Modeling of Microstructure Evolution of Ti6Al4V for Additive Manufacturing
Abstract
:1. Introduction
2. Microstructure Evolution of Ti6Al4V
- -
- Widmanstätten structure (Figure 1a): It is composed by lamellae, with a small retained amount of intra-lamellar , enriched by vanadium, typical of slow-medium cooling rates. lamellae are usually aligned to form colonies. A slight variant of this structure, characterized by thinner lamellae, is called “basket-weave” structure. In this work, no difference is made between these two structures and we will refer to them as Widmanstätten structures.
- -
- Grain boundary (Figure 1b): an allotriomorph crystal structure generally located at the grains boundaries.
- -
- Martensite (Figure 1c): it is a non-equilibrium phase with acicular shape similar to small needles, typical of fast cooling rates. There exists a variant of this structure called massive alpha, typical of medium-fast cooling rates, but, in this work, this difference is not considered. Both Martensite and Massive alpha present HCP crystal structure.
3. Microstructure Evolution Model
3.1. General Formulation for Diffusion-Controlled Transformations
3.1.1. Johnson-Mehl-Avrami-Kolmogorov Equation
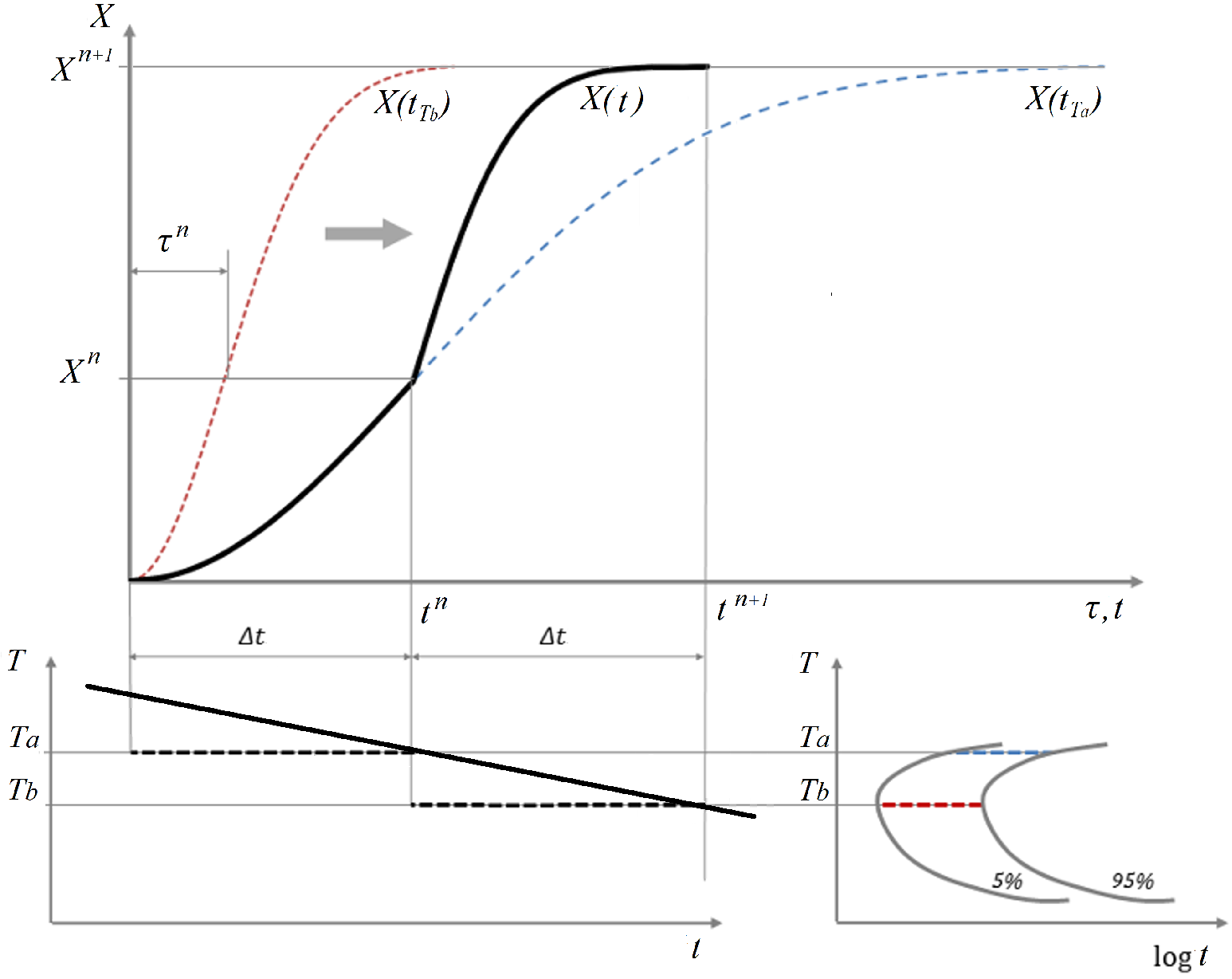
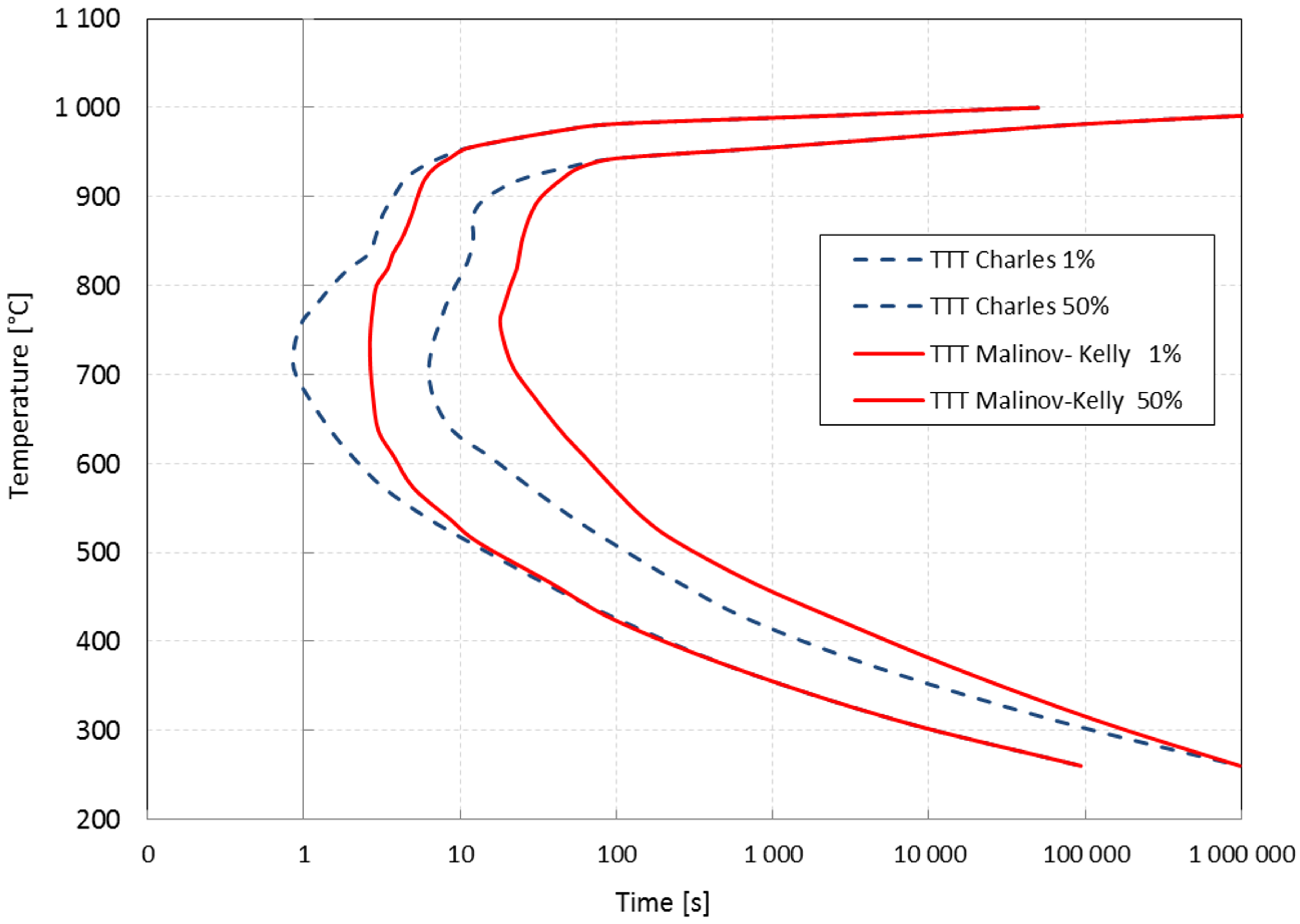
3.1.2. The TTT Curves
3.1.3. Additivity Rule
