Methods to Evaluate Corrosion in Buried Steel Structures: A Review
Abstract
:1. Introduction
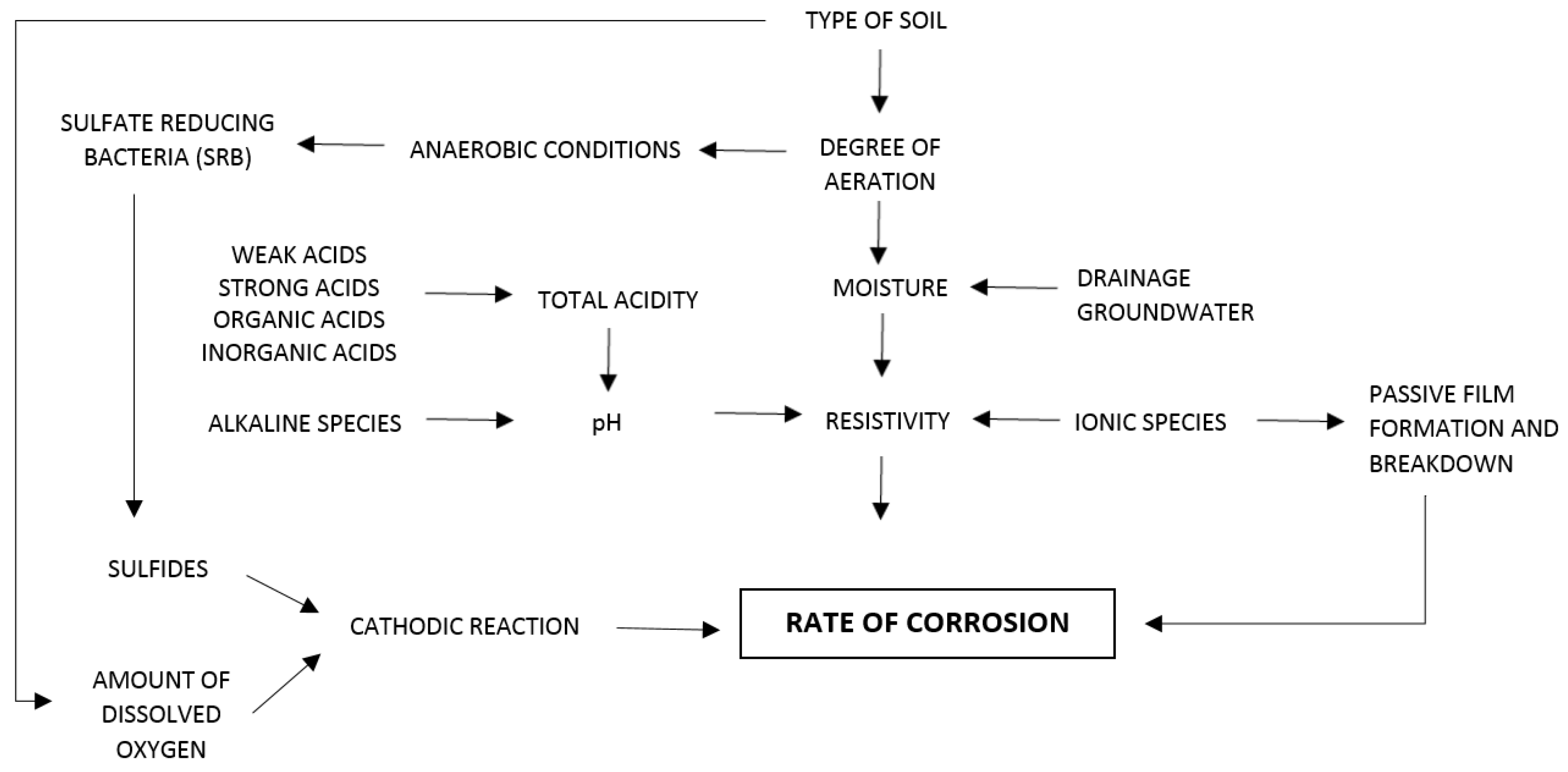
2. Corrosion in Buried Metal Structures
3. Corrosion in Soils
3.1. Soil Texture
3.2. Presence of Water
3.3. Aeration
3.4. Redox Potential
3.5. pH
3.6. Resistivity
3.7. Ions Content
3.8. Bacteria
4. Methods to Estimate the Corrosion of Soils
4.1. Type of Data
4.2. Qualitative Methods
4.3. Quantitative Methods
- Poor statistical design of the experiments. The statistical analysis was not a highly valued part and, consequently, measurement procedures were not developed.
