Enhanced Formability and Accelerated Precipitation Behavior of 7075 Al Alloy Extruded Rod by High Temperature Aging
Abstract
:1. Introduction
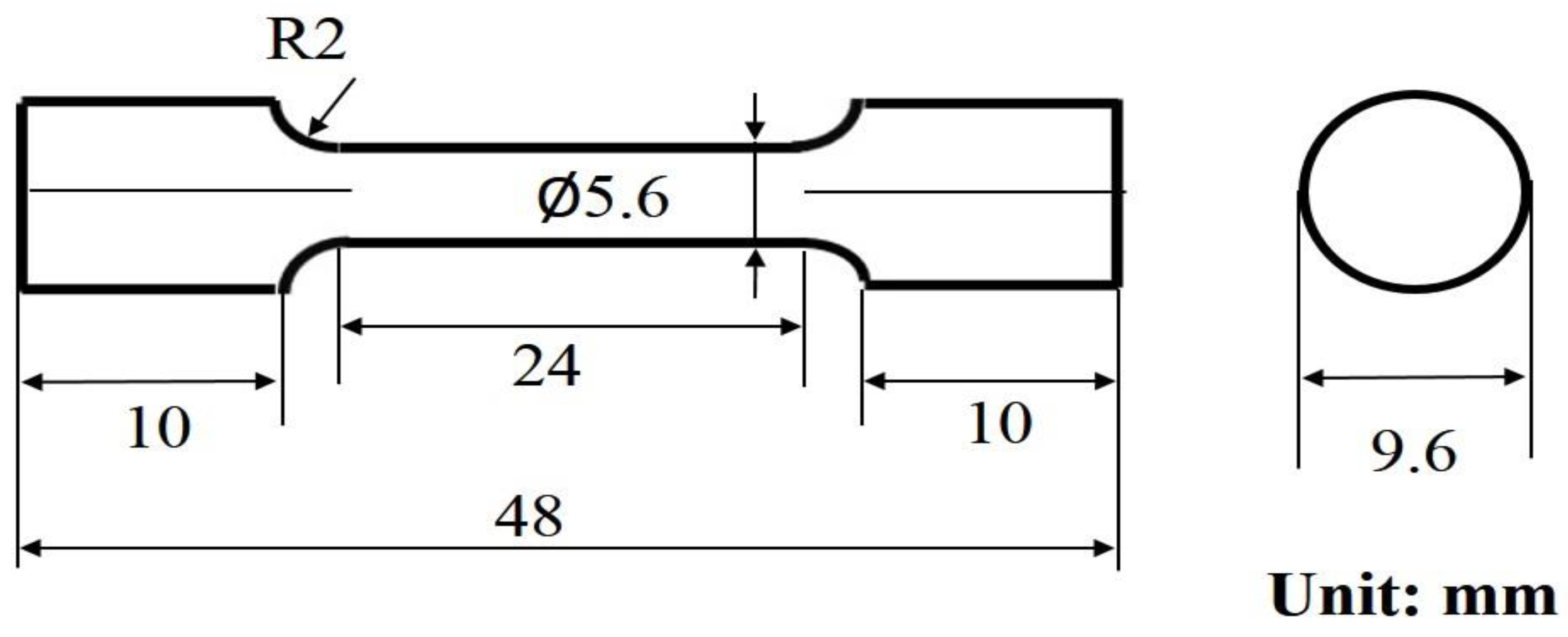