3.1.4. Modified JMAK Model Accounting for Initial Phase Fractions and Incomplete Transformations
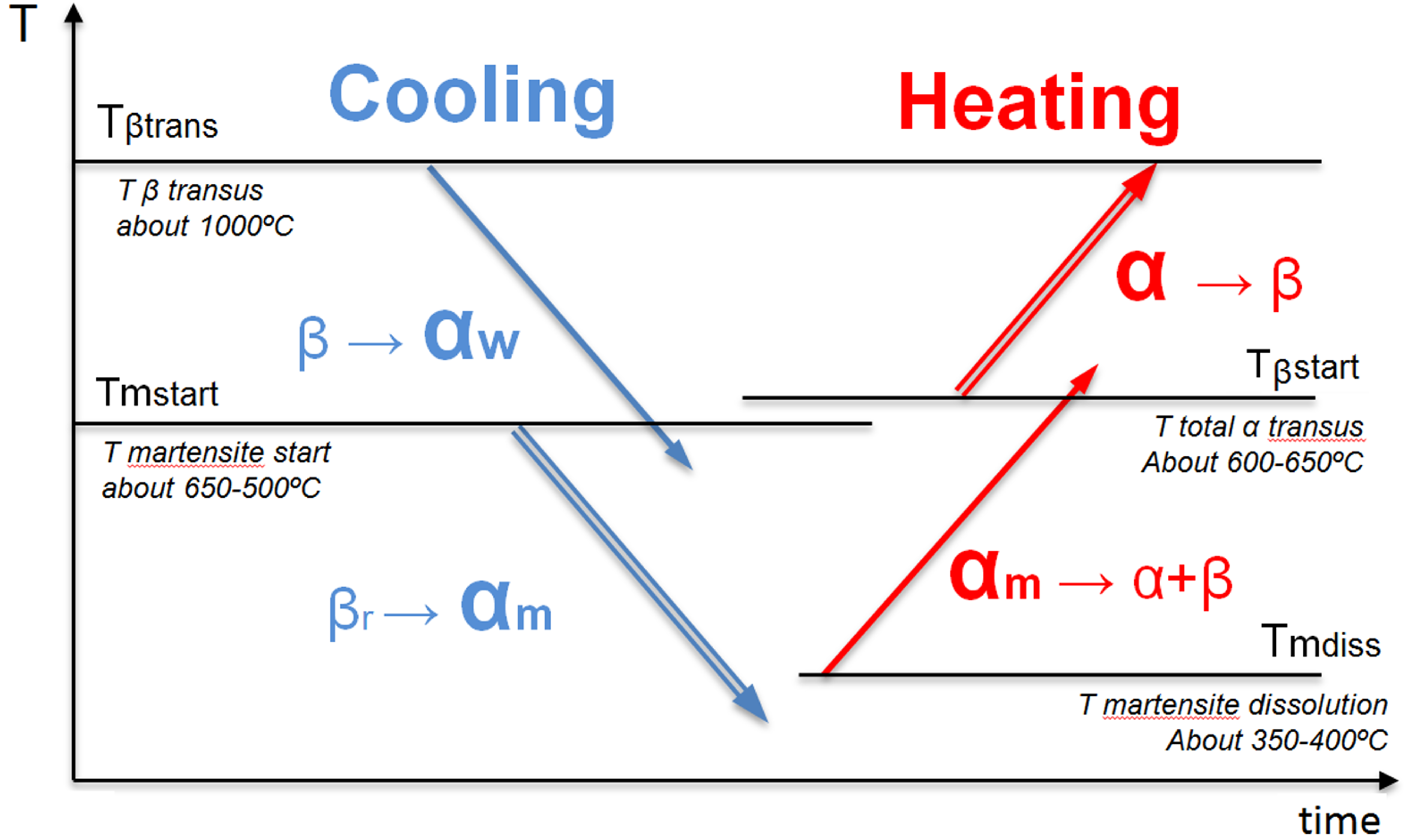
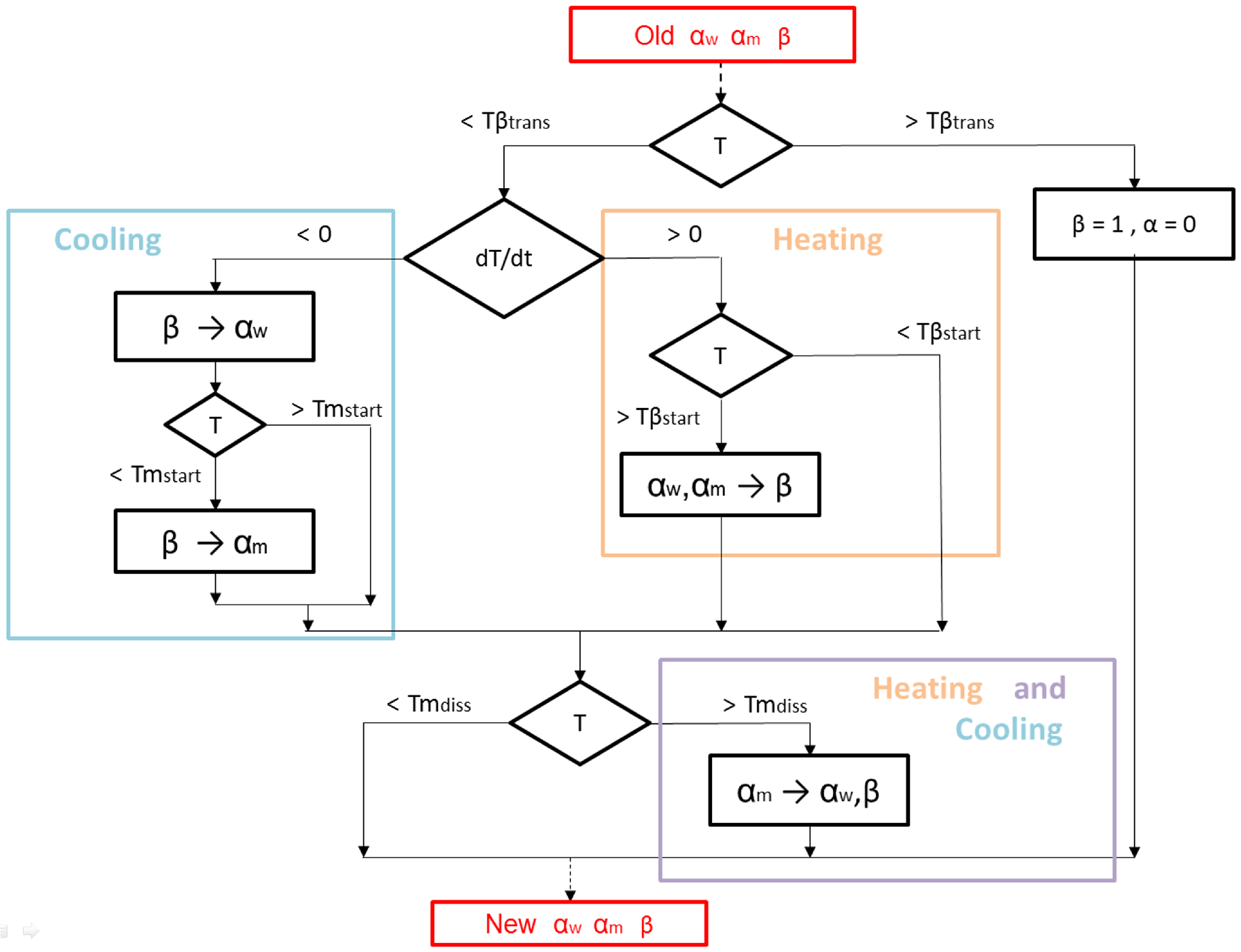
3.2. Microstructure Modeling of Ti6Al4V for AM Processes
- (I)
- Formation of Widmanstätten : diffusion-controlled transformation () for cooling processes below the -transus temperature .
- (II)
- Formation of Martensite : diffusionless transformation () for fast cooling processes below the martensite temperature .
- (III)
- Dissolution of Martensite : diffusion-controlled transformation ( → ) by heating process above .
- (IV)
- Dissolution of total (or re-formation of ): diffusionless transformation ( → ) by heating process above .