- It did not consider variations in the horizons of the soil. As a result, pipes buried in the same trench but at opposite ends could be exposed to very different conditions.
- Seasonal and annual variations were not contemplated due to the use of annual average values for some variables (temperature, precipitation, etc.). In addition, the exposure times and dates of the initiation of the experiments varied from place to place. The exact dates of burial and extraction of the samples are unknown, which is significant because corrosion damage can be concentrated in certain seasons. A sample with an exposure time of one year and three months can present a much higher degree of corrosion than a sample with an exposure time of one year, because the first has endured the most problematic season twice.
- Different depths of burial. At each site, the experiments were carried out by the most common methods of burial, instead of applying the same procedure of preparation of the experiment throughout the study, which introduces more uncertainty into the dataset.
- Use of unrepresentative values for some variables. For example, the average rainfall used corresponds to the nearest place with available data, and the information corresponds to the historical average instead of the burial period of the trial. Therefore, the effect of particularly rainy years is unknown.
- Many properties were measured in the laboratory instead of on site. The alterations generated in the soil by eliminating the samples influence the activity of water, carbon dioxide, oxygen, etc. It also influences the pH, so that these measures incorporate an inherent error.
- Incomplete information about the chemical analysis. Due to budgetary limitations, only a complete chemical analysis was performed on 26 of the 47 initial trial sites. In addition, the choice of these 26 sites did not follow any random sampling procedure, but those with lower resistivity were chosen.
- It did not consider the possible changes in soil conditions over time. Human activity, for example, could have changed the conditions in any of the sites during the years of the experiment, but there is no information about it.
4.4. Assessment of the Methods
5. Conclusions
Funding
Conflicts of Interest
References
- Norhazilan, M.N.; Nordin, Y.; Lim, K.S.; Siti, R.O.; Safuan, A.R.A.; Norhamimi, M.H. Relationship between soil properties and corrosion of carbon steel. J. Appl. Sci. Res. 2012, 8, 3. [Google Scholar]
- Ahammed, M. Probabilistic estimation of remaining life of a pipeline in the presence of active corrosion defects. Int. J. Press. Vessels Pip. 1998, 75, 321–329. [Google Scholar] [CrossRef]
- Doyle, G.; Seica, M.V.; Grabinsky, M.W. The Role of Soil in the External Corrosion of Cast Iron Water Mains in Toronto, Canada. Can. Geotech. J. 2003, 40, 225–236. [Google Scholar] [CrossRef]
- Hembara, O.V.; Andreikiv, O.E. Effect of hydrogenation of the walls of oil-and-gas pipelines on their soil corrosion and service life. Mater. Sci. 2012, 47, 598–607. [Google Scholar] [CrossRef]
- Scheweitzer, P.A. Corrosion Engineering Handbook: Fundamentals of Metallic Corrosion, 2nd ed.; Taylor and Francis Group: Abingdon, UK, 2006. [Google Scholar]
- De Baere, K.; Verstraelen, H.; Rigo, P.; Van Passel, S.; Lenaerts, S.; Potters, G. Study on alternative approaches to corrosion protection of ballast tanks using an economic model. Mar. Struct. 2013, 32, 1–17. [Google Scholar] [CrossRef]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Yang, L. Techniques for Corrosion Monitoring; Woodhead Publishing: Sawston, UK, 2008. [Google Scholar]
- Papadakis, G.A. Major hazard pipelines: A comparative study of onshore transmission accidents. J. Loss Prev. Process Ind. 1999, 12, 91–107. [Google Scholar] [CrossRef]
- Cooper, N.R.; Blakey, G.; Sherwin, C.; Ta, T.; Whiter, J.T.; Woodward, C.A. The use of GIS to develop a probability-based trunk main burst risk model. Urban Water 2000, 2, 97–103. [Google Scholar] [CrossRef]
- Kleiner, Y.; Rajani, B. Comprehensive review of structural deterioration of water mains: Statistical models. Urban Water 2001, 3, 131–150. [Google Scholar] [CrossRef]
- Hopkins, P. Transmission pipelines: How to improve their integrity and prevent failures. In Proceedings of the 2nd International Pipeline Technology Conference, Ostend, Belgium, 11–14 September 1995; Volume 1. [Google Scholar]
- Yahaya, N.; Noor, N.M.; Din, M.M.; Nor, S.H.M. Prediction of CO2 corrosion growth in submarine pipelines. Malays. J. Civ. Eng. 2009, 21, 69–81. [Google Scholar]
- Restrepo, C.E.; Simonoff, J.S.; Zimmerman, R. Causes, cost consequences, and risk implications of accidents in US hazardous liquid pipeline infrastructure. Int. J. Crit. Infrastruct. Prot. 2009, 2, 38–50. [Google Scholar] [CrossRef]
- Roche, M.; Bender, R. Corrosion Resistance of Steels, Nickel Alloys, and Zinc in Aqueous Media: Waste Water, Seawater, Drinking Water, High-Purity Water; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ferreira, C.A.M.; Ponciano, J.A. Determination of the soil corrosivity of samples from southeaster Brazilian region. In Proceedings of the Eurocorr 2006, Maastricht, The Netherland, 25–28 September 2006. [Google Scholar]
- ArcelorMittal Europe—Long Products. Sections and Merchant Bars. Corrosion Protection of Rolled Steel Sections Using Hot-Dip Galvanization. Available online: http://constructalia.arcelormittal.com/files/Corrosion_EN--e76d086608bf6809dc3b229b551ca70e.pdf (accessed on 4 May 2018).
