
2.2. Preparation of TiO2 Photoanode and Assembly of DSSCs
2.3. Characterization of Materials and DSSCs
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Laroussi, M.; Akan, T. Arc-free atmospheric pressure cold plasma jets: A review. Plasma Proc. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Wang, C.; Hsu, C.-C.; Cheng, I.-C. Ultrafast synthesis of carbon-nanotube counter electrodes for dye-sensitized solar cells using an atmospheric-pressure plasma jet. Carbon 2016, 98, 34–40. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Chang, H.; Liu, H.-W.; Yang, Y.-J.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-pressure-plasma-jet processed nanoporous TiO2 photoanodes and Pt counter-electrodes for dye-sensitized solar cells. RSC Adv. 2015, 5, 45662–45667. [Google Scholar] [CrossRef]
- Wan, T.-H.; Chiu, Y.-F.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.; Chen, J.-Z. Atmospheric-pressure plasma jet processed Pt-decorated reduced graphene oxides for counter-electrodes of dye-sensitized solar cells. Coatings 2016, 6, 44. [Google Scholar] [CrossRef]
- Wu, T.J.; Chou, C.Y.; Hsu, C.M.; Hsu, C.C.; Chen, J.Z.; Cheng, I.C. Ultrafast synthesis of continuous Au thin films from chloroauric acid solution using an atmospheric pressure plasma jet. RSC Adv. 2015, 5, 99654–99657. [Google Scholar] [CrossRef]
- Liu, L.; Ye, D.; Yu, Y.; Liu, L.; Wu, Y. Carbon-based flexible micro-supercapacitor fabrication via mask-free ambient micro-plasma-jet etching. Carbon 2017, 111, 121–127. [Google Scholar] [CrossRef]
- Kuok, F.-H.; Kan, K.-Y.; Yu, I.-S.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C. Application of atmospheric-pressure plasma jet processed carbon nanotubes to liquid and quasi-solid-state electrolyte supercapacitors. Appl. Surf. Sci. 2017, 425, 321–328. [Google Scholar] [CrossRef]
- Yang, C.-H.; Kuok, F.-H.; Liao, C.-Y.; Wan, T.-H.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Flexible reduced graphene oxide supercapacitor fabricated using a nitrogen dc-pulse atmospheric-pressure plasma jet. Mater. Res. Express 2017, 4, 025504. [Google Scholar] [CrossRef]
- Liu, H.W.; Liang, S.P.; Wu, T.J.; Chang, H.; Kao, P.K.; Hsu, C.C.; Chen, J.Z.; Chou, P.T.; Cheng, I.C. Rapid atmospheric pressure plasma jet processed reduced graphene oxide counter electrodes for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 15105–15112. [Google Scholar] [CrossRef] [PubMed]