2. Experiment Procedure
3. Results and Discussion
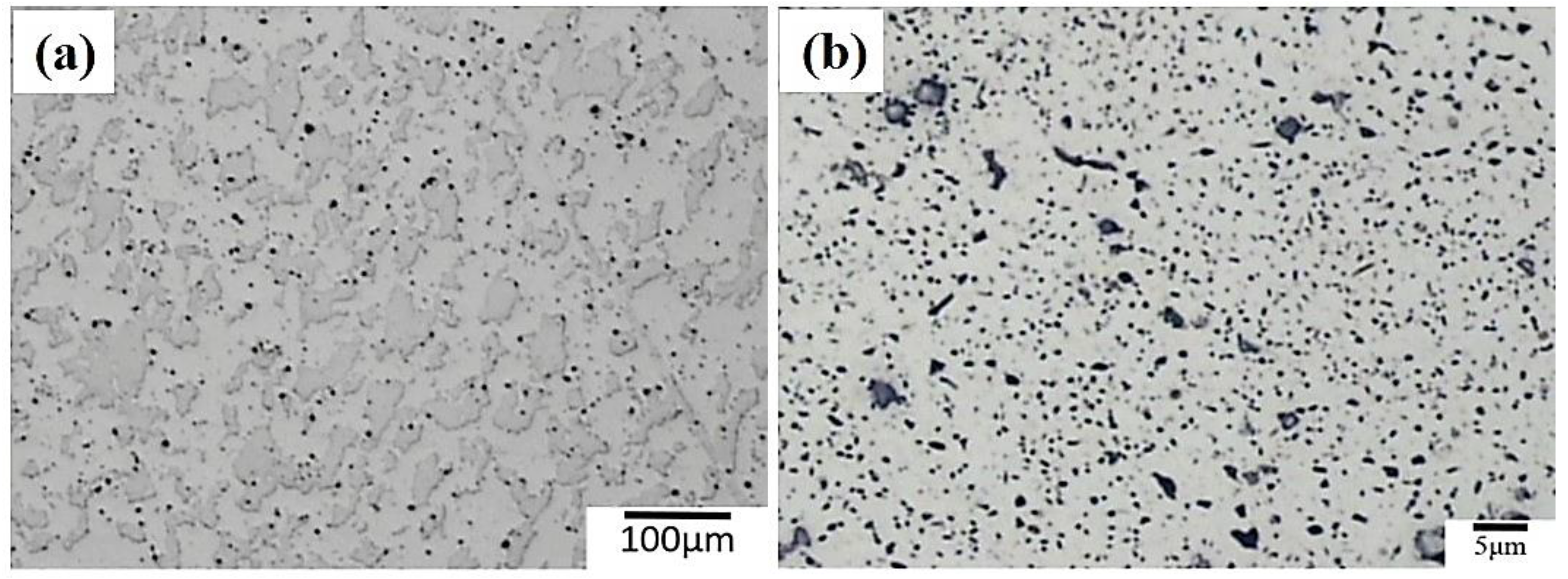
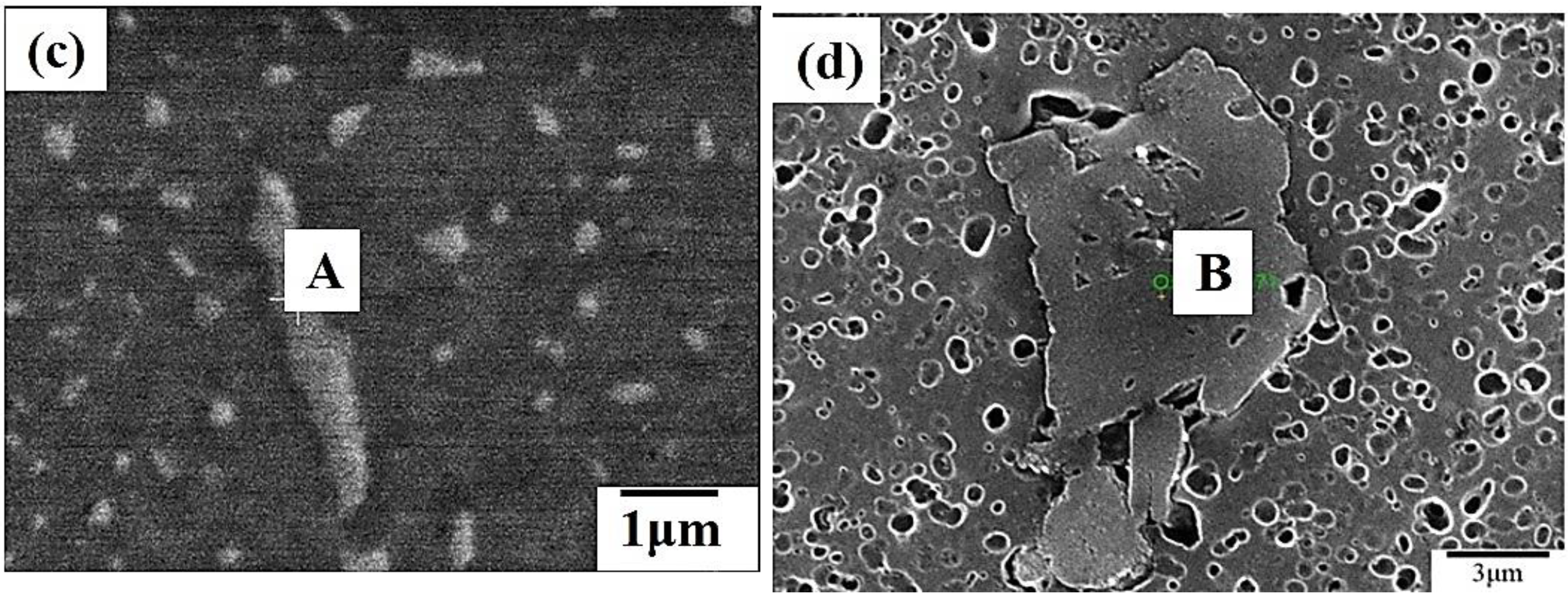