- Rim-Rukeh, A.; Awatefe, J.K. Investigation of soil corrosivity in the corrosion of low carbon steel pipe in soil environment. J. Appl. Sci. Res. 2006, 2, 466–469. [Google Scholar]
- Krivy, V.; Kubzova, M.; Kreislova, K.; Urban, V. Characterization of Corrosion Products on Weathering Steel Bridges Influenced by Chloride Deposition. Metals 2017, 7, 336. [Google Scholar] [CrossRef]
- Ferreira, C.A.M.; Ponciano, J.A.; Vaitsman, D.S.; Pérez, V. Evaluation of the corrosivity of the soil through its chemical composition. Sci. Total Environ. 2007, 388, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Costs and Prevention Strategies in the United States. Available online: https://www.nace.org/uploadedFiles/Publications/ccsupp.pdf (accessed on 4 May 2018).
- Cost of Corrosion and Prevention Strategies in the United States; C.C. Technologies Laboratories, Inc.: Dublin, OH, USA, 2001.
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; National Association of Corrosion Engineers (NACE) International: Houston, TX, USA, 2016. [Google Scholar]
- Butlin, K.R.; Vernon, W.H.; Whiskin, L.C. Investigations on Underground Corrosion; Special report No. 45; Iron and Steel Institute: London, UK, 1952. [Google Scholar]
- Schmitt, G. Global Needs for Knowledge Dissemination, Research, and Development in Materials Deterioration and Corrosion Control; World Corrosion Organization: New York, NY, USA, 2009. [Google Scholar]
- Alter, L.B.; Maestres, F.L. Corrosion and Protection; Polytechnic University of Catalonia: Barcelona, Spain, 2004; Volume 150. [Google Scholar]
- Davis, J.R. Corrosion: Understanding the Basics; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Chaker, V. Effects of Soil Characteristics on Corrosion; ASTM International: West Conshohocken, PA, USA, 1989. [Google Scholar]
- Schaschl, E.; Marsh, G.A. Some new views on soil corrosion. Mater. Protect. 1963, 2, 8–17. [Google Scholar] [CrossRef]
- Levlin, E. Corrosion of Water Pipe Systems Due to Acidification of Soil and Groundwater. Available online: https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/DE93500842.xhtml (accessed on 7 May 2018).
- Molina Pérez, L. Advanced Corrosion Study: Analysis of the Corrosion State of a Modernist Building in Barcelona and Study of Protection Mechanisms Based on Organic Coatings. Ph.D. Thesis, Universidad Politécnica de Catalunya, Barcelona, Spain, October 2011. [Google Scholar]
- Roberge, P.R. Corrosion Engineering: Principles and Practice; McGraw-Hill: Columbus, OH, USA, 2008. [Google Scholar]
- Payne, A.S. Control of Underground Corrosion; US Department of agriculture: Washington, DC, USA, 1999. [Google Scholar]
- Pritchard, O.; Hallett, S.H.; Farewell, T.S. Soil Corrosivity in the UK—Impacts on Critical Infrastructure; Infrastructure Transitions Research Consortium: Oxford, UK, 2013; pp. 1–55. [Google Scholar]
- Trethaway, K.R.; Chamberlain, J. Corrosion for Science and Engineering, 2nd ed.; Longman Group: Harlow, UK, 1995. [Google Scholar]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Eliyan, F.F.; Alfantazi, A. Mechanisms of corrosion and electrochemical significance of metallurgy and environment with corrosion of iron and steel in bicarbonate and carbonate solutions—A review. Corrosion 2014, 70, 880–898. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Corrosion of the Heat-Affected Zones (HAZs) of API-X100 pipeline steel in dilute bicarbonate solutions at 90 °C—An electrochemical evaluation. Corros. Sci. 2013, 74, 297–307. [Google Scholar] [CrossRef]
- Hudson, J.C.; Banfield, T.A.; Holden, H.A. Tests on the Corrosion of Buried Ferrous Metals. J. Iron Steel Inst. 1942, 146, 107. [Google Scholar]