- Kuok, F.H.; Liao, C.Y.; Wan, T.H.; Yeh, P.W.; Cheng, I.C.; Chen, J.Z. Atmospheric pressure plasma jet processed reduced graphene oxides for supercapacitor application. J. Alloys Compd. 2017, 692, 558–562. [Google Scholar] [CrossRef]
- Chen, S.L.; Wang, S.; Wang, Y.B.; Guo, B.H.; Li, G.Q.; Chang, Z.S.; Zhang, G.J. Surface modification of epoxy resin using He/CF4 atmospheric pressure plasma jet for flashover withstanding characteristics improvement in vacuum. Appl. Surf. Sci. 2017, 414, 107–113. [Google Scholar] [CrossRef]
- Munoz, J.; Bravo, J.A.; Calzada, M.D. Aluminum metal surface cleaning and activation by atmospheric-pressure remote plasma. Appl. Surf. Sci. 2017, 407, 72–81. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Cheng, I.-C.; Hsu, C.-C.; Chen, J.-Z. Dc-pulse atmospheric-pressure plasma jet and dielectric barrier discharge surface treatments on fluorine-doped tin oxide for perovskite solar cell application. J. Phys. D: Appl. Phys. 2017, 51, 025502. [Google Scholar] [CrossRef]
- Bose, A.C.; Shimizu, Y.; Mariotti, D.; Sasaki, T.; Terashima, K.; Koshizaki, N. Flow rate effect on the structure and morphology of molybdenum oxide nanoparticles deposited by atmospheric-pressure microplasma processing. Nanotechnology 2006, 17, 5976–5982. [Google Scholar] [CrossRef]
- Babayan, S.; Jeong, J.; Schütze, A.; Tu, V.; Moravej, M.; Selwyn, G.; Hicks, R. Deposition of silicon dioxide films with a non-equilibrium atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2001, 10, 573. [Google Scholar] [CrossRef]
- Patel, J.; Nemcova, L.; Maguire, P.; Graham, W.G.; Mariotti, D. Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-induced liquid chemistry. Nanotechnology 2013, 24, 245604. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Wan, T.-H.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-pressure plasma jet processed Pt/ZnO composites and its application as counter-electrodes for dye-sensitized solar cells. Appl. Surf. Sci. 2018, 436, 690–696. [Google Scholar] [CrossRef]
- Malecki, S.; Gargul, K. Low-waste recycling of spent CuO-ZnO-Al2O3 catalysts. Metals 2018, 8, 177. [Google Scholar] [CrossRef]
- Paiva, A.P. Recycling of palladium from spent catalysts using solvent extraction-some critical points. Metals 2017, 7, 505. [Google Scholar] [CrossRef]