3.2.1. Formation of Alpha Widmanstätten
3.2.2. Formation of Alpha Martensite
3.2.3. Dissolution of Alpha Martensite
3.2.4. Alpha to Beta Transformation
4. Numerical Results and Sensitivity Analysis to Material Data
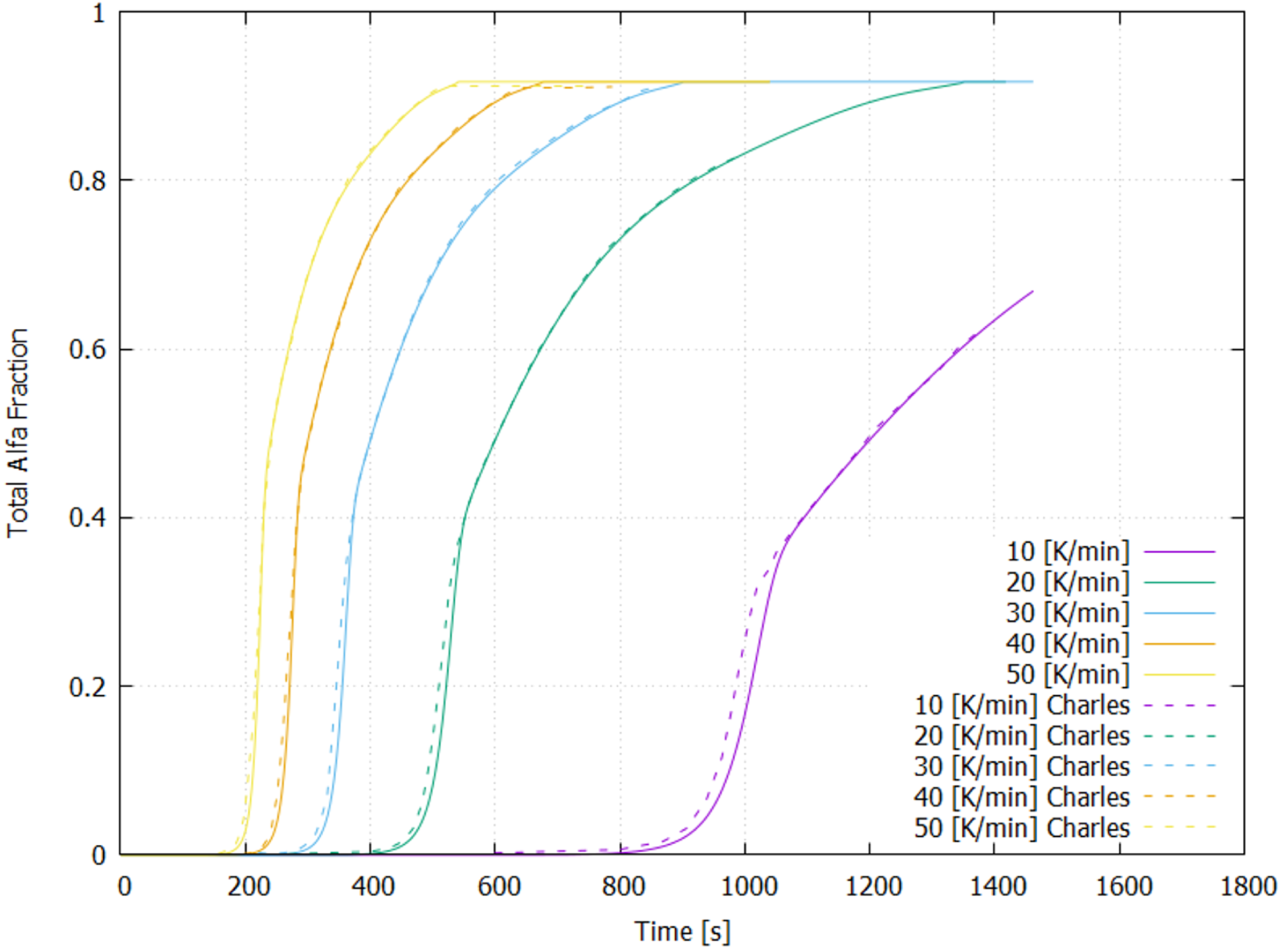
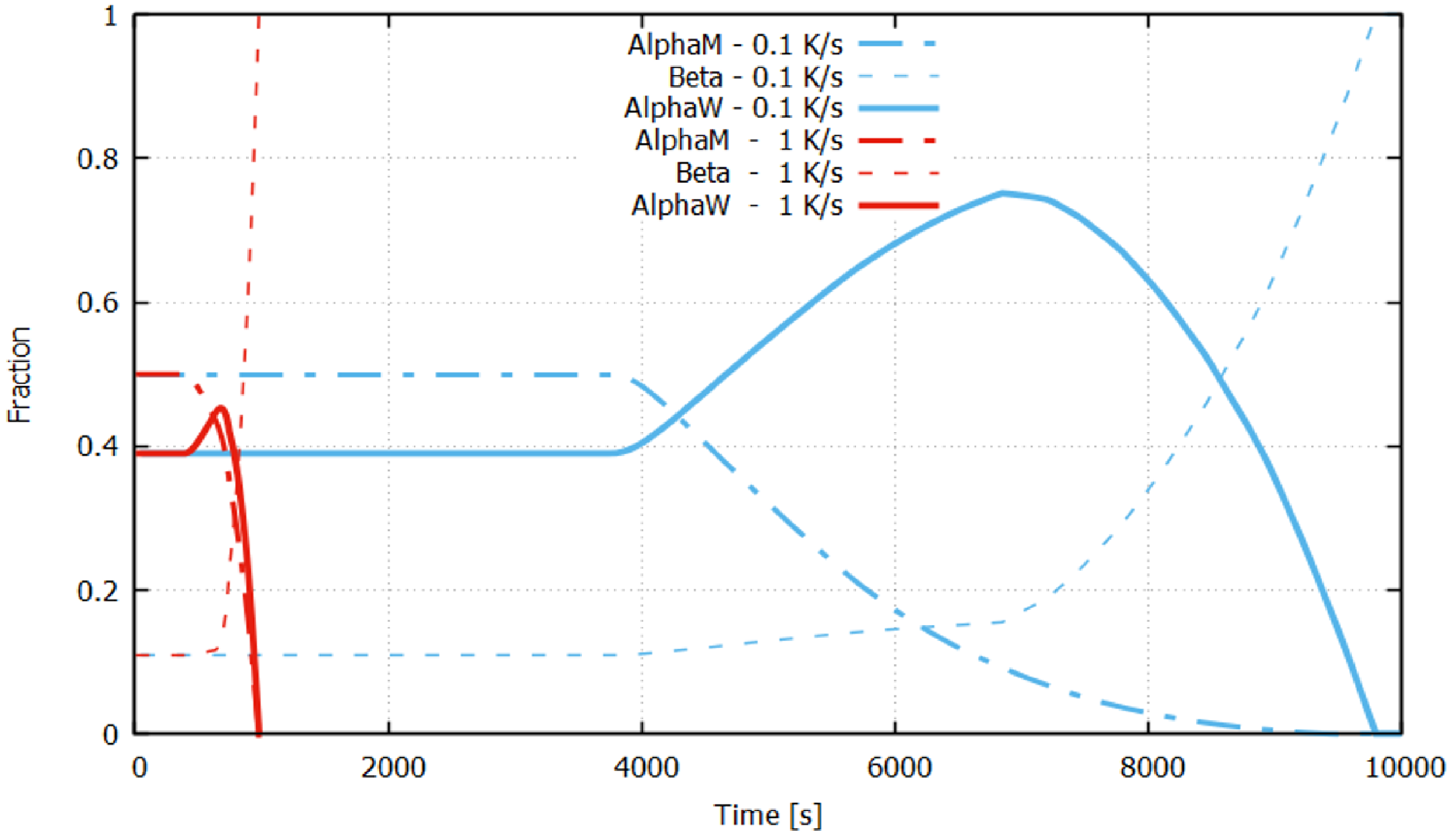
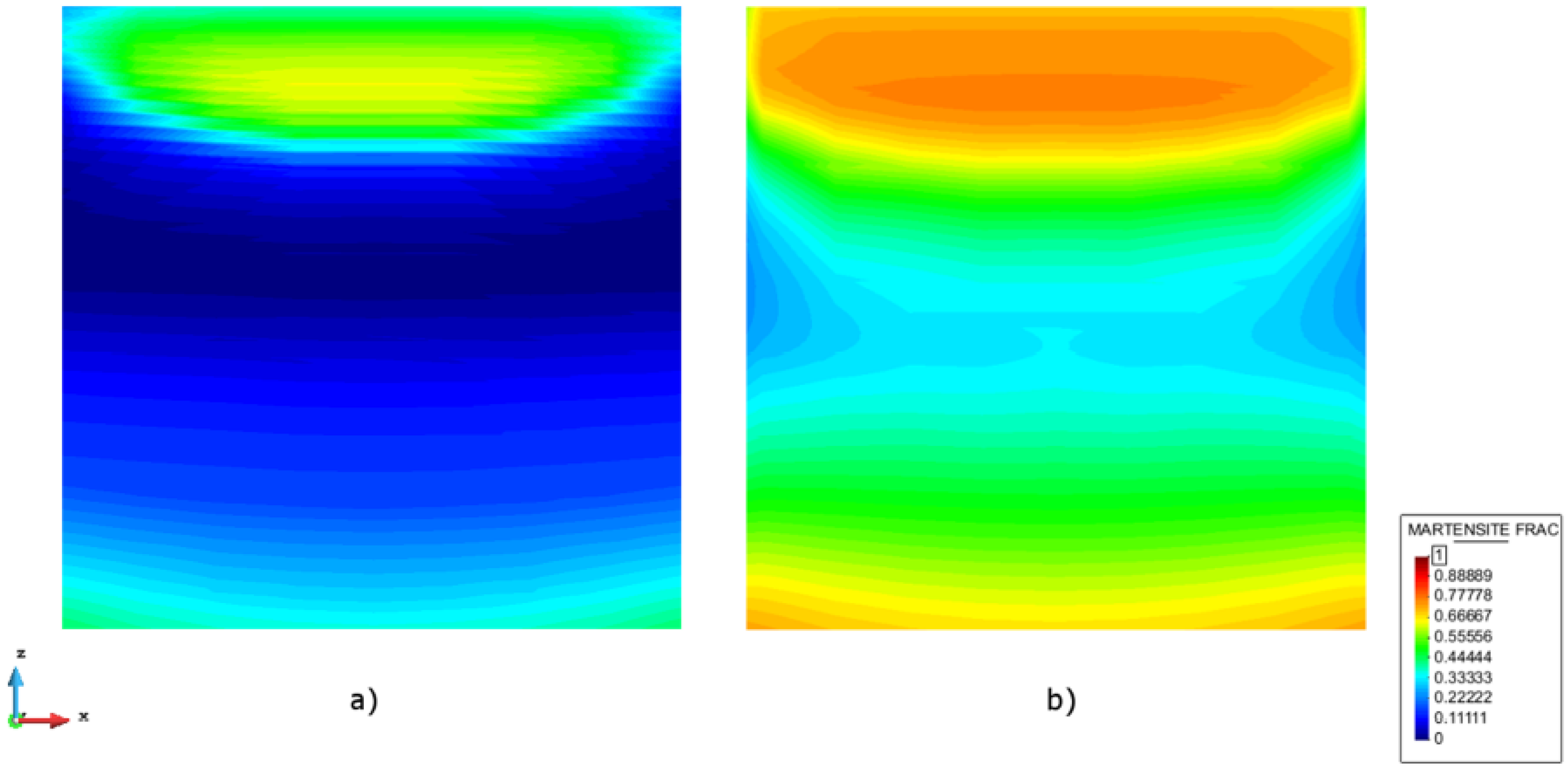
4.1. Cooling Process
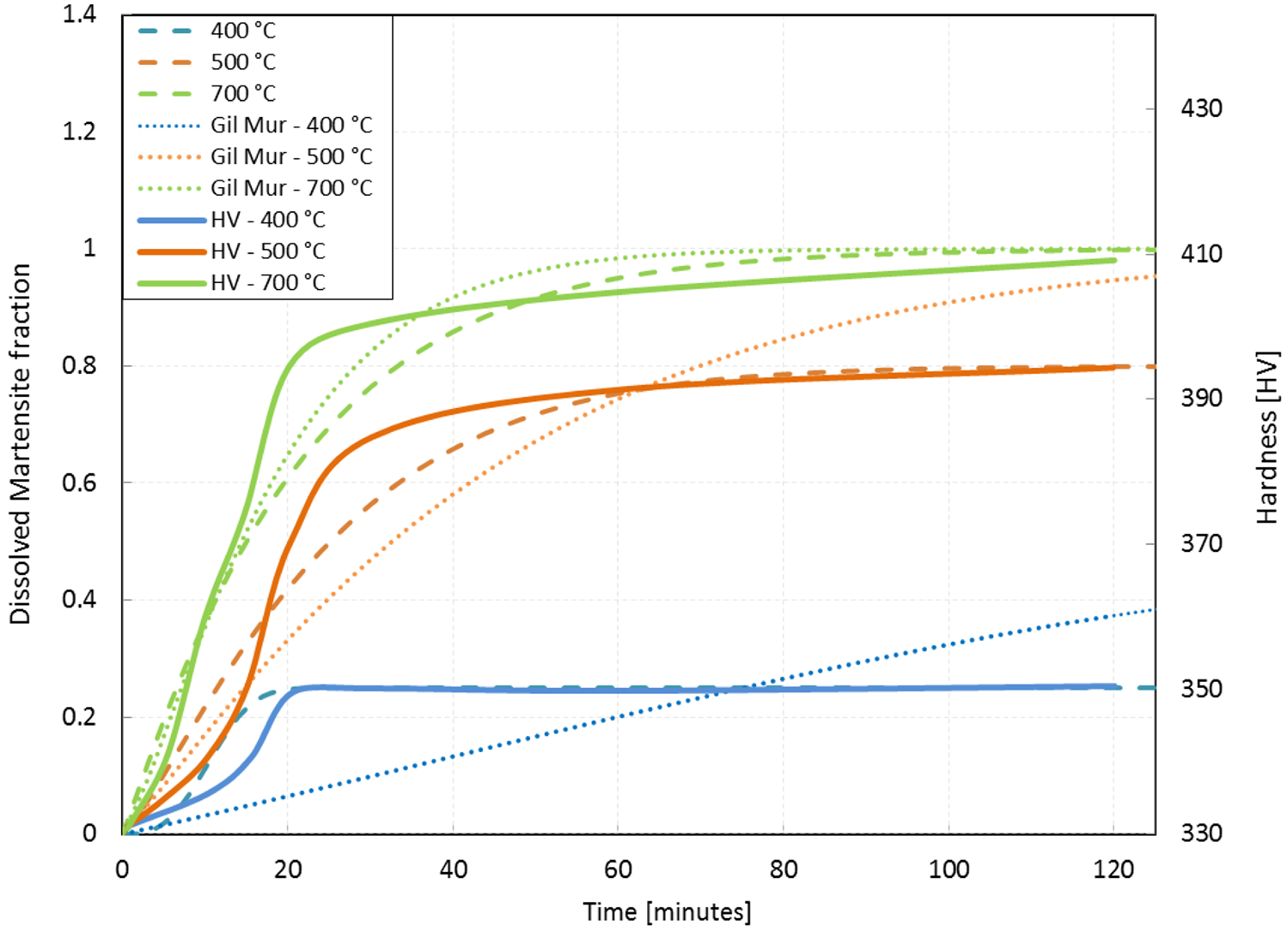
4.2. Re-Heating Process and Isothermal Treatment
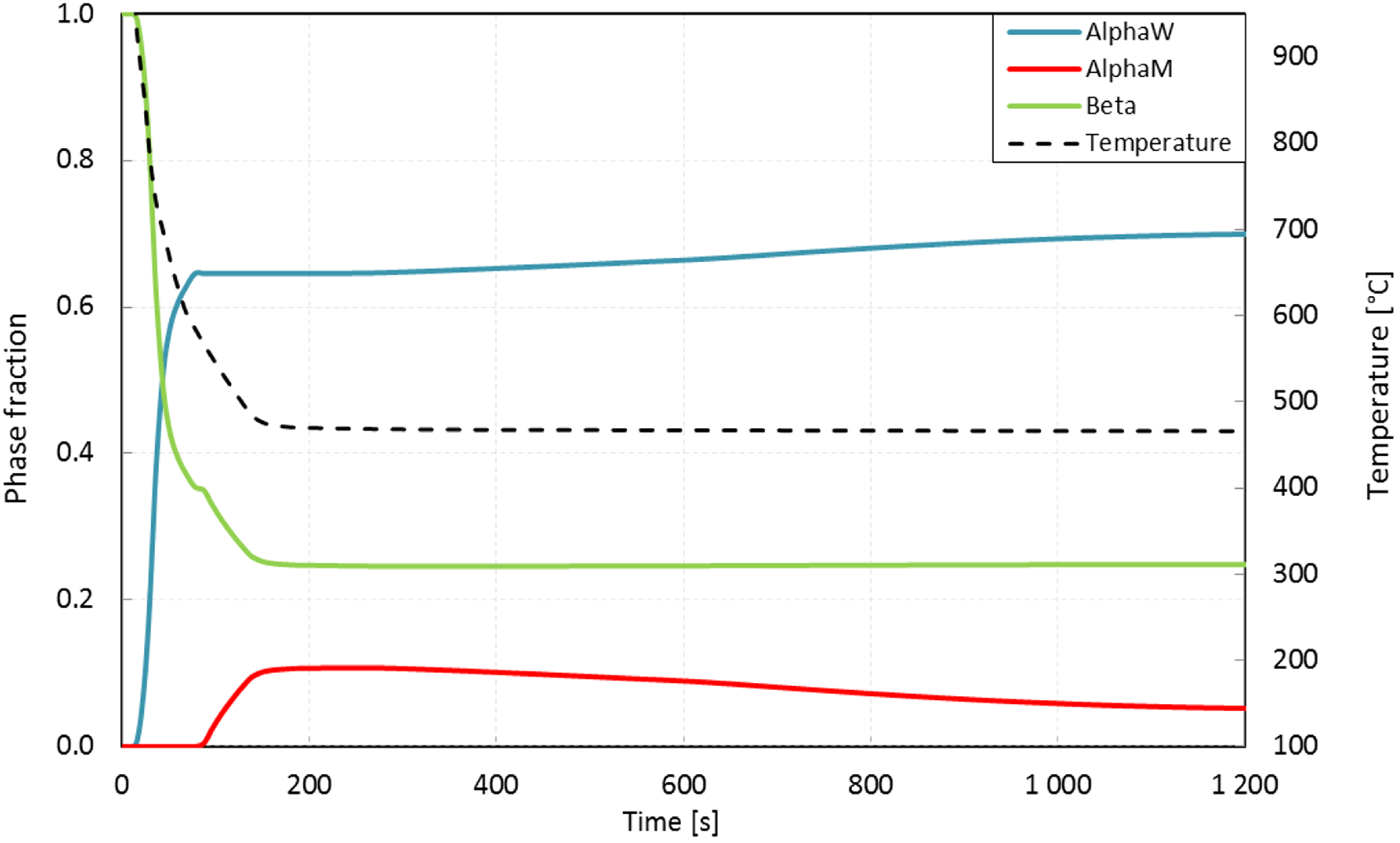
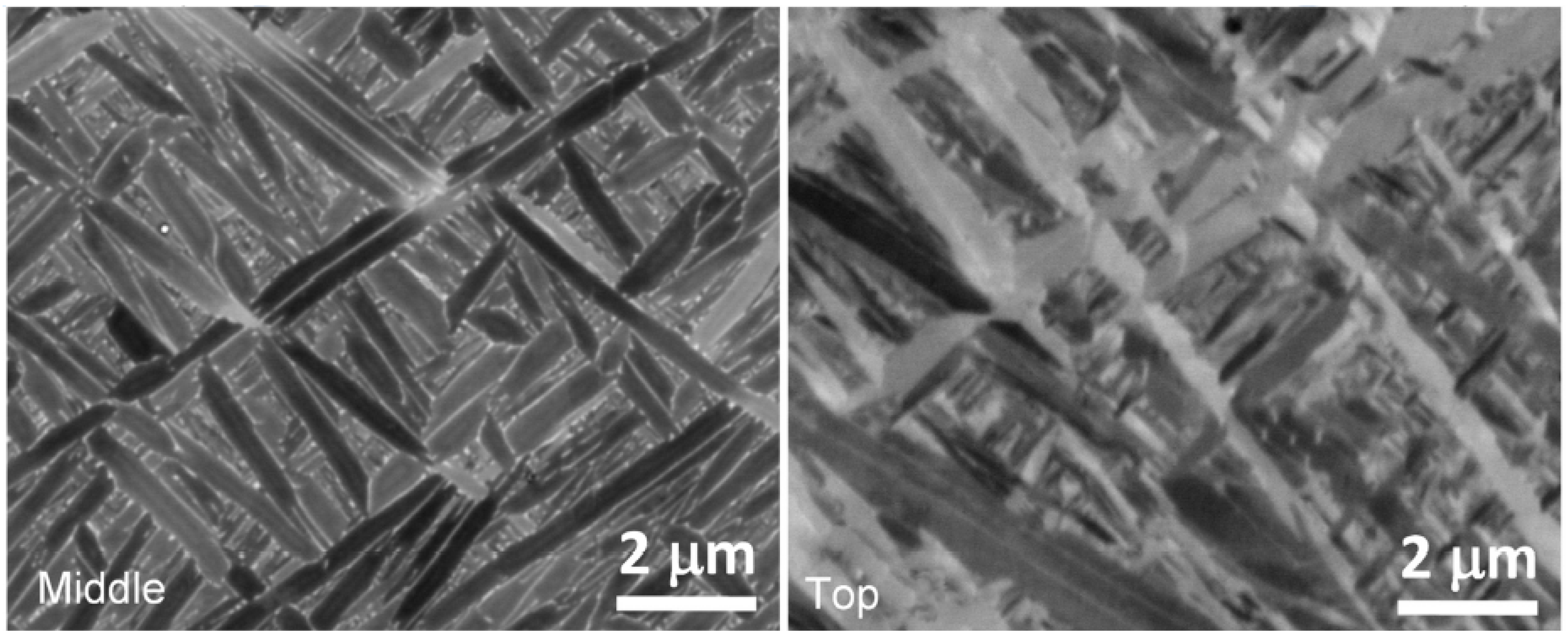
4.3. AM Processes-Experimental Validation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saboori, A.; Gallo, D.; Biamino, S.; Fino, P.; Lombardi, M. An Overview of Additive Manufacturing of Titanium Components by Directed Energy Deposition: Microstructure and Mechanical Properties. Appl. Sci. 2017, 7, 883. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015. [Google Scholar]
- Costa, L.; Vilar, R. Laser powder deposition. Rapid Prototyp. J. 2009, 15, 264–279. [Google Scholar] [CrossRef]
- Selcuk, C. Laser metal deposition for powder metallurgy parts. Powder Metall. 2011, 54, 94–99. [Google Scholar]
- Kar, S.; Searles, T.; Lee, E.; Viswanathan, G.B.; Tiley, J.; Banerjee, R.; Fraser, H.L. Modeling the tensile properties in β processed α/β Ti alloys. Metall. Mater. Trans. A 2006, 37, 559–566. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change: III. Granulation, phase change, and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Cahn, J. Transformation kinetics during continuous cooling. Acta Metall. 