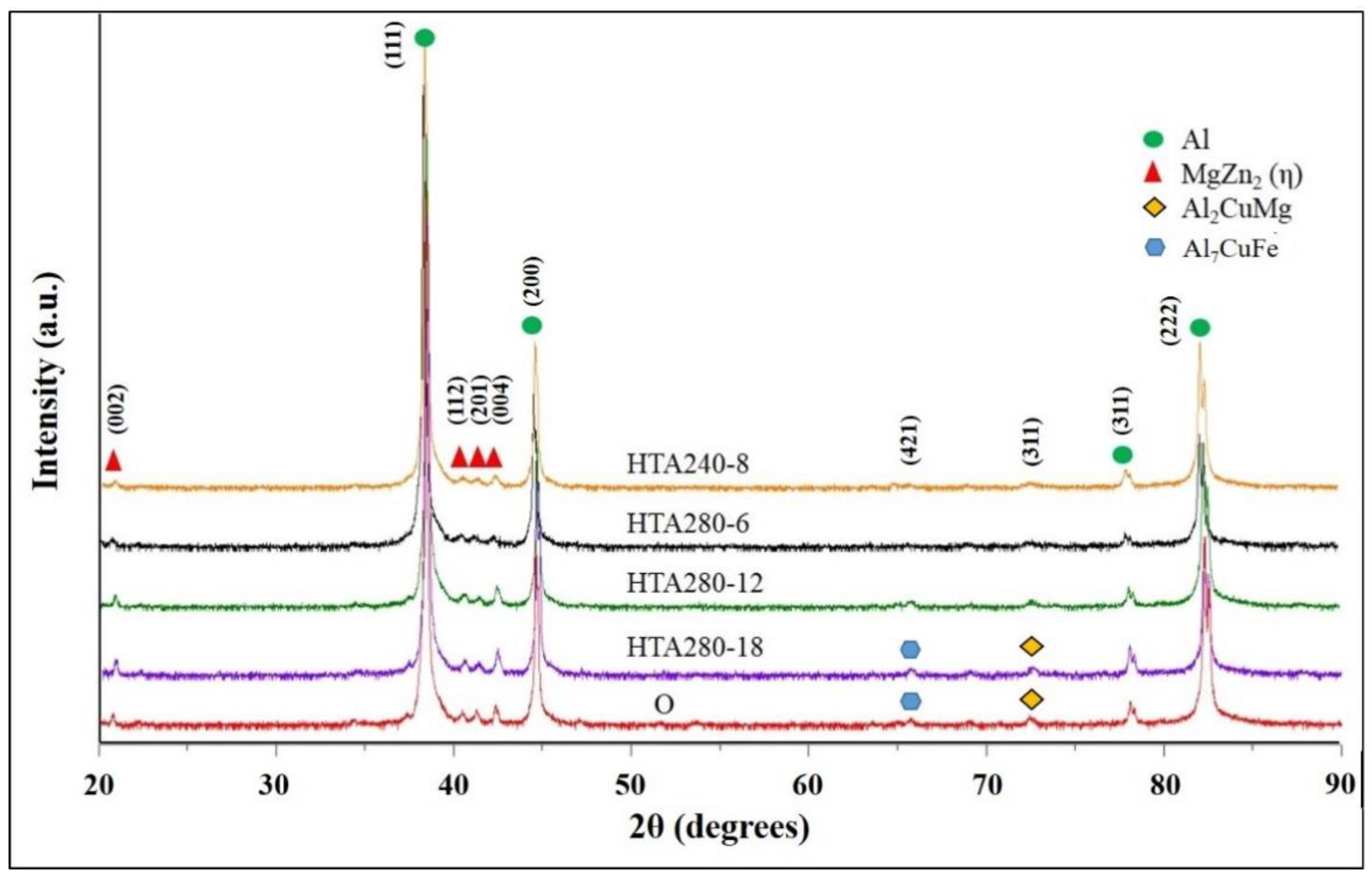
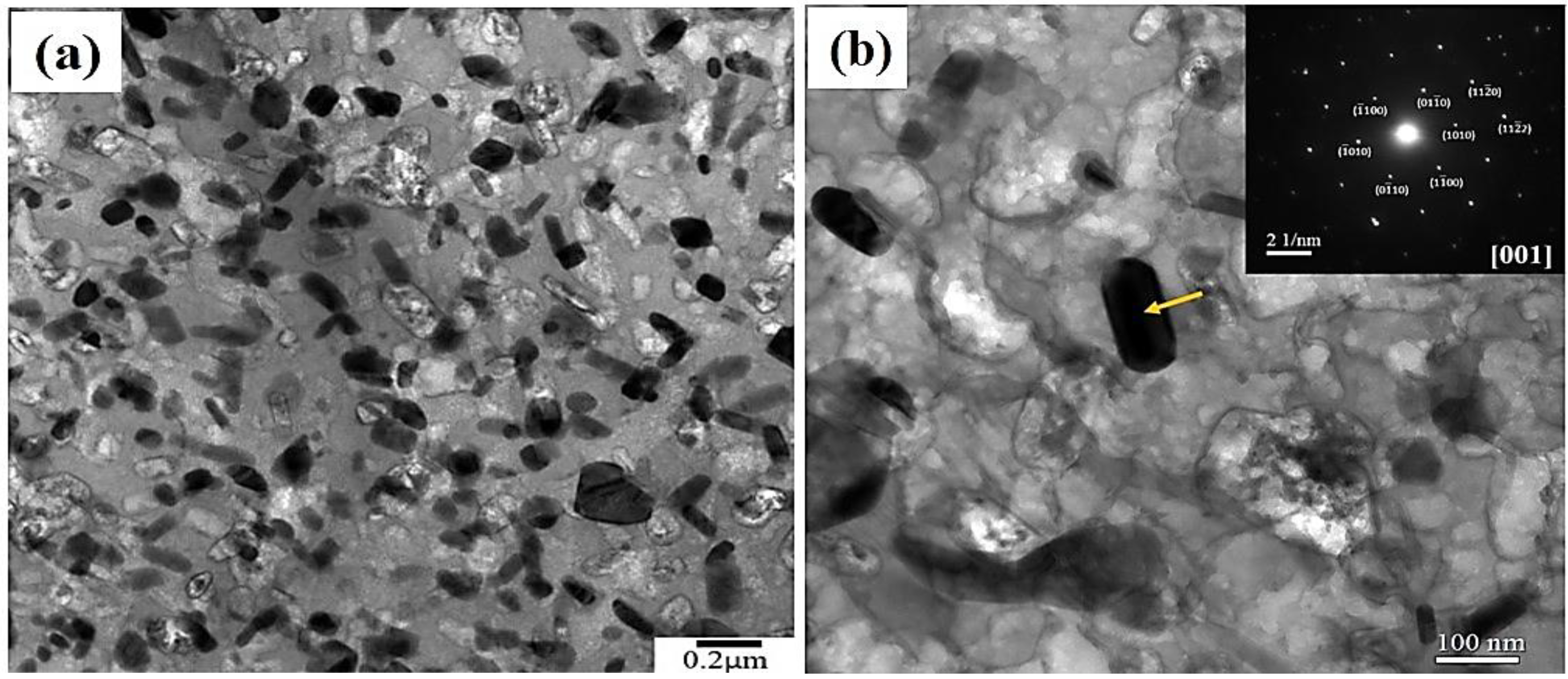
3.1. The Microstructure of 7075 Aluminum Alloy Extruded Rod after Various Post-Process Heat Treatments (PPHTs)