- Fujita, T.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Abe, H. In-situ TEM study of a nanoporous Ni-Co catalyst used for the dry reforming of methane. Metals 2017, 7, 406. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.H.; Wang, J.P.; Park, J.T.; Shin, S.M. Application of Co and Mn for a Co-Mn-Br or Co-Mn-C2H3O2 petroleum liquid catalyst from the cathode material of spent lithium ion batteries by a hydrometallurgical route. Metals 2017, 7, 439. [Google Scholar] [CrossRef]
- Tai, M.C.; Gentle, A.; de Silva, K.S.B.; Arnold, M.D.; van der Lingen, E.; Cortie, M.B. Thermal stability of nanoporous raney gold catalyst. Metals 2015, 5, 1197–1211. [Google Scholar] [CrossRef]
- Sabitu, S.T.; Goudy, A.J. Dehydrogenation kinetics and modeling studies of MgH2 enhanced by transition metal oxide catalysts using constant pressure thermodynamic driving forces. Metals 2012, 2, 219–228. [Google Scholar] [CrossRef]
- Song, H.; Hu, F.Y.; Peng, Y.; Li, K.Z.; Bai, S.P.; Li, J.H. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar] [CrossRef]
- O’regan, B.; Grfitzeli, M. A low-cost, high-efficiency solar cell based on dye-sensitized. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, J.-Y.; Wan, C.-C.; Wei, T.-C. High-performance and low platinum loading electrodeposited-Pt counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2011, 56, 1941–1946. [Google Scholar] [CrossRef]
- Bae, K.-H.; Park, E.; Dao, V.-D.; Choi, H.-S. PtZn nanoalloy counter electrodes as a new avenue for highly efficient dye-sensitized solar cells. J. Alloys Compd. 2017, 702, 449–457. [Google Scholar] [CrossRef]
- Dao, V.D.; Nang, L.V.; Kim, E.T.; Lee, J.K.; Choi, H.S. Pt nanoparticles immobilized on CVD-grown graphene as a transparent counter electrode material for dye-sensitized solar cells. ChemSusChem 2013, 6, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Chang, H.-Y.; Li, Y.-Y.; Huang, Y.-J.; Tsai, Y.-L.; Vittal, R.; Sheng, Y.-J.; Ho, K.-C. Electrocatalytic zinc composites as the efficient counter electrodes of dye-sensitized solar cells: Study on the electrochemical performances and density functional theory calculations. ACS Appl. Mater. Interfaces 2015, 7, 28254–28263. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Fang, G.; Yin, H.; Liu, X.; Liu, D.; Zhao, M.; Ke, W.; Tao, H.; Tang, Z. Pt–Ni alloy nanoparticles as superior counter electrodes for dye-sensitized solar cells: Experimental and theoretical understanding. Adv. Mater. 2014, 26, 8101–8106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, W.; Hu, Y.H. Efficient ZnO-based counter electrodes for dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 6622–6628. [Google Scholar] [CrossRef]
- Yang, Q.; Duan, J.; Yang, P.; Tang, Q. Counter electrodes from platinum alloy nanotube arrays with zno nanorod templates for dye-sensitized solar cells. Electrochim. Acta 2016, 190, 648–654. [Google Scholar] [CrossRef]
- Tang, Q.; Duan, J.; Duan, Y.; He, B.; Yu, L. Recent advances in alloy counter electrodes for dye-sensitized solar cells. A critical review. Electrochim. Acta 2015, 178, 886–899. [Google Scholar] [CrossRef]
- Nechiyil, D.; Vinayan, B.P.; Ramaprabhu, S. Tri-iodide reduction activity of ultra-small size ptfe nanoparticles supported nitrogen-doped graphene as counter electrode for dye-sensitized solar cell. J. Colloid Interface Sci. 2017, 488, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Jin, I.-K.; Dao, V.-D.; Larina, L.L.; Choi, H.-S. Optimum engineering of a PtSn alloys/reduced graphene oxide nanohybrid for a highly efficient counter electrode in dye-sensitized solar cells. J. Ind. Eng. Chem. 2016, 36, 238–244. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Gao, C.J.; Han, Q.J.; Wang, Z.; Wang, Y.R.; Zheng, H.K.; Wu, M.X. Large-scale high-efficiency dye-sensitized solar cells based on a Pt/carbon spheres composite catalyst as a flexible counter electrode. J. Catal. 2017, 346, 62–69. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, G.; Sun, S.; Liu, J.; Tang, S.; Li, H.; Zhou, B.; Xin, Q. Structure and chemical composition of supported Pt–Sn electrocatalysts for ethanol oxidation. Electrochim. Acta 2005, 50, 5384–5389. [Google Scholar] [CrossRef] [Green Version]
- Baranova, E.A.; Padilla, M.A.; Halevi, B.; Amir, T.; Artyushkova, K.; Atanassov, P. Electrooxidation of ethanol on PtSn nanoparticles in alkaline solution: Correlation between structure and catalytic properties. Electrochim. Acta 2012, 80, 377–382. [Google Scholar] [CrossRef]
- Pang, H.; Lu, J.; Chen, J.; Huang, C.; Liu, B.; Zhang, X. Preparation of SnO2-CNTs supported Pt catalysts and their electrocatalytic properties for ethanol oxidation. Electrochim. Acta 2009, 54, 2610–2615. [Google Scholar] [CrossRef]