1956, 4, 572–575. [Google Scholar] [CrossRef]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Kelly, S. Thermal and Microstructure Modeling of Metal Deposition Processes with Application to Ti6Al4V. Ph.D. Thesis, Faculty of Virginia, Polytechnic Institute and State University, Blacksburg, VA, USA, 2004. [Google Scholar]
- Charles Murgau, C.; Pederson, R.; Lindgren, L. A model for Ti-6Al-4V microstructure evolution for arbitrary temperature changes. Model. Simul. Mater. Sci. Eng. 2012, 20, 055006. [Google Scholar] [CrossRef]
- Fachinotti, V.; Cardona, A.; Baufeld, B.; Van der Biest, O. Evolution of microstructure during shaped metal deposition. Mecánica Comput. 2010, 29, 4927–4934. [Google Scholar]
- Chiumenti, M.; Cervera, M.; Salmi, A.; Agelet de Saracibar, C.; Dialami, N.; Matsui, K. Finite element modeling of multi-pass welding and shaped metal deposition processes. Comput. Methods Appl. Mech. Eng. 2010, 199, 2343–2359. [Google Scholar] [CrossRef]
- Agelet de Saracibar, C.; Lundbäck, A.; Chiumenti, M.; Cervera, M. Shaped Metal Deposition Processes. In Encyclopedia of Thermal Stresses; Hetnarski, R.B., Ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014; pp. 4346–4355. [Google Scholar]
- Chiumenti, M.; Neiva, E.; Salsi, E.; Cervera, M.; Badia, S.; Moya, J.; Chen, Z.; Lee, C.; Davies, C. Numerical Modelling and Experimental Validation in Selective Laser Melting. Addit. Manuf. 2017, 18, 171–185. [Google Scholar] [CrossRef]
- Charles Murgau, C. Microstructure Model for Ti-6Al-4V Used in Simulation of Additive Manufacturing. Ph.D. Thesis, University of Luleå, Luleå, Sweden, 2016. [Google Scholar]