- Parker, M.E.; Peattie, E.G. Pipe Line Corrosion and Cathodic Protection: A Practical Manual for Corrosion Engineers, Technicians, and Field Personnel; Gulf Professional Publishing: Houston, TX, USA, 1984. [Google Scholar]
- Logan, K.H.; Coe, M.; Brush, W.W. The Bureau of Standards soil-Corrosion investigation. J. Am. Water Works Assoc. 1929, 21, 311–317. [Google Scholar]
- Osella, A.; Favetto, A.; López, E. Currents induced by geomagnetic storms on buried pipelines as a cause of corrosion. J. Appl. Geophys. 1998, 38, 219–233. [Google Scholar] [CrossRef]
- Yan, M.; Sun, C.; Xu, J.; Ke, W. Anoxic Corrosion Behavior of Pipeline Steel in Acidic Soils. Ind. Eng. Chem. Res. 2014, 53, 17615–17624. [Google Scholar] [CrossRef]
- Burstein, G.T.; Shrir, L.L.; Jarman, R.A. Corrosion 2 Volume Set, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1994. [Google Scholar]
- Ternary Diagram of the Soil Composition, Redrawn from the USDA. Credit Mikenorton. Available online: https://commons.wikimedia.org/wiki/File:SoilTexture_USDA.png (accessed on 9 May 2018).
- Jeannin, M.; Calonnec, D.; Sabot, R.; Refait, P. Role of a clay sediment deposit on the corrosion of carbon steel in 0.5 mol L−1 NaCl solutions. Corros. Sci. 2010, 52, 2026–2034. [Google Scholar] [CrossRef]
- Ismail, A.I.M.; El-Shamy, A.M. Engineering behavior of soil materials on the corrosion of mild steel. Appl. Clay Sci. 2009, 42, 356–362. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion; McMillan: New York, NY, USA, 1992. [Google Scholar]
- Gupta, S.K.; Gupta, B.K. The critical soil moisture content in the underground corrosion of mild steel. Corros. Sci. 1979, 19, 171–178. [Google Scholar] [CrossRef]
- Pereira, R.F.C.; Oliveira, E.S.D.; Lima, M.A.G.A.; Cezar Brazil, S.L.D. Corrosion of Galvanized Steel under Different Soil Moisture Contents. Mater. Res. 2015, 18, 563–568. [Google Scholar] [CrossRef]
- Phear, A.; Dew, C.; Ozsoy, B.; Wharmby, N.J.; Judge, J.; Barley, A.D. Soil Nailing—Best Practice Guidance; Construction Industry Research & Information Association: London, UK, 2005. [Google Scholar]
- Veleva, L. Soils and Corrosion (Chapter 32). In Corrosion Tests and Standards: Application and Interpretation, 2nd ed.; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Fiedler, S.; Veparskas, M.; Richardson, J.L. Soil Redox Potential: Importance, Field Measurements, and Observations, in Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, UK, 2007; Volume 94, pp. 1–54. [Google Scholar]
- Yahaya, N.; Noor, N.M.; Othman, S.R.; Sing, L.K.; Din, M.M. New Technique for Studying Soil-Corrosion of Underground Pipeline. J. Appl. Sci. 2011, 11, 1510–1518. [Google Scholar] [CrossRef]
- Tiba, T.; De Oliveira, E.M. Utilization of cathodic protection for transmission towers through photovoltaic generation. Renew. Energy 2012, 40, 150–156. [Google Scholar] [CrossRef]
- Bradford, S.A. The Practical Handbook of Corrosion Control in Soils; CASTI Publishing Inc.: Edmonton, AB, Canada, 2000. [Google Scholar]
- Zhu, Q.J.; Cao, A.; Wang, Z.F.; Song, J.W.; Chen, S.L. Fundamental Aspects of Stray Current Corrosion on Buried Pipeline. Adv. Mater. Res. 2011, 146–147, 70–74. [Google Scholar] [CrossRef]
- Bayliss, D.A.; Deacon, D.H. Steelwork Corrosion Control; CRC Press: London, UK, 2002. [Google Scholar]
- Palmer, J.D. Environmental Characteristics Controlling the Soil Corrosion of Ferrous Piping. In Effects of Soil Characteristics on Corrosion; ASTM International: West Conshohocken, PA, USA, 1989. [Google Scholar]