- Ordonez, L.C.; Roquero, P.; Sebastian, P.J.; Ramirez, J. Carbon-supported platinum-molybdenum electro-catlysts for methanol oxidation. Catal. Today 2005, 107, 46–52. [Google Scholar] [CrossRef]
- Jiang, L.H.; Sun, G.Q.; Zhou, Z.H.; Xin, Q. Preparation and characterization of PtSn/C anode electrocatalysts for direct ethanol fuel cell. Catal. Today 2004, 93, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Pang, Y.J.; Gu, L.L.; Yao, Y.; Su, Q.; Ji, W.J.; Au, C.T. Structurally defined SnO2 substrates, nanostructured Au/SnO2 interfaces, and their distinctive behavior in benzene and methanol oxidation. J. Catal. 2017, 349, 183–196. [Google Scholar] [CrossRef]
- Lee, B.; Sakamoto, Y.; Hirabayashi, D.; Suzuki, K.; Hibino, T. Direct oxidation of methane to methanol over proton conductor/metal mixed catalysts. J. Catal. 2010, 271, 195–200. [Google Scholar] [CrossRef]
- Xie, J.H.; Falcone, D.D.; Davis, R.J. Restructuring of supported PtSn bimetallic catalysts during aqueous phase oxidation of 1,6-hexanediol. J. Catal. 2015, 332, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B Chem. 2006, 114, 283–295. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Khelashvili, G.; Behrens, S.; Hinsch, A.; Habicht, W.; Schild, D.; Eichhöfer, A.; Sastrawan, R.; Skupien, K.; Dinjus, E.; Bönnemann, H. Preparation and characterization of low platinum loaded Pt: SnO2 electrocatalytic films for screen printed dye solar cell counter electrode. Thin Solid Films 2007, 515, 4074–4079. [Google Scholar] [CrossRef]
- Sun, H.; Ullah, R.; Chong, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Room-light-induced indoor air purification using an efficient Pt/N-TiO2 photocatalyst. Appl. Catal. B Environ. 2011, 108, 127–133. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Ji, S.; Linkov, V.; Wang, R. PtSn/C catalysts for ethanol oxidation: The effect of stabilizers on the morphology and particle distribution. J. Power Sources 2014, 247, 142–150. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Chen, Y.; Zhou, Y.; Lu, T.; Tang, Y. Platinum–cobalt alloy networks for methanol oxidation electrocatalysis. J. Mater. Chem. 2012, 22, 23659–23667. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Zhu, L.; Fang, J.; Xu, J.; Xu, P.; Li, X.; Liu, C.