- Sieniawski, J.; Ziaja, W.; Kubiak, K.; Motyka, M. Microstructure and Mechanical Properties of High Strength Two-Phase Titanium Alloys. In Titanium Alloys—Advances in Properties Control; Sieniawski, J., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar] [Green Version]
- Donachie, M. Titanium: A Technical Guide; ASM International Materials: Park, OH, USA, 2000. [Google Scholar]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Gil, F.J.; Ginebra, M.P.; Manero, J.M.; Planell, J.A. Formation of alpha-Widmanstatten structure: Effects of grain size and cooling rate on theWidmanstatten morphologies and on the mechanical properties in Ti6Al4V alloy. J. Alloy. Compd. 2001, 329, 142–152. [Google Scholar] [CrossRef]
- Gil Mur, F.X.; Rodríguez, F.D.; Planell, J.A. Influence of tempering temperature and time on the alpha prime Ti-6Al-4V martensite. J. Alloy. Compd. 1996, 234, 287–289. [Google Scholar] [CrossRef]
- Charles Murgau, C.; Jarvstrat, N. Modelling Ti-6Al-4V microstructure by evolution laws implemented as finite element subroutine. In Proceedings of the 8th International Conference on Trends in Welding Research, Pine Mountain, GA, USA, 1–6 June 2008. [Google Scholar]
- Johnson, W.; Mehl, R. Reaction Kinetics in processes of nucleation and growth. Trans. Am. Inst. Min. Metall. Eng. 1939, 135, 416–458. [Google Scholar]
- Kolmogorov, A. Statistical theory of crystallization of metals (in Russian). Izv. Akad. Nauk SSSR Ser. Math. 1937, 1, 355–359. [Google Scholar]