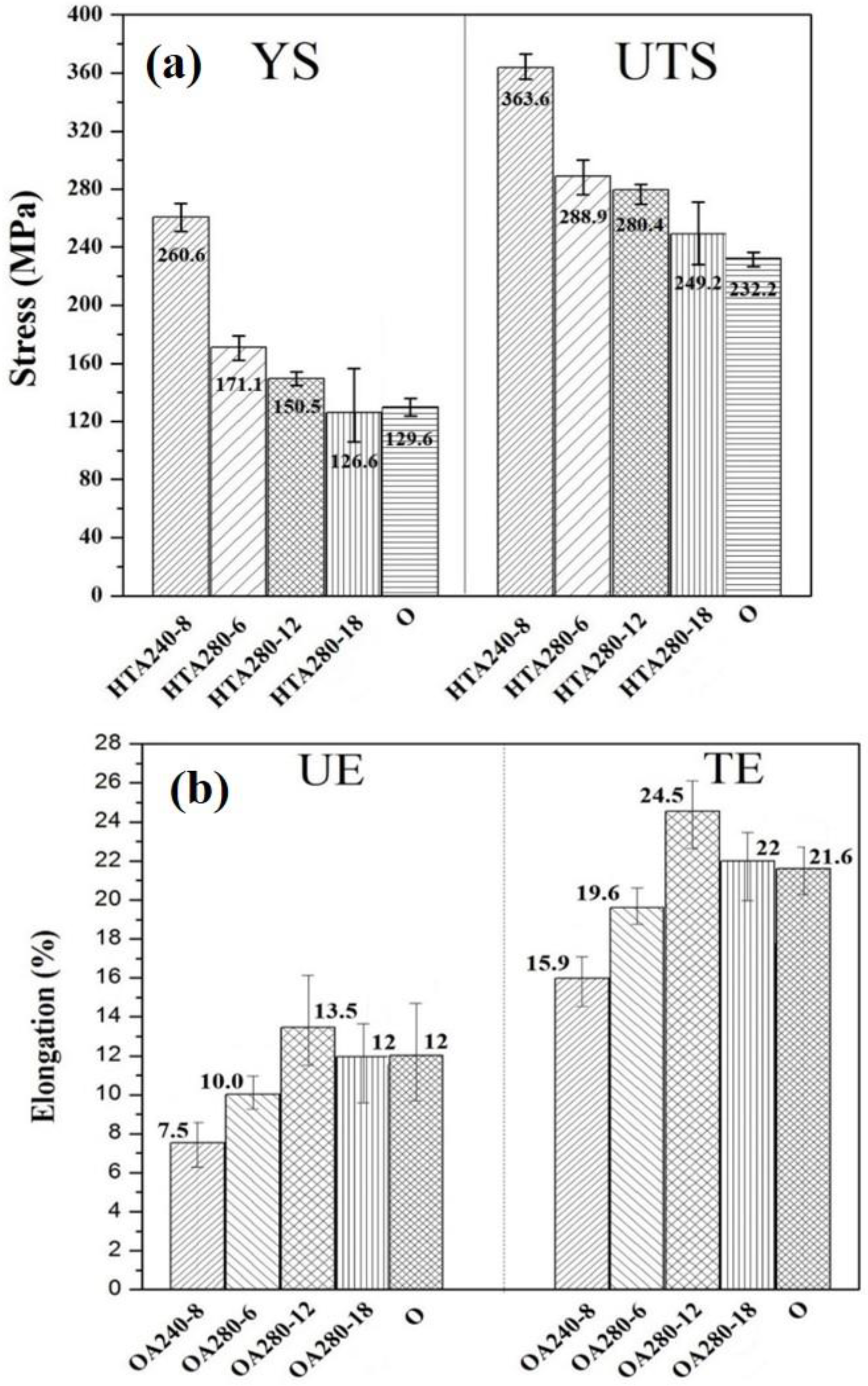
3.2. Tensile Properties of 7075 Aluminum Alloy Extruded Rod after Various PPHTs at Room Temperature