- Busby, J.P.; Entwisle, D.; Hobbs, P.; Jackson, P.; Johnson, N.; Lawley, R.; Linley, K.; Mayr, T.; Palmer, R.; Rines, M.; et al. A GIS for the planning of electrical earthing. Q. J. Eng. Geol. Hydrogeol. 2012, 45, 379–390. [Google Scholar] [CrossRef]
- Wenner, F. A method for measuring Earth resistivity. J. Wash. Acad. Sci. 1915, 5, 561–563. [Google Scholar] [CrossRef]
- Li, D.G.; Bai, Z.Q.; Zhu, J.W.; Zheng, M.S. Influence of temperature, chloride ions and chromium element on the electronic property of passive film formed on carbon steel in bicarbonate/carbonate buffer solution. Electrochim. Acta 2007, 52, 7877–7884. [Google Scholar] [CrossRef]
- Arzola, S.; Palomar-Pardave, M.E.; Genesca, J. Effect of resistivity on the corrosion mechanism of mild steel in sodium sulfate solutions. J. Appl. Electrochem. 2003, 33, 1233–1237. [Google Scholar]
- Edyvean, R.G.J. Hydrogen sulphide—A corrosive metabolite. Int. Biodeterior. 1991, 27, 109–120. [Google Scholar]
- Lee, W.; Characklis, W.G. Corrosion of mild steel under anaerobic biofilm. Corrosion 1993, 49, 186–199. [Google Scholar] [CrossRef]
- Fontana, M.G.; Staehle, R.W. Advances in Corrosion Science and Technology; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Soriano, C.; Alfantazi, A. Corrosion behavior of galvanized steel due to typical soil organics. Constr. Build. Mater. 2016, 102, 904–912. [Google Scholar] [CrossRef]
- Neff, D.; Dillmann, P.; Descostes, M.; Beranger, G. Corrosion of iron archaeological artefacts in soil: Estimation of the average corrosion rates involving analytical techniques and thermodynamic calculations. Corros. Sci. 2006, 48, 2947–2970. [Google Scholar] [CrossRef]
- Fleming, H.C. Economical and technical overview. In Microbiologically Influenced Corrosion of Materials; Heitz, E., Fleming, H.C., Sand, W., Eds.; Springer-Verlag: New York, NY, USA, 1996; pp. 6–14. [Google Scholar]
- Maruthamuthu, S.; Kumar, B.D.; Ramachandran, S.; Anandkumar, B.; Palanichamy, S.; Chandrasekaran, M.; Subramanian, P.; Palaniswamy, N. Microbial Corrosion in Petroleum Product Transporting Pipelines. Ind. Eng. Chem. Res. 2011, 50, 8006–8015. [Google Scholar] [CrossRef]
- Bastin, E.S. The Problem of the Natural Reduction of Sulphates. AAPG Bull. 1926, 10, 1270–1299. [Google Scholar]
- Beech, I.B.; Gaylarde, C.C. Recent advances in the study of biocorrosion: An overview. Rev. Microbiol. 1999, 30, 117–190. [Google Scholar] [CrossRef]
- Javaherdashti, R. Microbiologically Influenced Corrosion: An Engineering Insight; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Wu, T.; Xu, J.; Sun, C.; Yan, M.; Yu, C.; Ke, W. Microbiological corrosion of pipeline steel under yield stress in soil environment. Corros. Sci. 2014, 88, 291–305. [Google Scholar] [CrossRef]
- Mehanna, M.; Basséguy, R.; Délia, M.L.; Bergel, A. Effect of Geobacter sulfurreducens on the microbial corrosion of mild steel, ferritic and austenitic stainless steels. Corros. Sci. 2009, 51, 2596–2604. [Google Scholar] [CrossRef] [Green Version]
- Kobrin, G. A Practical Manual on Microbiologically Influenced Corrosion; NACE International: Houston, TX, USA, 1993. [Google Scholar]
- Von Wolzogen Kühr, C.A.H.; Van der Vlugt, L.S. Water (The Hage), 1934, 16, 147. Corrosion 1961, 17, 293. [Google Scholar]
- Li, S.Y.; Kim, Y.G.; Jeon, K.S.; Kho, Y.T.; Kang, T. Microbiologically Influenced Corrosion of Carbon Steel Exposed to Anaerobic Soil. Corrosion 2001, 57, 815–828. [Google Scholar] [CrossRef]
- Cunha Lins, V.F.; Magalhães Ferreira, M.L.; Saliba, P.A. Corrosion Resistance of API X52 Carbon Steel in Soil Environment. J. Mater. Res. Technol. 2012, 1, 161–166. [Google Scholar] [CrossRef]
- CP 2—Cathodic Protection Technician Course Manual; NACE International: Houston, TX, USA, 2006.