-C. Pt35Cu65 nanoarchitecture: A highly durable and effective electrocatalyst towards methanol oxidation. Nanotechnology 2015, 26, 135706. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; De Souza, R.F.; Romano, M.A.; D’Villa-Silva, M.; Calegaro, M.L.; Hammer, P.; Neto, A.O.; Santos, M.C. PtSnIr/C anode electrocatalysts: Promoting effect in direct ethanol fuel cells. J. Braz. Chem. Soc. 2012, 23, 1146–1153. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; Somodi, F.; Borbáth, I.; Margitfalvi, J.L.; Peña, M.A.; Fierro, J.L.G.; Rojas, S. Controlled synthesis of Pt-Sn/C fuel cell catalysts with exclusive Sn–Pt interaction: Application in co and ethanol electrooxidation reactions. Appl. Catal. B Environ. 2009, 91, 83–91. [Google Scholar] [CrossRef]
- De la Fuente, J.G.; Rojas, S.; Martínez-Huerta, M.; Terreros, P.; Pena, M.; Fierro, J. Functionalization of carbon support and its influence on the electrocatalytic behaviour of Pt/C in H2 and Co electrooxidation. Carbon 2006, 44, 1919–1929. [Google Scholar] [CrossRef]
- Vilella, I.; De Miguel, S.; Scelza, O. Hydrogenation of citral on Pt and PtSn supported on activated carbon felts (ACF). Latin Am. Appl. Res. 2005, 35, 51–57. [Google Scholar]
- Vilella, I.M.; de Miguel, S.R.; de Lecea, C.S.-M.; Linares-Solano, Á.; Scelza, O.A. Catalytic performance in citral hydrogenation and characterization of PtSn catalysts supported on activated carbon felt and powder. Appl. Catal. A Gen. 2005, 281, 247–258. [Google Scholar] [CrossRef]
- Crabb, E.M.; Marshall, R.; Thompsett, D. Carbon monoxide electro-oxidation properties of carbon-supported PtSn catalysts prepared using surface organometallic chemistry. J. Electrochem. Soc. 2000, 147, 4440–4447. [Google Scholar] [CrossRef]
- Gong, F.; Wang, H.; Wang, Z.-S. Self-assembled monolayer of graphene/Pt as counter electrode for efficient dye-sensitized solar cell. Phys. Chem. Chem. Phys. 2011, 13, 17676–17682. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.; Wu, J.; Xiao, Y.; Lin, J.; Huang, M.; Lan, Z.; Fan, L. Functionalized graphene/poly (3, 4-ethylenedioxythiophene): Polystyrenesulfonate as counter electrode catalyst for dye-sensitized solar cells. Energy 2013, 54, 315–321. [Google Scholar] [CrossRef]