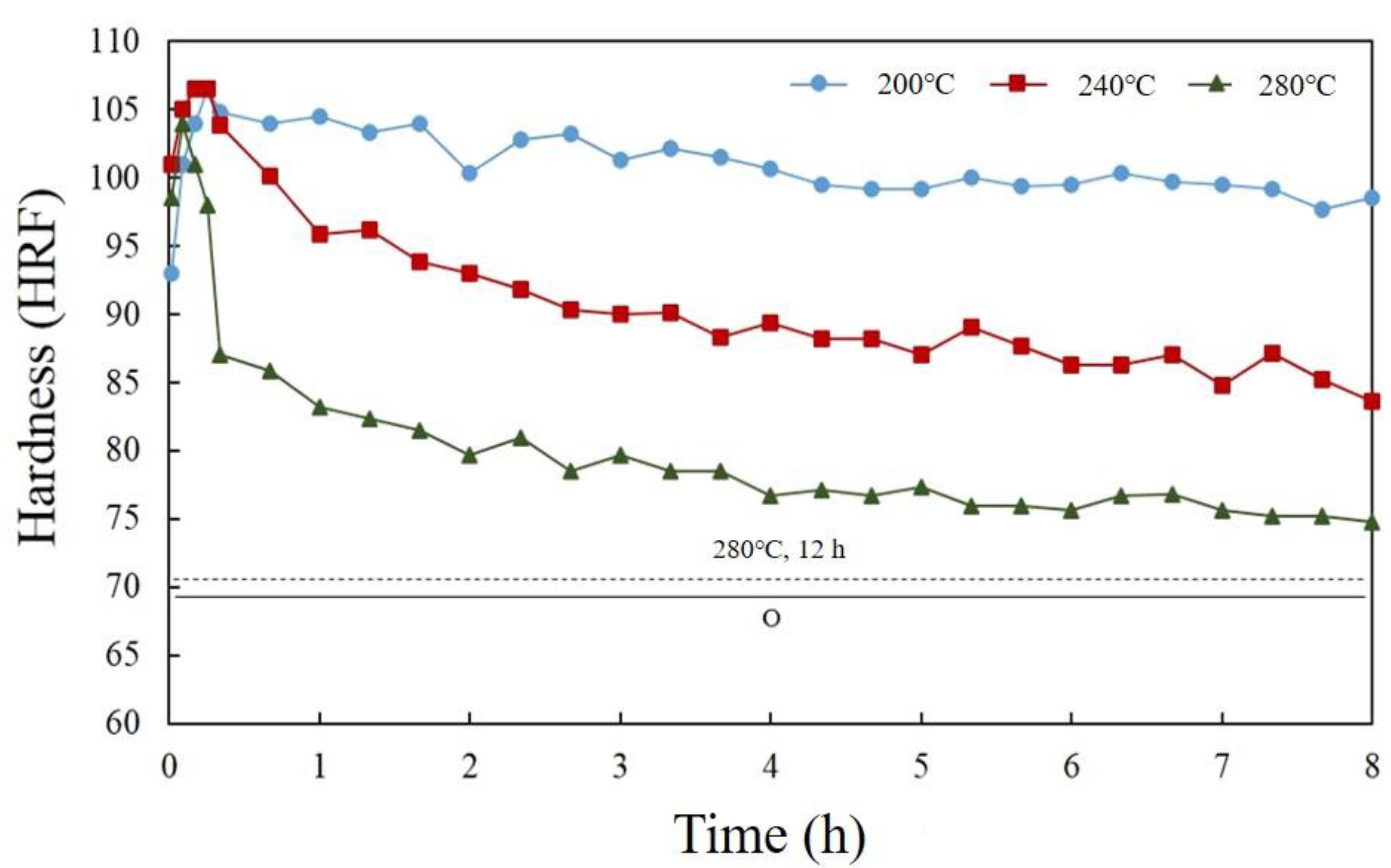
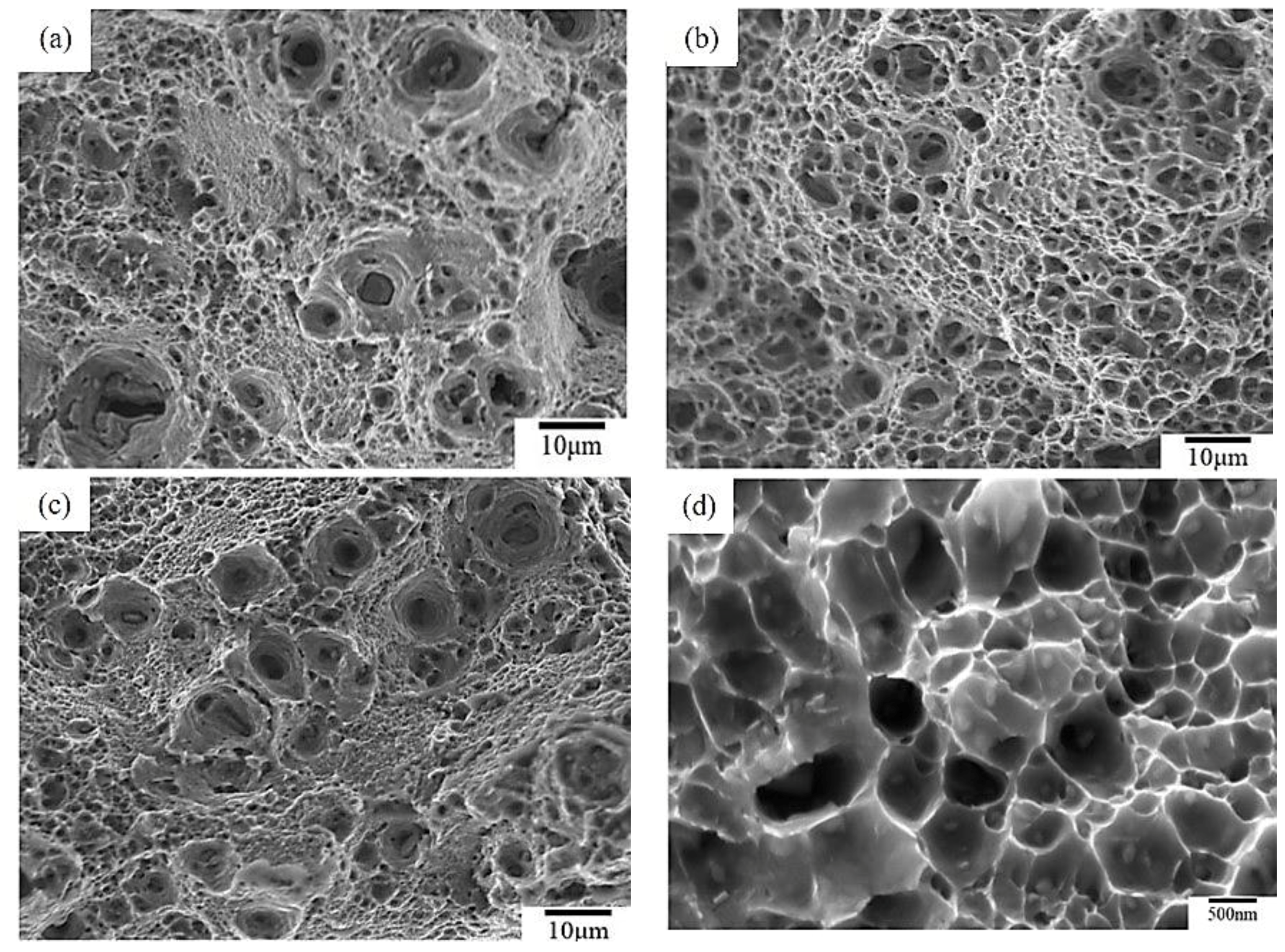
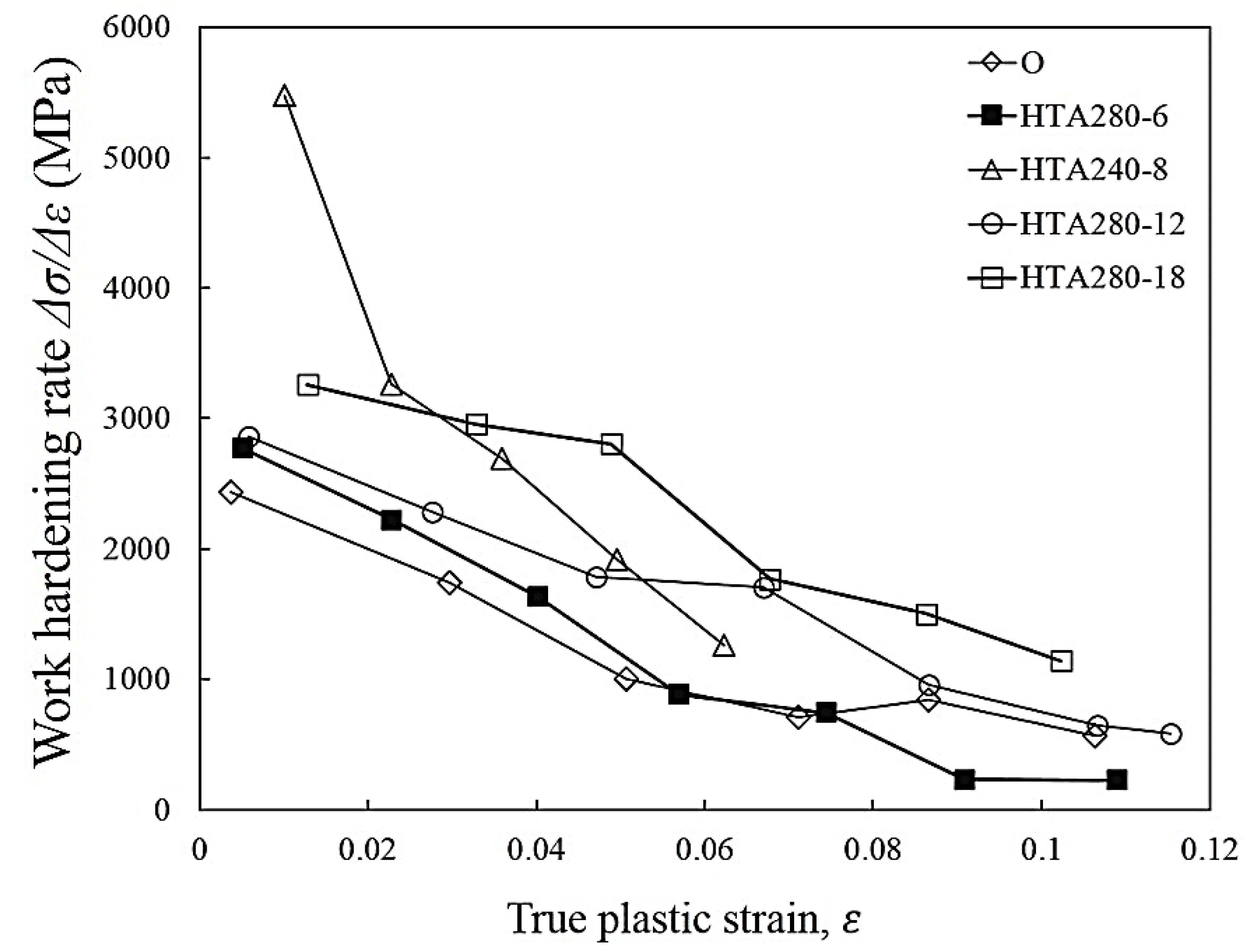
3.3. The Tensile Fracture and the Working Hardness of PPHT Specimens