- Ahmed, T.; Rack, H.J. Phase transformations during cooling in α + β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Castro, R.; Seraphin, L. Contribution in the metallographic and structural study of the alloy of titanium Ti4Al6V. Mem. Sci. Rev. Metall. 1966, 63, 1025–1058. [Google Scholar]
- Malinov, S.; Guo, Z.; Sha, W.; Wilson, A. Differential scanning calorimetry study and computer modeling of β to α phase transformation in a Ti-6Al-4V alloy. Metall. Mater. Trans. A 2001, 32, 879–887. [Google Scholar] [CrossRef]
- Malinov, S.; Markovsky, P.; Sha, W.; Guo, Z. Resistivity study and computer modelling of the isothermal transformation kinetics of Ti–6Al–4V and Ti-6Al-2Sn-4Zr-2Mo-0.08Si alloys. J. Alloy. Compd. 2001, 314, 181–192. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S.; Zhang, W.; DebRoy, T. In-situ observations of phase transformations in the fusion zone of Ti-6Al-4V alloy transient welds using synchrotron radiation. In Mathematical Modelling of Weld Phenomena; Cerjak, H., Ed.; Verlag der Technische Universitat Graz: Graz, Austria, 2005; Volume 7. [Google Scholar]
- Babu, S.S.; Kelly, S.M.; Specht, E.D.; Palmer, T.A.; Elmer, J.W. Measurement of phase transformation kinetics during repeated thermal cycling of Ti-6Al-4V using time-resolved X-ray diffraction. In Proceedings of the International Conference on Solid-Solid Phase Transformations in Inorganic Materials, Phoenix, AZ, USA, 29 May–3 June 2005. [Google Scholar]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Xia, K.; Quian, M. Additive manufacturing of strong and ductile Ti-6Al-4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]




| Temperature (°C) | Alpha Fraction at Equilibrium αeq |
|---|---|