- Yin, X.; Xue, Z.; Liu, B. Electrophoretic deposition of Pt nanoparticles on plastic substrates as counter electrode for flexible dye-sensitized solar cells. J. Power Sources 2011, 196, 2422–2426. [Google Scholar] [CrossRef]
- Sigdel, S.; Dubey, A.; Elbohy, H.; Aboagye, A.; Galipeau, D.; Zhang, L.; Fong, H.; Qiao, Q. Dye-sensitized solar cells based on spray-coated carbon nanofiber/TiO2 nanoparticle composite counter electrodes. J. Mater. Chem. A 2014, 2, 11448–11453. [Google Scholar] [CrossRef]
- Wang, Q.; Moser, J.-E.; Grätzel, M. Electrochemical impedance spectroscopic analysis of dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 14945–14953. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Thummalakunta, L.; Cook, C.; Peh, C.; Wong, A.; Ke, L.; Ho, G. Co-existence of LiI and KI in filler-free, quasi-solid-state electrolyte for efficient and stable dye-sensitized solar cell. J. Power Sources 2011, 196, 1651–1656. [Google Scholar] [CrossRef]
- Jeong, H.; Pak, Y.; Hwang, Y.; Song, H.; Lee, K.H.; Ko, H.C.; Jung, G.Y. Enhancing the charge transfer of the counter electrode in dye-sensitized solar cells using periodically aligned platinum nanocups. Small 2012, 8, 3757–3761. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Kim, S.J.; Gomes, R.; Bhaumik, A. High performance dye-sensitized solar cell by using porous polyaniline nanotubes as counter electrode. Chem. Eng. J. 2015, 260, 393–398. [Google Scholar] [CrossRef]
- Yue, G.; Wu, J.; Xiao, Y.; Huang, M.; Lin, J.; Lin, J.-Y. High performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 1495–1501. [Google Scholar] [CrossRef]
- Zheng, X.J.; Guo, J.H.; Shi, Y.T.; Xiong, F.Q.; Zhang, W.H.; Ma, T.L.; Li, C. Low-cost and high-performance CoMoS4 and NiMoS4 counter electrodes for dye-sensitized solar cells. Chem. Commun. 2013, 49, 9645–9647. [Google Scholar] [CrossRef] [PubMed]


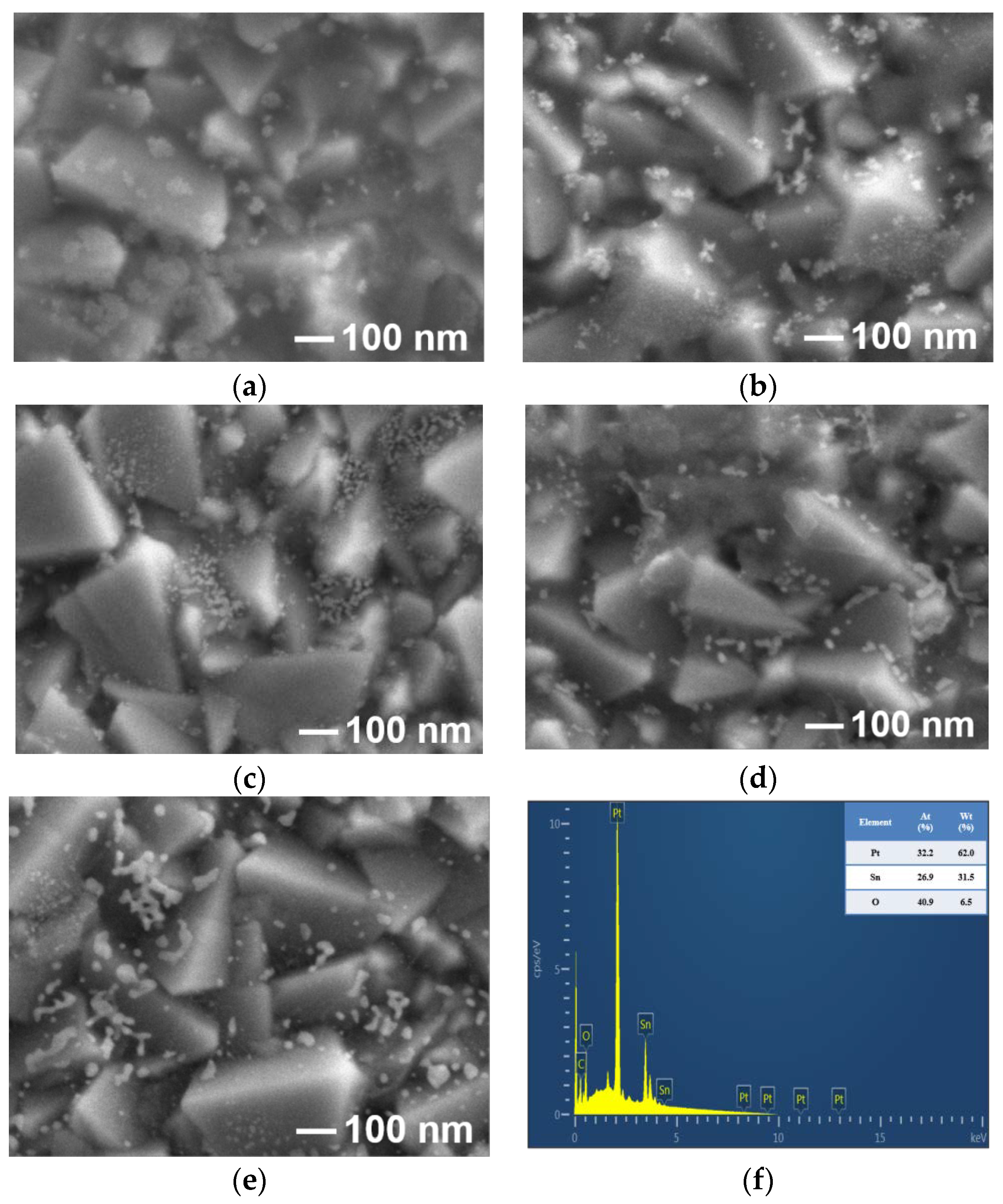
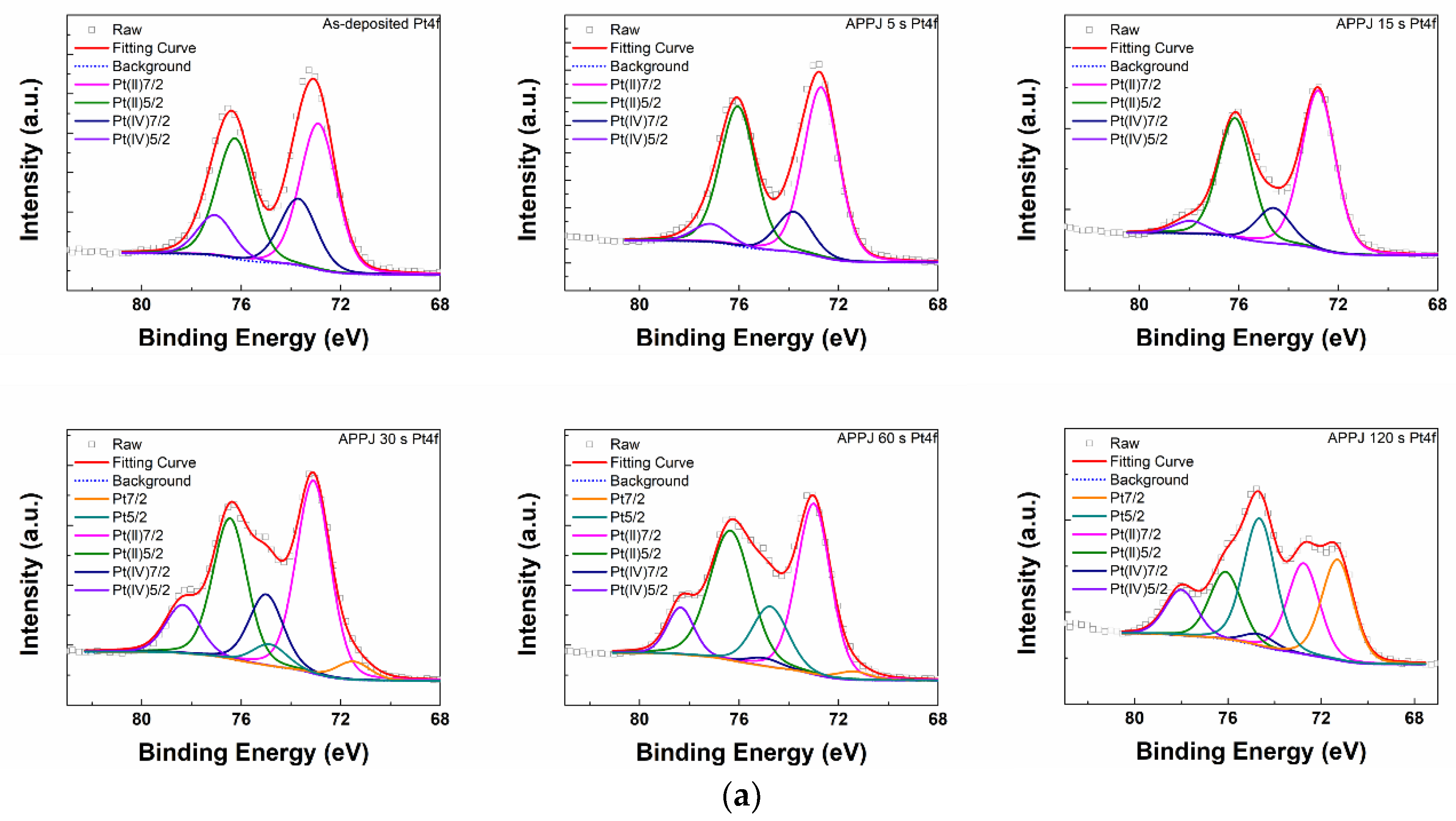

| APPJ Pt4f (%) | Pt 7/2 | Pt 5/2 | Pt(II) 7/2 | Pt(II) 5/2 | Pt(IV) 7/2 | Pt(IV) 5/2 |
|---|---|---|---|---|---|---|
| 0 s | - | - | 39.19 | 32.34 | 17.90 | 10.58 |
| 5 s | - | - | 46.17 | 37.94 | 10.90 | 4.99 |
| 15 s | - | - | 48.87 | 36.29 | 10.87 | 3.97 |
| 30 s | 3.72 | 4.38 | 39.16 | 28.52 | 14.39 | 9.82 |
| 60 s | 1.87 | 14.34 | 38.04 | 35.64 | 1.80 | 8.31 |
| 120 s | 22.66 | 28.72 | 20.46 | 15.21 | 2.69 | 10.26 |
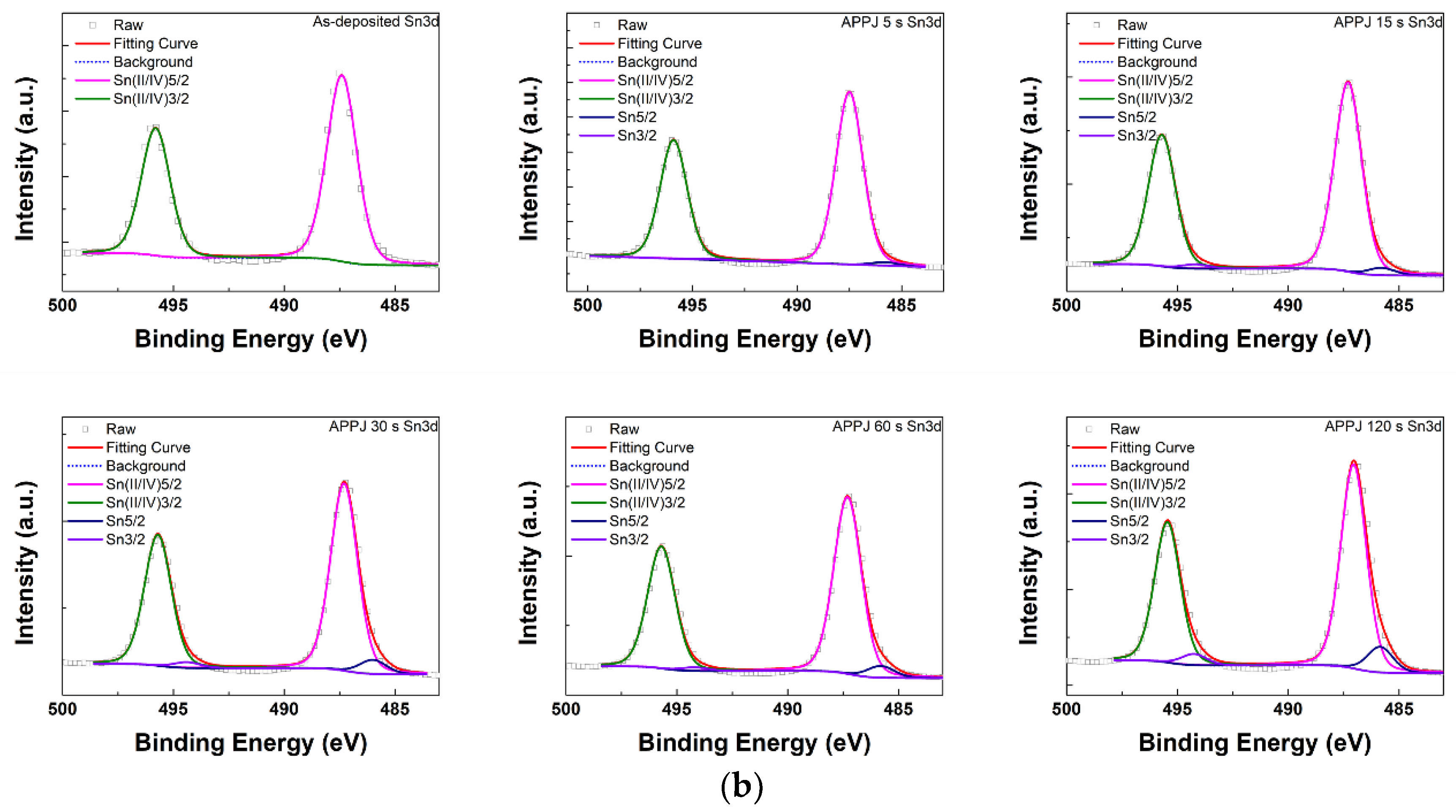