4. Conclusions
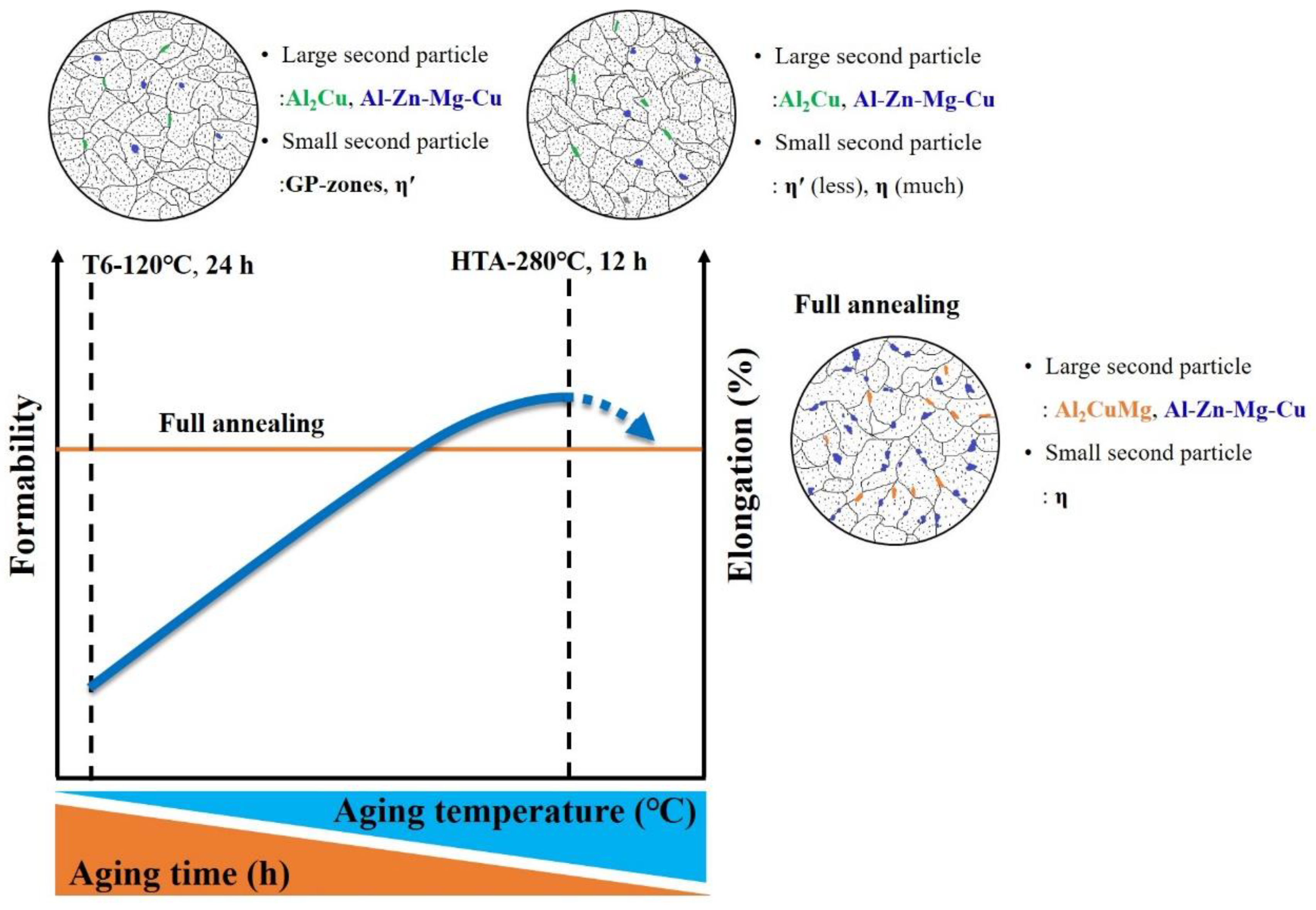
- High temperature aging can decrease precipitate hardening by the formation of an η phase.