| 1000 | |
| 988 | |
| 967 | |
| 940 | |
| 913 | 0.39 |
| 898 | |
| 863 | |
| 829 | |
| 799 | |
| 767 | |
| 742 | |
| 710 | |
| 684 | |
| 667 | |
| 650 |
| Cooling Rate [K/s] | Observed Microstructures | Calculated αm Martensite Final Fraction | ||
|---|---|---|---|---|
| Ahmed et al. [24] | Sieniawski et al. [16] | Charles et al. TTT | Malinov and Kelly TTT | |
| 3.5 | (trace) | (trace) | 0.0001% | 3% |
| 9 | 0.04% | 30% | ||
| 18 | (trace) | 4% | 57% | |
| 48 | 61% | 77% | ||
| Temperature (°C) | [s] | [s] | |
|---|---|---|---|
| 320 | 0 | ||
| 400 | 500 | 1200 | |
| 500 | 520 | 3600 | |
| 700 | 1 | 450 | 3000 |
| Sample | P (W) | t (μm) | v (mm s−1) | h (mm) | E (J mm−3) | Measured Phase Fractions | Calculated αm |
|---|---|---|---|---|---|---|---|
| 375 | 60 | 1029 | 0.12 | 50.62 | 0.13 | ||
| 375 | 60 | 1029 | 0.18 | 33.74 | and | 0.57 |
| Temperature (°C) | (s) | (s) | |
|---|---|---|---|
| 320 | 0 | ||
| 400 | 300 | 800 | |
| 500 | 250 | 300 | |
| 700 | 1 | 30 | 60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salsi, E.; Chiumenti, M.; Cervera, M. Modeling of Microstructure Evolution of Ti6Al4V for Additive Manufacturing. Metals 2018, 8, 633. https://doi.org/10.3390/met8080633
Salsi E, Chiumenti M, Cervera M. Modeling of Microstructure Evolution of Ti6Al4V for Additive Manufacturing. Metals. 2018; 8(8):633. https://doi.org/10.3390/met8080633
Chicago/Turabian StyleSalsi, Emilio, Michele Chiumenti, and Miguel Cervera. 2018. "Modeling of Microstructure Evolution of Ti6Al4V for Additive Manufacturing" Metals 8, no. 8: 633. https://doi.org/10.3390/met8080633




