Enhancement of the Young’s Modulus through Infrared Heat Treatment: A Study of the Microstructure and the Mass Effect of Real Body 6082 Aluminum Forgings
Abstract
:1. Introduction
2. Materials and Experimental Process
3. Results and Discussion
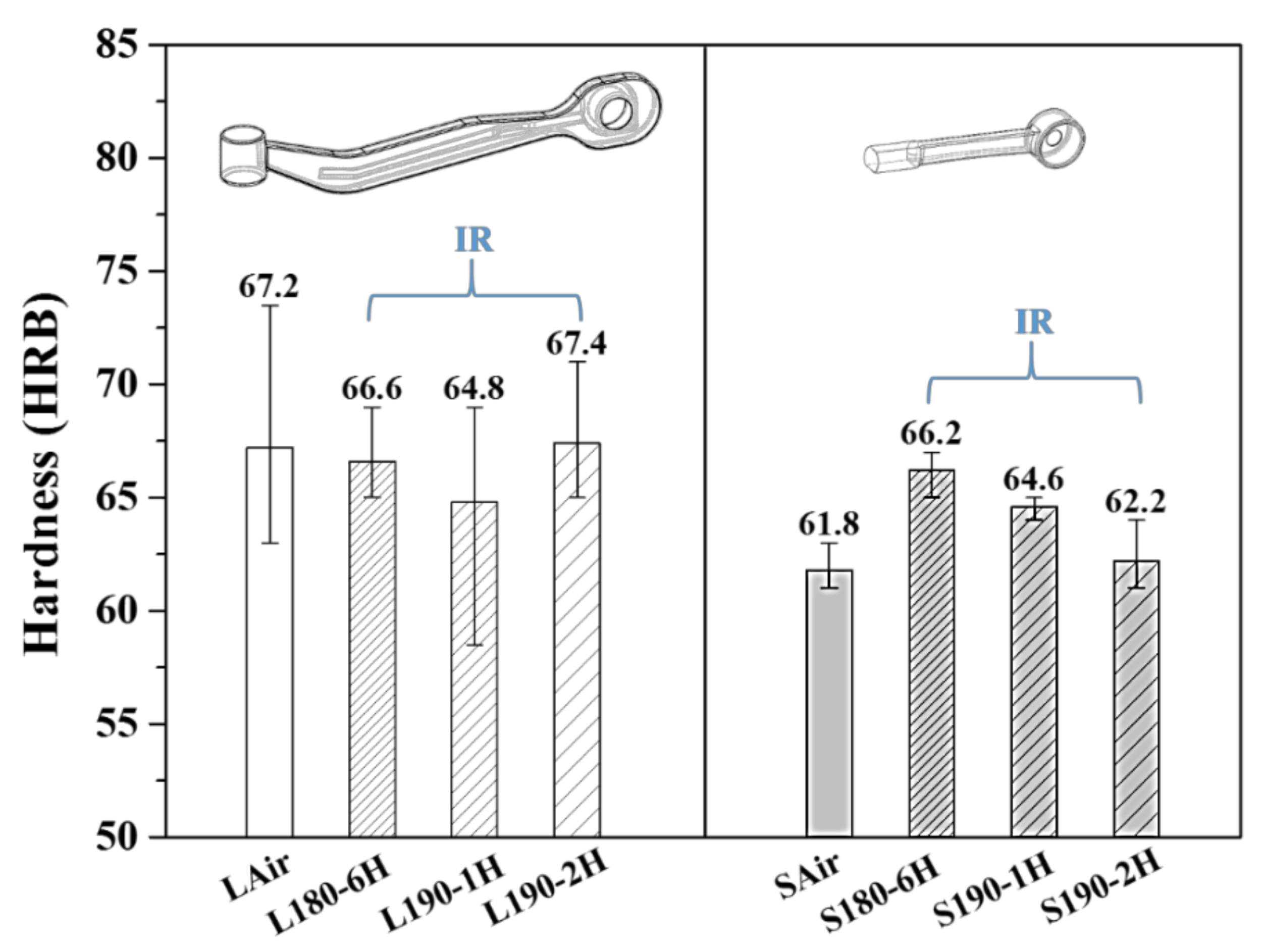
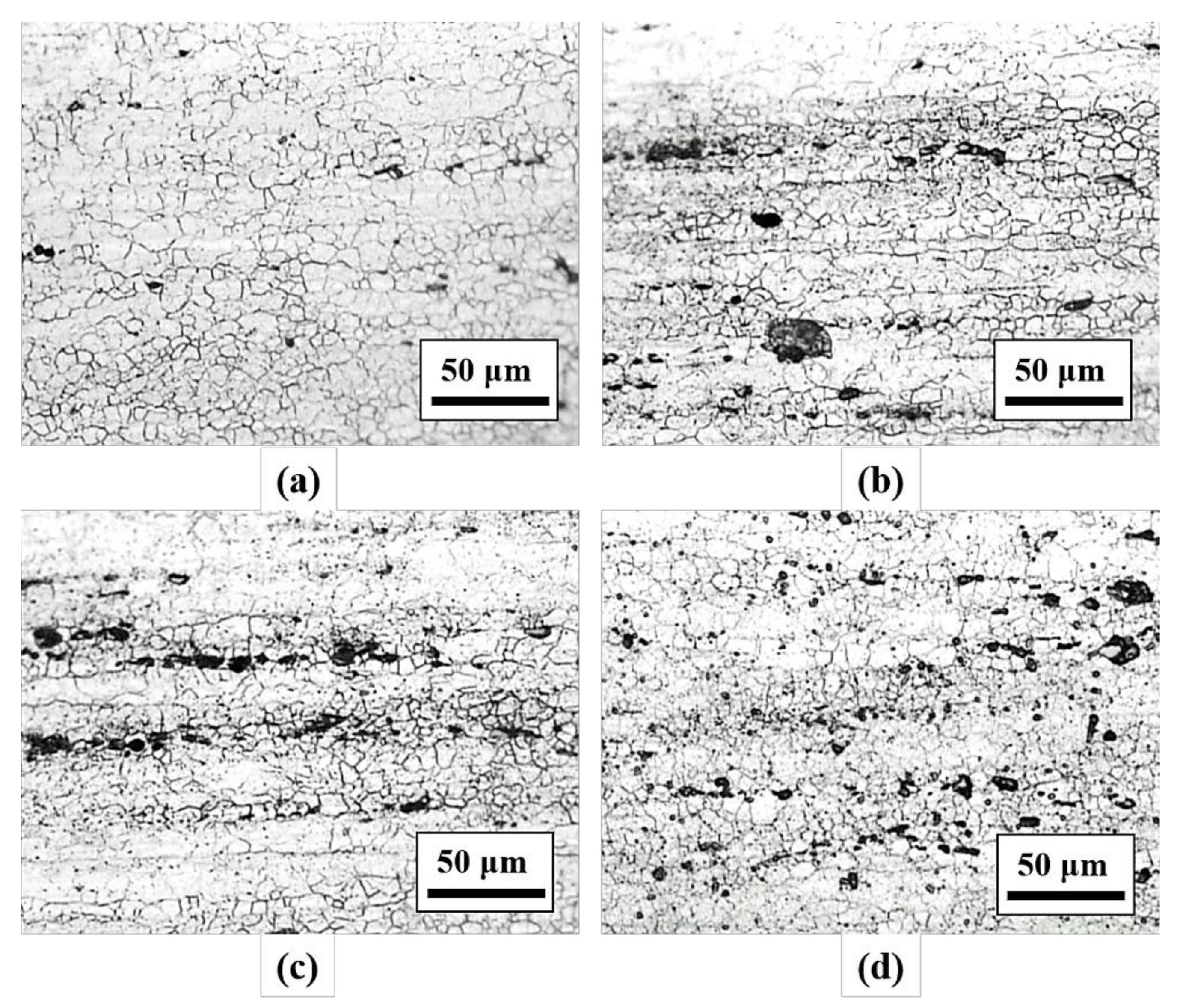