- The high temperature aging at 280 °C, 12 h had fewer large particles (Al-Cu phase) in the Al matrix than full annealing did. The nucleation of microvoid and microcrack was located frequently around the large particles. The defects form cracks mainly and cause the fractures of the sample. Hence, the high temperature aging at 280 °C, 12 h presented to be superior over full annealing in regard to the work hardening rate and formability.
- High temperature aging can increase ductility only in a temperature range. At aging temperatures beyond 280 °C, the dissolution of η becomes endothermic, inducing natural aging and lower ductility.
Author Contributions
Funding
Conflicts of Interest
References
- De Sanctis, M. Structure and properties of rapidly solidified ultrahigh strength Al–Zn–Mg–Cu alloys produced by spray deposition. Mater. Sci. Eng. A 1991, 141, 103–121. [Google Scholar] [CrossRef]
- Starke, E.A., Jr.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerospace Sci. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Liu, J. Advanced Aluminum and Hybrid Aerostructures for Future Aircraft. Mater. Sci. Forum. 2006, 519–521, 1233–1238. [Google Scholar] [CrossRef]
- Mondolfo, L.F.; Gjostein, N.A.; Levinson, D.W. Structural Changes during the Aging in an Al-Mg-Zn Alloy. JOM 1956, 10, 1378–1385. [Google Scholar] [CrossRef]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy, 1st ed.; American Society for Metals: Geauga County, OH, USA, 1984; pp. 145–158. ISBN 0-87170-176-6. [Google Scholar]
- Gjønnes, J.; Simensen, C. An Electron Microscope Investigation of the Microstructure in an Aluminium-Zinc-Magnesium Alloy. Acta Metall. 1970, 18, 881–890. [Google Scholar] [CrossRef]
- Degischer, H.P.; Lacom, W.; Zahra, A.M.; Zahra, C.Y. Decomposition Processes in an Al-5% Zn-1% Mg Alloy. II.-Electronmicroscopic Investigations. Z. Metallk. 1980, 71, 231–238. [Google Scholar]
- Friauf, J.B. The Crystal Structure of Magnesium Di-Zincide. Phys. Rev. 1927, 29, 34–40. [Google Scholar] [CrossRef]
- Komura, Y.; Tokunaga, K. Structural Studies of Stacking Variants in Mg-base Friauf-Laves phases. Acta Cryst. 1980, B36, 1548–1554. [Google Scholar] [CrossRef]
- Dorward, R.C.; Hasse, K.R. Flaw Growth of 7075, 7475, 7050 and 7049 Aluminum Plate in Stress Corrosion Environments; Research Final Report: Contact No. NAS8-30890; Kaiser Aluminum & Chemical Corporation: Foothill Ranch, CA, USA, 1976. [Google Scholar]
- Oñoro, J. The stress corrosion cracking behaviour of heat-treated Al-Zn-Mg-Cu alloy in modified salt spray fog testing. Mater. Corros. 2010, 61, 125–129. [Google Scholar]
- Gruhl, W. Stress Corrosion Cracking of High Strength Aluminum Alloys. Z. Metallkd. 1984, 75, 819–826. [Google Scholar]
- Park, J.K. Influence of Retrogression and Reaging Treatments on the Strength and Stress Corrosion Resistance of Aluminium alloy 7075-T6. Mater. Sci. Eng. A 1988, 103, 223–231. [Google Scholar] [CrossRef]
- Sessler, J.; Welss, V. Metallurgy. Materials Data Handbook: Aluminum Alloy 7075, 1st ed.; National Aeronautics and Space Administration: Huntsville, AL, USA, 1967; Chapter 3; pp. 9–23. [Google Scholar]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy, 1st ed.; American Society for Metals: Geauga County, OH, USA, 1984; pp. 152–153. ISBN 0-87170-176-6. [Google Scholar]
- Ertürk, T.; Kazazoglu, E. Effect of aging on bulk formability of aluminum alloys. In Formability of Metallic meaterials—2000, 1st ed.; Newby, J.R., Niemeier, B.A., Eds.; American Society for Testing and Materials: Chicago, IL, USA, 1982; pp. 19–34. [Google Scholar]
- Deschamps, A.; Niewczas, M.; Bley, F.; Brechet, Y.; Embury, J.D.; Le Sinq, L.; Livet, F.; Simon, J.P. Low-temperature dynamic precipitation in a supersaturated Al-Zn-Mg alloy and related strain hardening. Philos. Mag. 1999, 79, 2485–2504. [Google Scholar] [CrossRef]
- Kim, W.J.; Chung, C.S.; Ma, D.S.; Hong, S.I.; Kim, H.K. Optimization of strength and ductility of 2024 Al by equal channel angular pressing (ECAP) and post-ECAP aging. Scr. Mater. 2003, 49, 333–338. [Google Scholar] [CrossRef]
- Rajan, K.; Wallace, W.; Beddoes, J.C. Microstructural study of a high-strength stress-corrosion resistant 7075 aluminium alloy. J. Mater. Sci. 1982, 17, 2817–2824. [Google Scholar] [CrossRef]