- Lorenz, W.J.; Mansfeld, F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- Bullard, S.J.; Covino, B.S.; Russell, J.H.; Holcomb, G.R.; Cramer, S.D.; Ziomek-Moroz, M.E.D. Laboratory Evaluation of an Electrochemical Noise System for Detection of Localized and General Corrosion of Natural Gas Transmission Pipelines; NACE International: Houston, TX, USA, 2003. [Google Scholar]
- Baboian, R. Corrosion Tests and Standards: Application and Interpretation; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Ewing, S. Corrosion of metals in soils as a factor in the selection of pipe materials. Oil Gas J. 1937, 36, 51–59. [Google Scholar]
- Logan, K.H. Engineering Significance of National Bureau of Standards Soil-Corrosion Data; National Bureau of Standards: Gaithersburg, MD, USA, 1939. [Google Scholar]
- Neighbours, W. A new method for measuring potentials of polarized electrodes in soil corrosion cells. Corrosion 1955, 11, 28–30. [Google Scholar] [CrossRef]
- Hickling, A. Studies in Electrode Polarization. Part I. The Accurate Measurement of the Potential of a Polarized Electrode. Trans. Faraday Soc. 1937, 33, 1540–1546. [Google Scholar] [CrossRef]
- Denison, I.A.; Darnielle, R.B. Observations on the Behavior of Steel Corroding under Cathodic Control in Soils. Trans. Electrochem. Soc. 1939, 76, 199–214. [Google Scholar] [CrossRef]
- Haynes, G.S. Laboratory Corrosion Tests and Standards; ASTM International: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Robles Hernandez, K.K. Rail Base Corrosion Detection and Prevention; Transportation Technology Centre, Inc.: Pueblo, CO, USA, 2007. [Google Scholar]
- Peabody, A.W. Peabody’s Control of Pipeline Corrosion; NACE International: Houston, TX, USA, 2001. [Google Scholar]
- ASTM G187-12a. Standard Test Method for Measurement of Soil Resistivity Using the Two-Electrode Soil Box Method; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Bhattarai, J. Study on the corrosive nature of soil towards the buried-structures. Sci. World 2013, 11, 43–47. [Google Scholar] [CrossRef]
- Starkey, R.L.; Wight, K.M. Anaerobic Corrosion of Iron in Soil: Soil Science; LWW: Philadelphia, PA, USA, 1946; Volume 62. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Pourbaix Diagram of Iron, Redrawn from the USDA. Credit Mikenorton. Available online: https://commons.wikimedia.org/wiki/File:Pourbaix_Diagram_of_Iron.svg (accessed on 9 May 2018).
- EN 12501-2. Protection of Metallic Materials against Corrosion. CORROSION Likelihood in Soil. Part 2: Low Alloyed and Unalloyed Ferrous Materials; European Standard Store: Pilsen, Czech Republic, 2003. [Google Scholar]
- ANSI/AWWA C105. Ductile-Iron Pipe Standard; American Water Works Association: Denver, CO, USA, 2012. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: Columbus, OH, USA, 2000. [Google Scholar]
- Spickelmire, B. Corrosion considerations for ductile iron pipe. Mater. Perform. 2002, 41, 16–23. [Google Scholar]
- DVGW GW 9:2011. Evaluation of Soils in View of Their Corrosion Behavior towards Buried Pipelines and Vessels of Non-Alloyed Iron Materials; German Technical and Scientific Association for Gas and Water: Bonn, Germany, 2011. [Google Scholar]
- Eyre, D.; Lewis, D.A. Soil Corrosivity Assessment; Transport and Road Research Laboratory: Wokingham, UK, 1987. [Google Scholar]
- DMRB BD 42/00. DMRB Volume 2: Highway Structures: Design. Part 2; UK Highways Agency: Guildford, UK, 2000. [Google Scholar]
- DIN 50929-3. Probability of Corrosion of Metallic Materials When Subject to Corrosion from the Outside. Buried and Underwater Pipelines and Structural Components; German Institute for Standardization: Berlin, Germany, 1985. [Google Scholar]
- Richardson, T.J. Shreir’s Corrosion; Elsevier: New York City, NY, USA, 2009. [Google Scholar]
- Handbook of Steel Drainage & Highway Construction Products, 5th ed.; American Iron and Steel Institute: Washington, DC, USA, 1994.