3.1. Hardness Values and Microstructural Observation
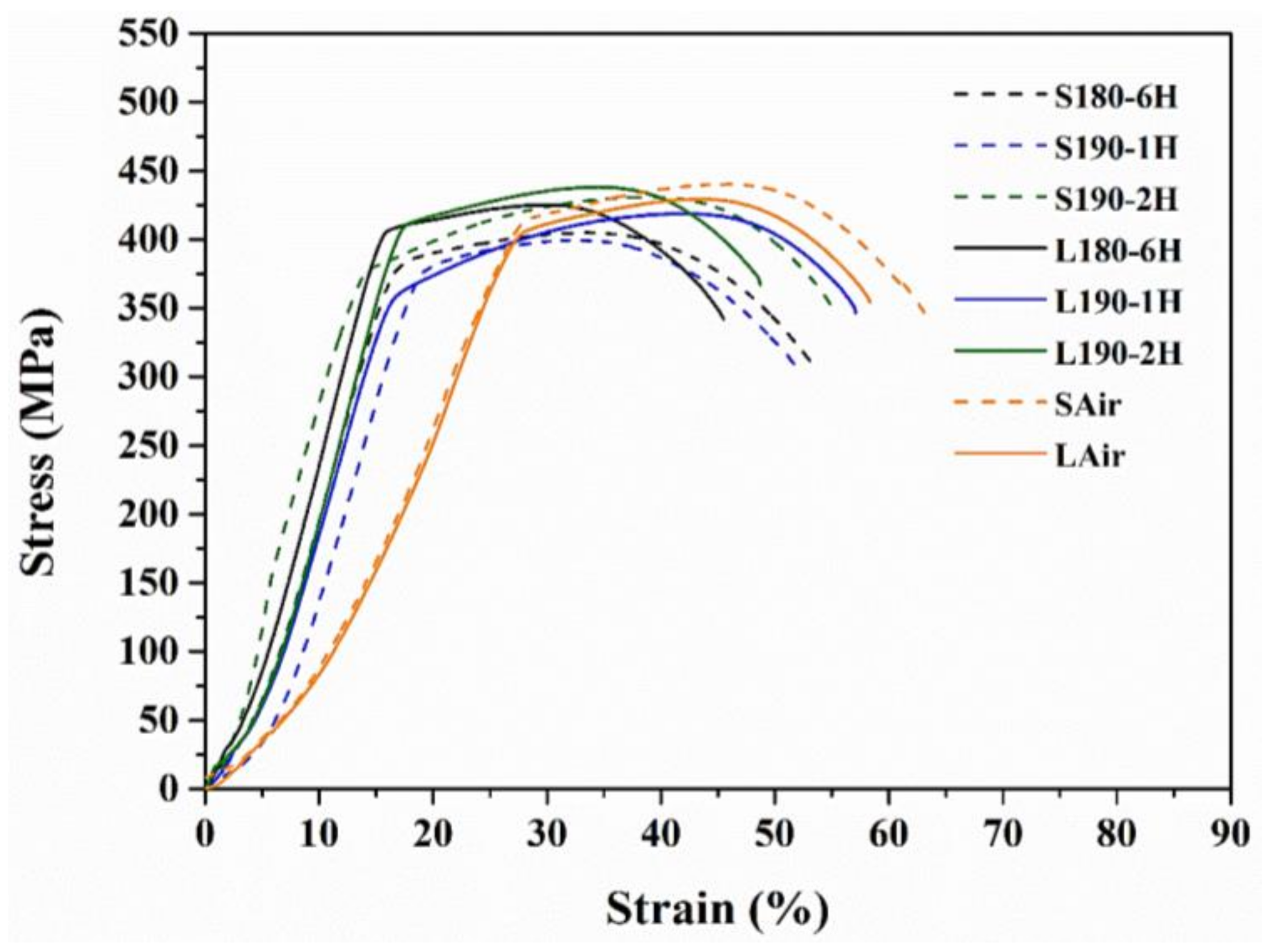
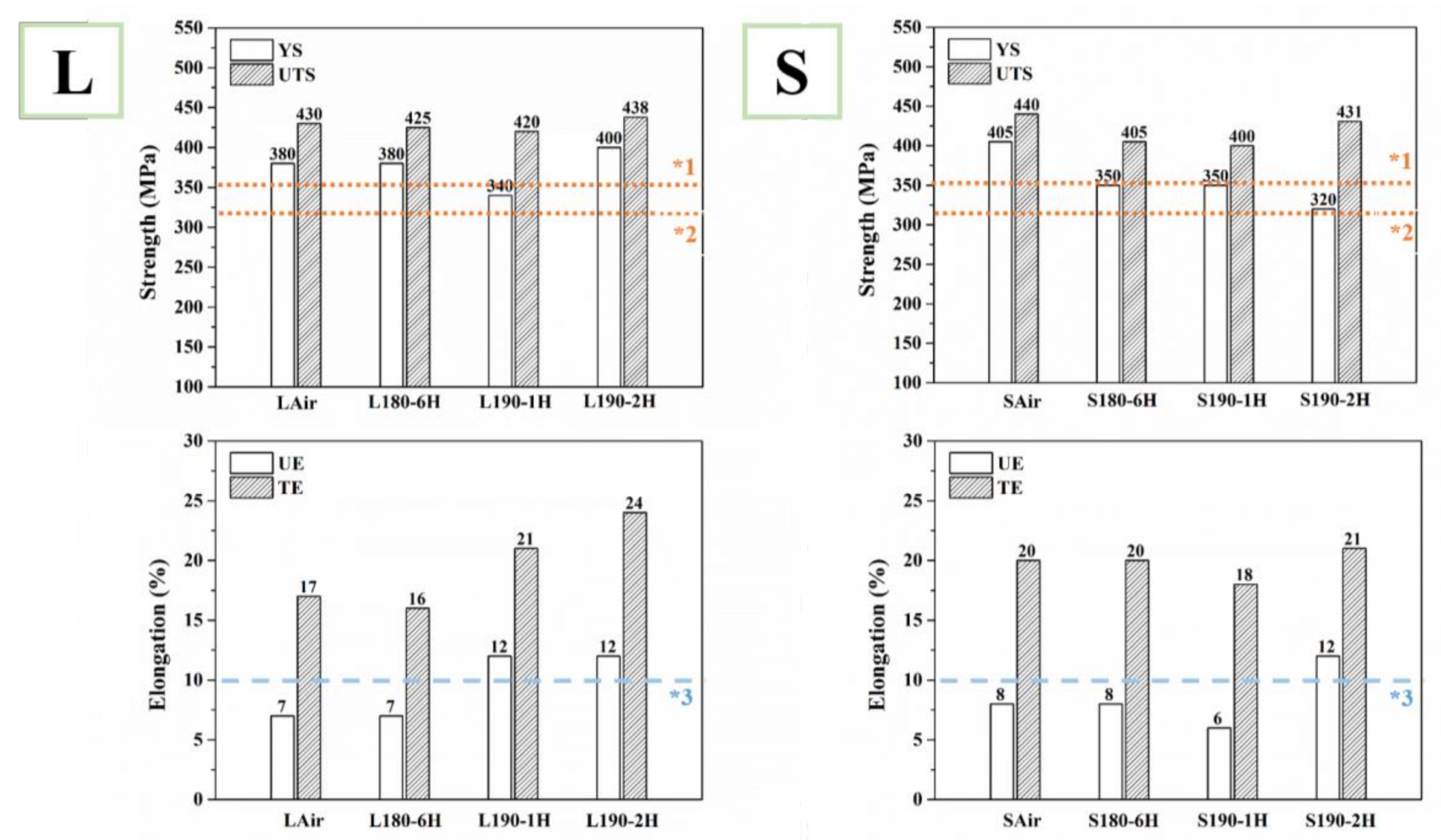