- Staley, J.T. Aging kinetics of aluminum alloy 7050. Metall. Mater. Trans. B 1974, 5, 929–932. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, Y.; Nie, Z. Effect of Zn Content on the Microstructure and Properties of Super-High Strength Al-Zn-Mg-Cu alloys. Metall. Mater. Trans. A 2013, 44, 3910–3920. [Google Scholar] [CrossRef]
- Deiasi, R.; Adler, P.N. Calorimetric Studies in 7000 Series Aluminum Alloys: I. Matrix Precipitate Characterization of 7075. Metall. Mater. Trans. A 1977, 8, 1177–1183. [Google Scholar]
- Liang, R.; Khan, A.S. A critical review of experimental results and constitutive models for BCC and FCC metals over a wide range of strain rates and temperatures. Int. J. Plast. 1999, 15, 963–980. [Google Scholar] [CrossRef]
- Embury, J.D.; Nicholson, R.B. The nucleation of precipitates: The system Al-Zn-Mg. Acta Metall. 1965, 13, 403–416. [Google Scholar] [CrossRef]
- Wert, J.A. Identification of precipitates in 7075 Al after high-temperature aging. Scr. Mater. 1981, 15, 445–447. [Google Scholar] [CrossRef]
- Chang, Y.L.; Hung, F.Y.; Lui, T.S. Enhancing the tensile yield strength of A6082 aluminum alloy with rapid heat solutionizing. Mater. Sci. Eng. A 2017, 702, 438–445. [Google Scholar] [CrossRef]
- Fujita, H.; Tabata, T. Discontinuous deformation in Al-Mg alloys under various conditions. Acta Metall. 1977, 25, 793–800. [Google Scholar] [CrossRef]
- Misha, R.S.; Mahoney, M.W.; McFadden, S.X.; Mara, N.A.; Mukherjee, A.K. High strain rate superplasticity in a friction stir processed 7075 Al alloy. Scr. Mater. 2000, 42, 163–168. [Google Scholar] [CrossRef]
- Chen, C.M.; Kovacevic, R. Finite element modeling of friction stir welding-thermal and thermomechanical analysis. Int. J. Mach. Tools Manuf. 2003, 43, 1319–1326. [Google Scholar] [CrossRef]
- Worthington, P.J.; Brindley, B.J. Serrated yielding in substitutional alloys. Philos. Mag. 1969, 19, 1175–1178. [Google Scholar] [CrossRef]
- Rodriguez, P. Serrated plastic flow. Bull. Mat. Sci. 1984, 6, 653–663. [Google Scholar] [CrossRef]
- Li, X.M.; Starink, M.J. Identification and analysis of intermetallic phases in overaged Zr-containing and Cr-containing Al–Zn–Mg–Cu alloys. J. Alloys Compd. 2010, 509, 471–476. [Google Scholar] [CrossRef]
- Pardoeny, T.; Brechet, Y. Influence of microstructure-driven strain localization on the ductile fracture of metallic alloys. Philos. Mag. 2004, 84, 268–297. [Google Scholar] [CrossRef]
- Hecker, S.S. Formability of aluminum alloy sheets. J. Eng. Mater. Technol. 1975, 97, 66–73. [Google Scholar] [CrossRef]





| Zn | Mg | Cu | Cr | Fe | Si | Al |
|---|---|---|---|---|---|---|
| 5.80 | 2.53 | 1.64 | 0.23 | 0.09 | 0.05 | Bal. |
| Symbol | Procedure |
|---|---|
| HTA240-8 | Heating at 490 ℃ for 1 h then quenching, and heating at 240 ℃ for 8 h and air cooling. |
| HTA280-6 | Heating at 490 °C for 1 h then quenching, and heating at 280 ℃ for 6 h and air cooling. |
| HTA280-12 | Heating at 490 °C for 1 h then quenching, and heating at 280 ℃ for 12 h and air cooling. |
| HTA280-18 | Heating at 490 °C for 1 h then quenching, and heating at 280 ℃ for 18 h and air cooling. |
| O | Heating at 415 °C for 2 h then furnace cooling at 20 ℃/h cooling rate to 230 ℃, holding for 6 h and furnace cooling. |
| Al | Zn | Mg | Cu | |
|---|---|---|---|---|
| A | 59.4 | 0.6 | 21.0 | 19.0 |
| B | 81.2 | 0.7 | 1.4 | 16.7 |
| Al | Zn | Mg | Cu | |
|---|---|---|---|---|
| A | 87.7 | 0 | 1.1 | 11.2 |
| B | 60.0 | 5.06 | 2.71 | 31.32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, M.-H.; Hung, F.-Y.; Lui, T.-S.; Lai, J.-J. Enhanced Formability and Accelerated Precipitation Behavior of 7075 Al Alloy Extruded Rod by High Temperature Aging. Metals 2018, 8, 648. https://doi.org/10.3390/met8080648
Ku M-H, Hung F-Y, Lui T-S, Lai J-J. Enhanced Formability and Accelerated Precipitation Behavior of 7075 Al Alloy Extruded Rod by High Temperature Aging. Metals. 2018; 8(8):648. https://doi.org/10.3390/met8080648
Chicago/Turabian StyleKu, Ming-Hsiang, Fei-Yi Hung, Truan-Sheng Lui, and Jyun-Jhih Lai. 2018. "Enhanced Formability and Accelerated Precipitation Behavior of 7075 Al Alloy Extruded Rod by High Temperature Aging" Metals 8, no. 8: 648. https://doi.org/10.3390/met8080648