- Robinson, J. Predicting the In-Ground Performance of Galvanized Steel; Mount Townsend Solutions Pty Ltd.: Jesmond, Australia, 2005. [Google Scholar]
- Heim, G.; Schwen, K.W. Corrosion in Aqueous Solutions and Soil. In Handbook of Cathodic Corrosion Protection, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 139–152. [Google Scholar]
- Roberge, P.R. Corrosion of Steel-H Piles at Nuclear Generating Stations; Canadian Nuclear Safety Commission: Ottawa, ON, Canada, 2016. [Google Scholar]
- The Design Decision Model for Corrosion Control of Ductile Iron Pipelines; Ductile Iron Pipe Research Association: Birmingham, AL, USA, 2016.
- Schwerdtfeger, W.J. Soil resistivity as related to underground corrosion and cathodic protection. Highw. Res. Rec. 1966, 110, 20–21. [Google Scholar] [CrossRef]
- Romanoff, M. Underground Corrosion; National Bureau of Standards Circular 579; National Bureau of Standards: Washington, DC, USA, 1957.
- Booth, G.H.; Cooper, A.W.; Cooper, P.M.; Wakerley, D.S. Criteria of Soil Aggressiveness towards Buried Metals. I. Experimental Methods. Br. Corros. J. 1967, 2, 104–108. [Google Scholar] [CrossRef]
- Ricker, R.E. Analysis of Pipeline Steel Corrosion Data from NBS (NIST) Studies Conducted between 1922–1940 and Relevance to Pipeline Management. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, J.; Caleyo, F.; Valor, A.; Hallen, J.M. Predictive Model for Pitting Corrosion in Buried Oil and Gas Pipelines. Corrosion 2009, 65, 332–342. [Google Scholar] [CrossRef]
- Ikechukwu, A.S.; Ugochukwu, N.H.; Ejimofor, R.A.; Obioma, E. Correlation between soil properties and external corrosion growth rate of carbon steel. Int. J. Eng. Sci. 2014, 3, 38–47. [Google Scholar]
- Stratful, R.F. A new test for estimating soil corrosivity based on investigation of metal highway culverts. Corrosion 1961, 17, 493–496. [Google Scholar] [CrossRef]
- Wang, H.; Yajima, A.; Liang, R.Y.; Castaneda, H. A clustering approach for assessing external corrosion in a buried pipeline based on hidden Markov random field model. Struct. Saf. 2015, 56, 18–29. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Isakhani-Zakaria, M.; Derakhshani, M.; Samadian, M.; Sharifi-Rasaey, H.; Razmjoo, A. Investigation on corrosion rate and a novel corrosion criterion for gas pipelines affected by dynamic stray current. J. Nat. Gas Sci. Eng. 2015, 26, 453–460. [Google Scholar] [CrossRef]
- Nguyen Dang, D.; Lanarde, L.; Jeannin, M.; Sabot, R.; Refait, P. Influence of soil moisture on the residual corrosion rates of buried carbon steel structures under cathodic protection. Electrochim. Acta 2015, 176, 1410–1419. [Google Scholar] [CrossRef]





| Variable | Relation |
|---|---|
| Soil texture | Direct |
| Presence of water | Direct |
| Aeration | Direct |
| pH | Inverse |
| Resistivity | Inverse |
| Redox potential | Direct |
| Ion contents | Direct |
| Bacteria | Direct |
| Soil Resistivity (Ω.cm) | NACE | ASTM |
|---|---|---|
| >10,000 | Negligible | Very mildly corrosive |
| 5001–10,000 | Mildly corrosive | Mildly corrosive |
| 2001–5000 | Mildly corrosive | Moderately corrosive |
| 1001–2000 | Moderately corrosive | Severely corrosive |
| 501–1000 | Corrosive | Extremely corrosive |
| 0–500 | Very corrosive | Extremely corrosive |
| pH | Resistivity (Ω.cm) | Corrosion |
|---|---|---|
| <3.5 | Any | High |
| 3.5–4.5 | <4500 >4500 | High Medium-High |
| 4.5–5.5 | <4500 4500–5000 >5000 | High Medium-High Medium |
| 5.5–6.0 | <1000 1000–5000 5000–10,000 >10,000 | High Medium-High Medium Medium-Low |
| 6.0–9.5 | <1000 1000–3000 3000–10,000 10,000–20,000 >20,000 | High Medium-High Medium Medium-Low Low |
| Assigned Point | Resistivity (Ω.cm) | pH | Redox Potential (mV) | Sulphides | Moistures |
|---|---|---|---|---|---|
| 0 | >3000 | 4–8.5 | >100 | Negative | Good drainage |