| APPJ Sn3d (%) | Sn 5/2 | Sn 3/2 | Sn(II/IV) 5/2 | Sn(II/IV) 3/2 |
|---|---|---|---|---|
| 0 s | - | - | 60.19 | 39.81 |
| 5 s | 1.13 | 0.36 | 58.33 | 40.19 |
| 15 s | 2.21 | 1.31 | 56.95 | 39.53 |
| 30 s | 4.17 | 1.72 | 55.35 | 38.76 |
| 60 s | 3.72 | 1.14 | 56.27 | 38.87 |
| 120 s | 6.72 | 2.93 | 53.51 | 36.84 |
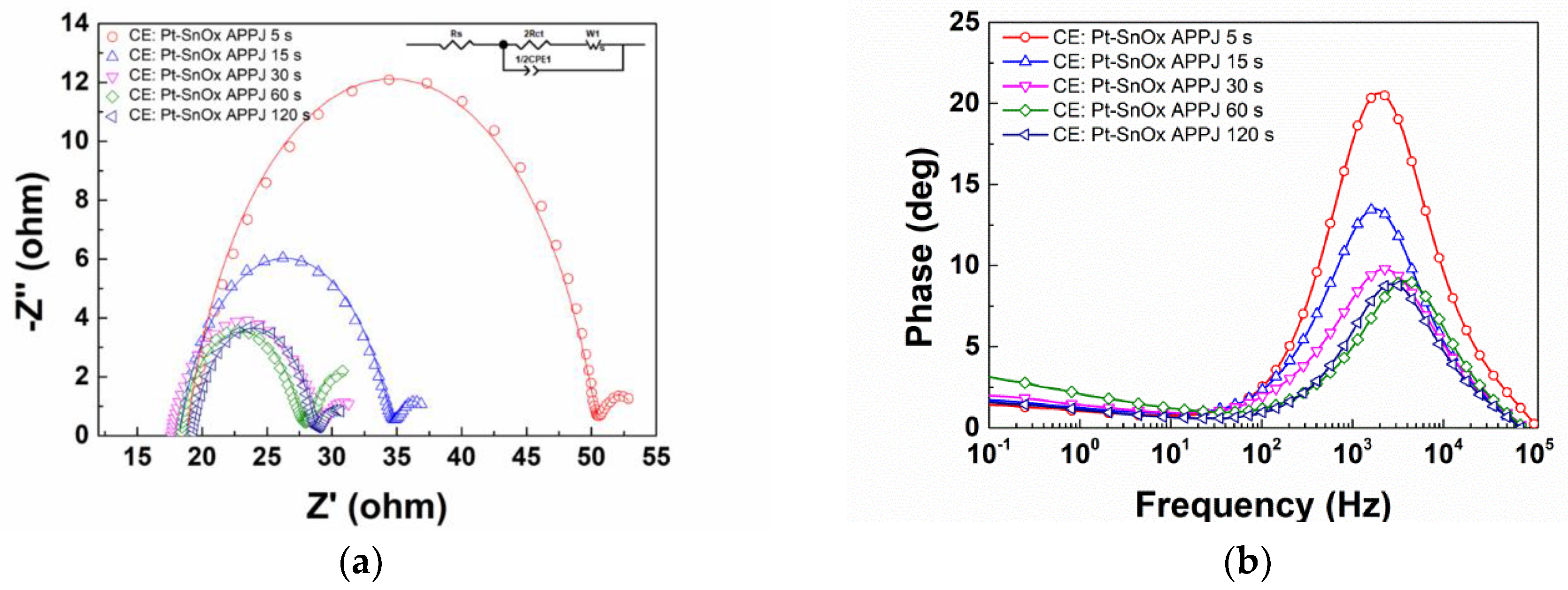
| Counter Electrode | Rs (Ω) | Rct (Ω) | CPE1-T (μF/cm2) | CPE1-P | W1 | J0 a (mA/cm2) | J0 b (mA/cm2) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| W1-R(Ω) | W1-T(s) | W1-P | ||||||||
| Pt-SnOx APPJ | 5 s | 18.8 | 15.74 | 35.6 | 0.835 | 3.23 | 2.13 | 0.5 | 0.82 | 1.04 |
| 15 s | 18.2 | 8.18 | 83.1 | 0.808 | 2.82 | 1.99 | 0.5 | 1.58 | 1.39 | |
| 30 s | 17.55 | 5.8 | 95 | 0.75 | 2.73 | 2.25 | 0.5 | 2.23 | 1.67 | |
| 60 s | 18.25 | 4.72 | 105 | 0.815 | 5.04 | 3.15 | 0.5 | 2.74 | 1.85 | |
| 120 s | 19.28 | 4.69 | 105.5 | 0.84 | 2.21 | 1.8 | 0.5 | 2.75 | 2.08 | |
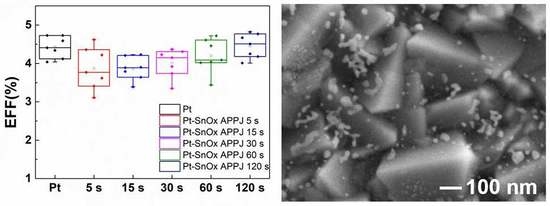
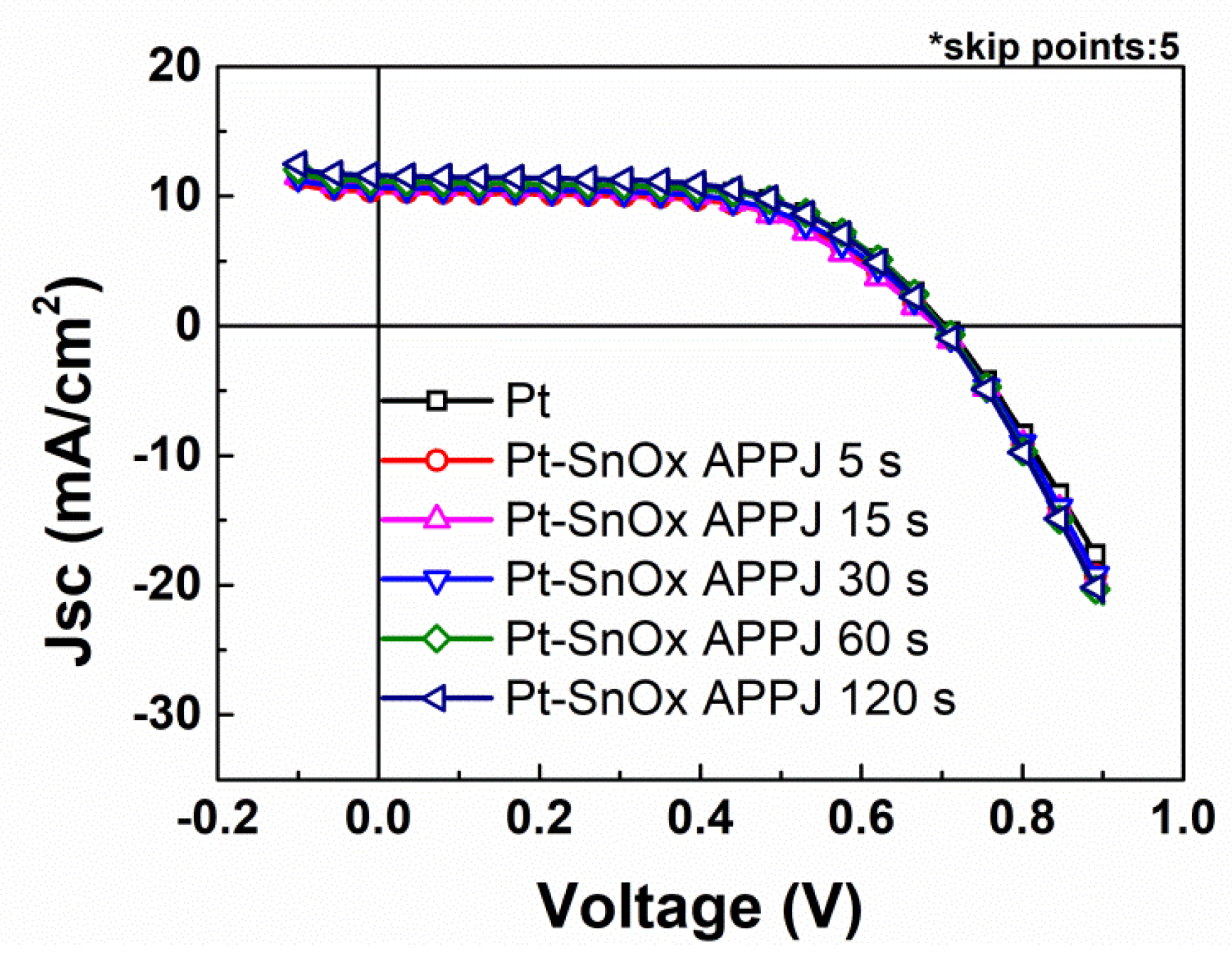
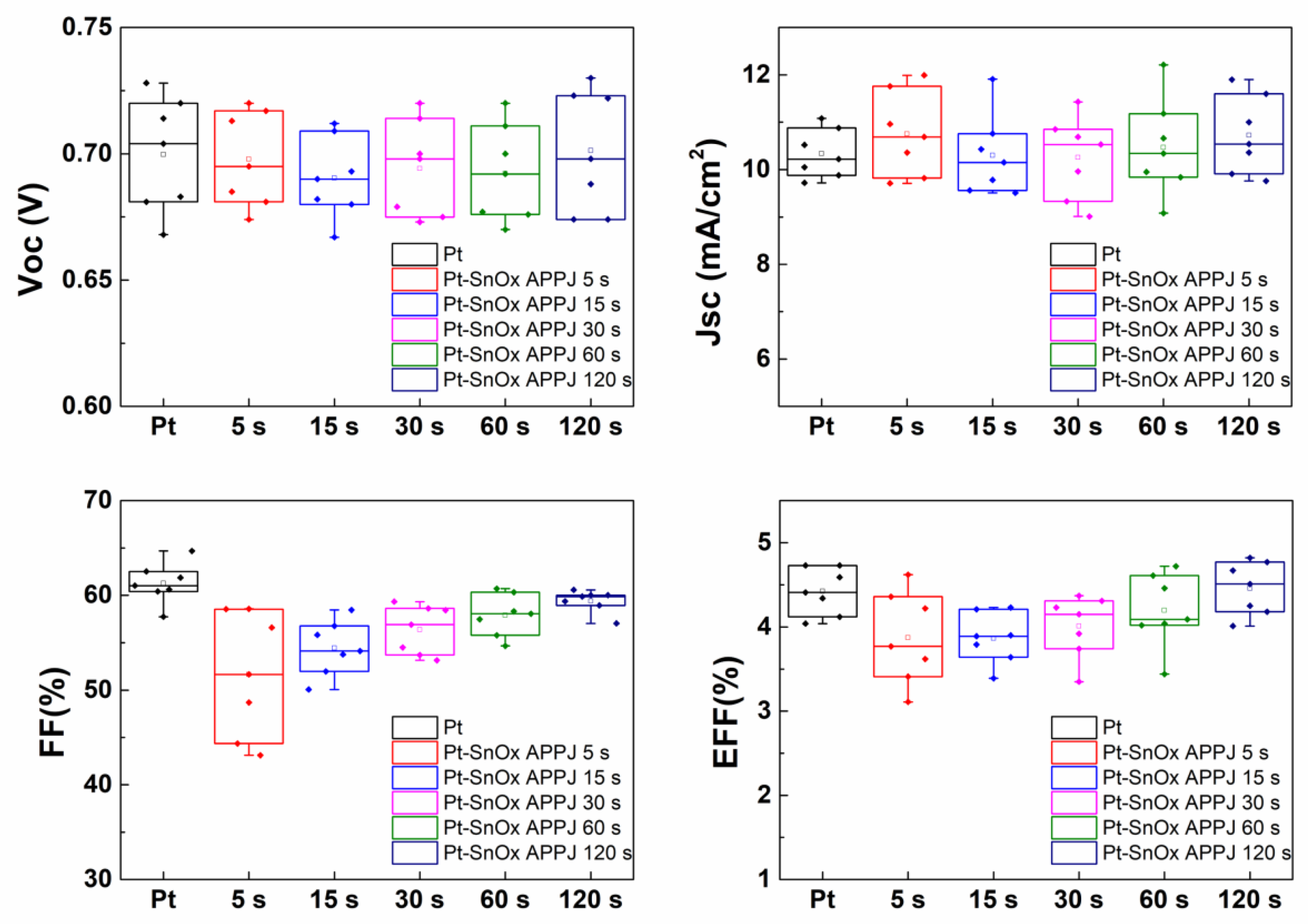
| Condition | Voc (V) | Jsc (mA/cm2) | FF (%) | EFF (%) | |
|---|---|---|---|---|---|
| Pt | 0.70 ± 0.02 | 10.34 ± 0.47 | 61.27 ± 1.91 | 4.42 ± 0.26 | |
| Pt-SnOx APPJ | 5 s | 0.70 ± 0.01 | 10.76 ± 0.82 | 51.65 ± 6.03 | 3.87 ± 0.58 |
| 15 s | 0.69 ± 0.02 | 10.30 ± 0.78 | 54.43 ± 2.65 | 3.86 ± 0.28 | |
| 30 s | 0.69 ± 0.02 | 10.26 ± 0.80 | 56.38 ± 2.37 | 4.01 ± 0.34 | |
| 60 s | 0.69 ± 0.02 | 10.47 ± 0.94 | 57.91 ± 2.04 | 4.20 ± 0.41 | |
| 120 s | 0.70 ± 0.02 | 10.72 ± 0.75 | 59.41 ± 1.08 | 4.46 ± 0.29 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Huang, T.-M.; Cheng, I.-C.; Hsu, C.-C.; Chen, J.-Z. Time Evolution Characterization of Atmospheric-Pressure Plasma Jet (APPJ)-Synthesized Pt-SnOx Catalysts. Metals 2018, 8, 690. https://doi.org/10.3390/met8090690
Lee C-C, Huang T-M, Cheng I-C, Hsu C-C, Chen J-Z. Time Evolution Characterization of Atmospheric-Pressure Plasma Jet (APPJ)-Synthesized Pt-SnOx Catalysts. Metals. 2018; 8(9):690. https://doi.org/10.3390/met8090690
Chicago/Turabian StyleLee, Chia-Chun, Tzu-Ming Huang, I-Chun Cheng, Cheng-Che Hsu, and Jian-Zhang Chen. 2018. "Time Evolution Characterization of Atmospheric-Pressure Plasma Jet (APPJ)-Synthesized Pt-SnOx Catalysts" Metals 8, no. 9: 690. https://doi.org/10.3390/met8090690