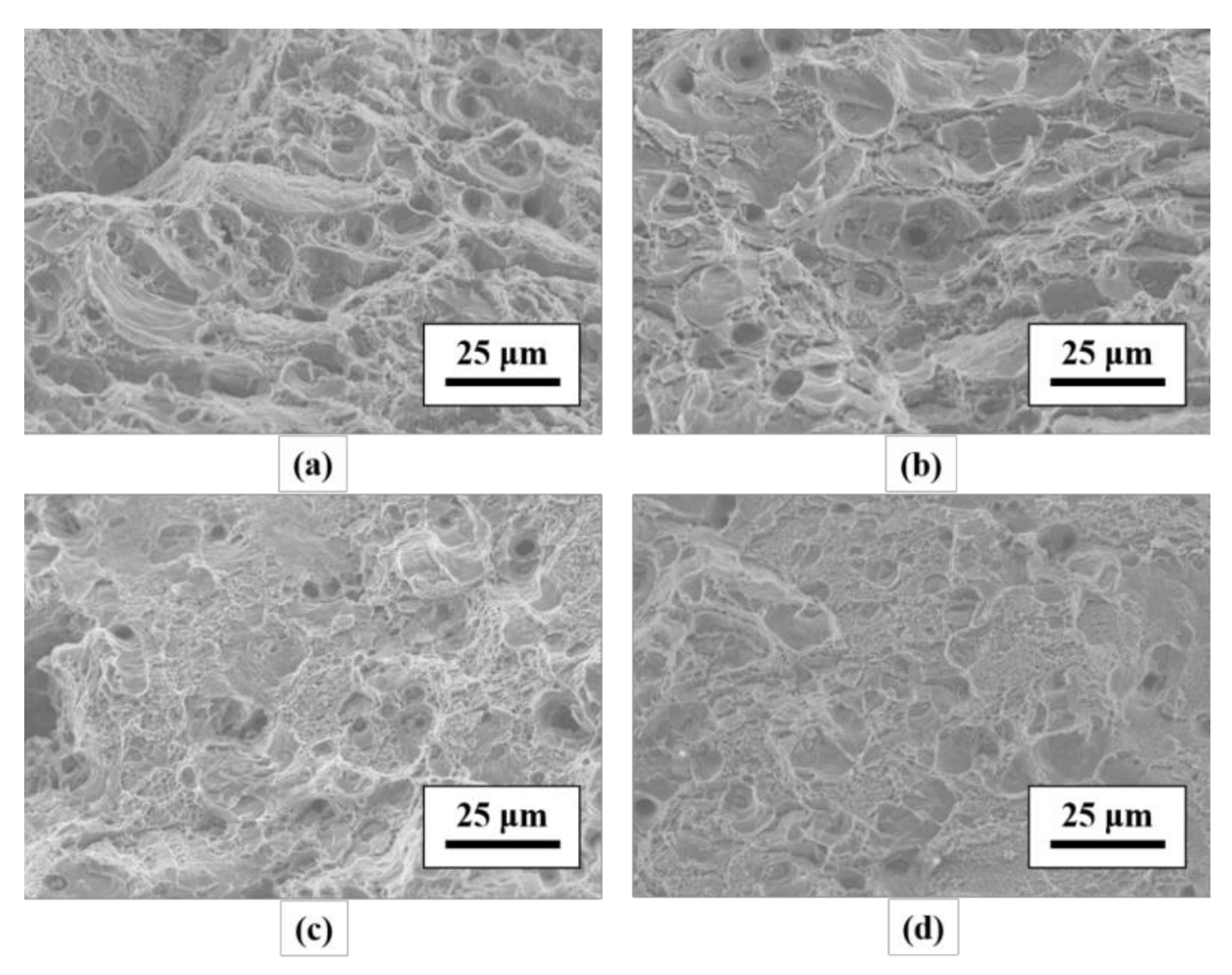
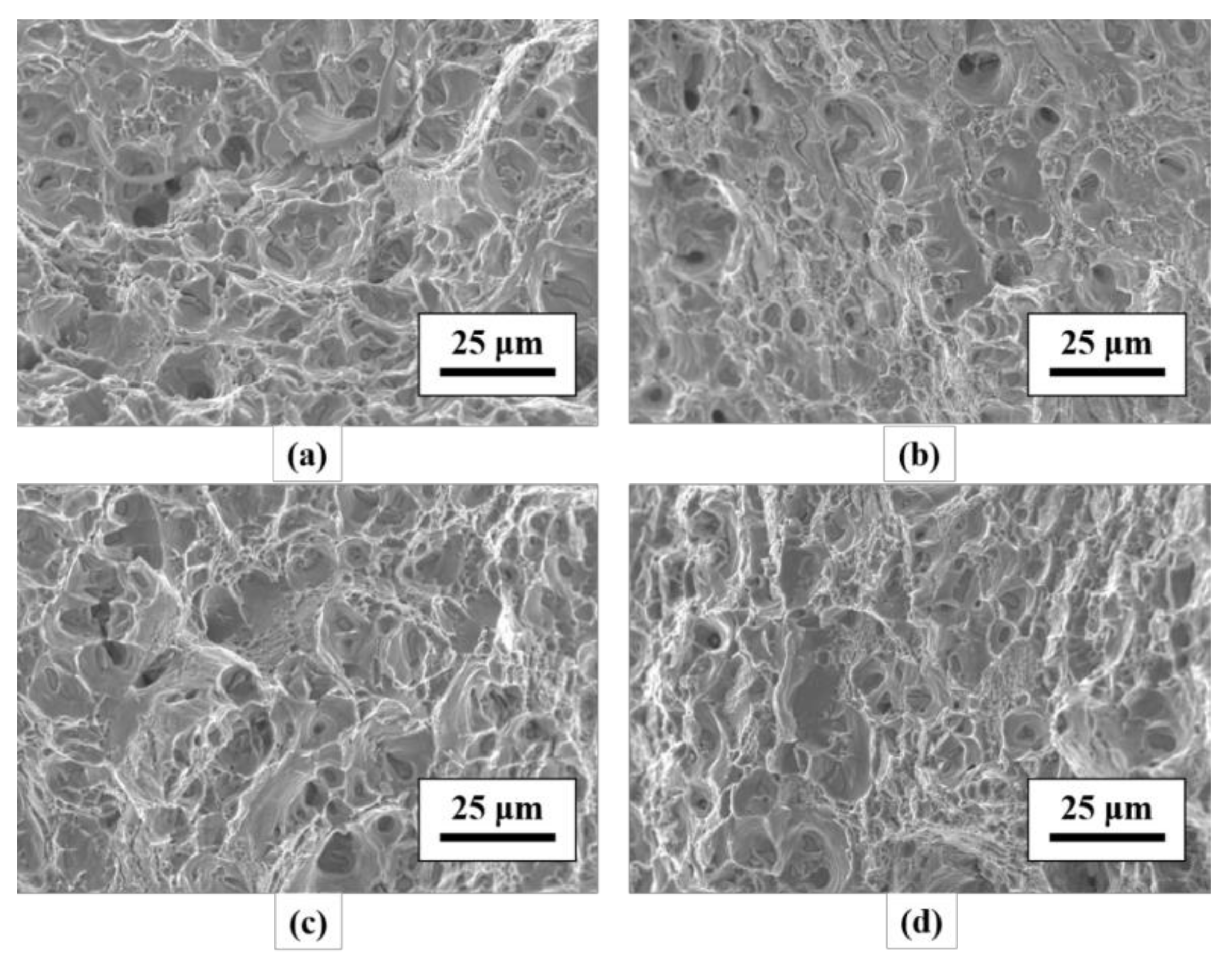
3.2. Tensile Mechanical Properties and Fracture Observation