| 1 | 2500–3000 | Fair drainage | |||
| 2 | 2500–2100 | Trace | Poor drainage | ||
| 3 | 2–4 >8.5 | ||||
| 3.5 | 50–100 | Positive | |||
| 4 | 0–50 | ||||
| 5 | 2100–1800 | 0–2 | <0 | ||
| 8 | 1800–1500 | ||||
| 10 | <1500 |
| Rating Number | Parameter |
|---|---|
| R1 | Soil type |
| R2 | Resistivity (Ω) |
| R3 | Water content (%) |
| R4 | pH |
| R5 | Buffering capacity |
| R6 | Sulphide content (mg/kg) |
| R7 | Neutral salts (mmol/kg) |
| R8 | Sulphates (mmol/kg) |
| R9 | Groundwater |
| R10 | Horizontal homogeneity |
| R11 | Vertical homogeneity |
| R12 | Redox potential |
| Likelihood | Consequences | Proposed action |
|---|---|---|
| <10 | Any | Standard protection |
| 10–20 | <30 1035.7x−1.05 > C > 240.25x−0.7 >1035.7x−1.05 | Standard protection PE (Polyethylene) PE+bonded joints |
| 20–35 | <25 1596.1x−1.08 > C > 1035.7x−1.05 >1596.1x−1.08 | PE (Polyethylene) PE+bonded joints PE+bonded joints or Cathodic protection |
| 35–40 | <30 1177.8x−0.89 > C > 1596.1x−1.08 >1177.8x−0.89 | PE+bonded joints PE+bonded joints or cathodic protection Cathodic protection |
| 40–45 | <1177.8x−0.89 >1177.8x−0.89 | PE+bonded joints or Cathodic protection Cathodic protection |
| 45–50 | Any | Cathodic protection |
| Soil | Duration of Exposure | Loss in Weight | Maximum Penetration | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Type | Open Hearth Iron | Wrought Iron | Bessemer Steel | Open Hearth Iron | Wrought Iron | Bessemer Steel | |
| Years | oz/ft2 | oz/ft2 | oz/ft2 | Mils | Mils | Mils | ||
| 52 | Lake Charles clay loam | 2.0 | 3.1 | 3.4 | 2.7 | 66 | 62 | 40 |
| 5.4 | 14.7 | 14.6 | 13.5 | 116 | 123 | 118 | ||
| 7.5 | 19.0 | 19.0 | 16.9 | 116 | 176 | 163 | ||
| Model | Type | Description | Methods | Soil Factors |
|---|---|---|---|---|
| Qualitative | Univariate | Direct Relationship | NACE | Resistivity |
| ASTM | Resistivity | |||
| Soil redox potential | ||||
| pH | ||||
| Multivariate | Direct Relationship | Pourbaix diagrams | Potential, pH | |
| EN 12501-2:2003 | Resistivity, pH | |||
| Point scales | AWWA C-105 | Resistivity, pH, redox potential, sulfides, moisture | ||
| DVGW | Soil composition, ground-water level at buried position, resistivity, moisture content, pH, sulfide and hydrogen sulfide, carbonate, chloride, sulfate, cinder and coke | |||
| DIN 50929 | Soil type and extent of contamination, resistivity, pH, moisture, soil condition (disturbed/undisturbed), buffer capacity, combined chloride/sulfate content, sulfate content, sulfide content, presence of ground water, vertical/horizontal homogeneity, external stray current | |||
| Dechema Soil Corrosivity Worksheet | Soil type, resistivity, water content, pH, buffering capacity, sulfide content, neutral salts, sulfates, groundwater, horizontal homogeneity, vertical homogeneity, redox potential | |||
| Risk matrix | Design Decision Model | Resistivity, pH, redox potential, sulfides, moisture | ||
| Quantitative | Multivariate | Field data | Romanoff Tables | Aeration, electrolyte, electrical factors, miscellaneous (man-made alterations, bacteria) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arriba-Rodriguez, L.-d.; Villanueva-Balsera, J.; Ortega-Fernandez, F.; Rodriguez-Perez, F. Methods to Evaluate Corrosion in Buried Steel Structures: A Review. Metals 2018, 8, 334. https://doi.org/10.3390/met8050334
Arriba-Rodriguez L-d, Villanueva-Balsera J, Ortega-Fernandez F, Rodriguez-Perez F. Methods to Evaluate Corrosion in Buried Steel Structures: A Review. Metals. 2018; 8(5):334. https://doi.org/10.3390/met8050334
Chicago/Turabian StyleArriba-Rodriguez, Lorena-de, Joaquin Villanueva-Balsera, Francisco Ortega-Fernandez, and Fernando Rodriguez-Perez. 2018. "Methods to Evaluate Corrosion in Buried Steel Structures: A Review" Metals 8, no. 5: 334. https://doi.org/10.3390/met8050334