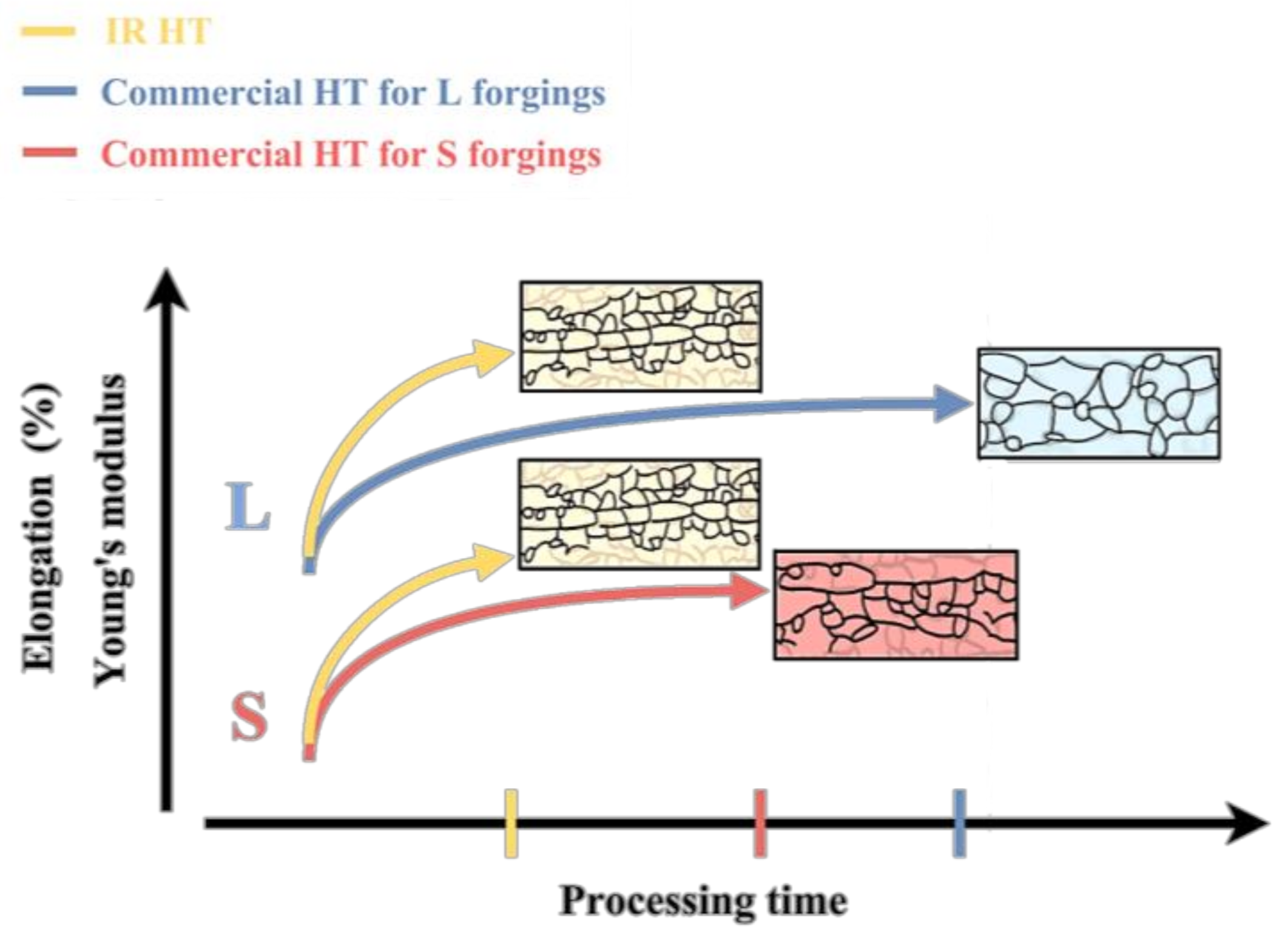
3.3. Young’s Modulus Rise Effect
4. Conclusions
- The variability in the mechanical properties of L and S primarily affected by different forging processes can be reduced through an IR heat treatment. An air furnace heat treatment can’t achieve the same results.
- For IR heat treatment specimens, the best IR heat treatment condition for L and S forging is a solution heat treatment for 30 min at 560 °C and artificial aging for 2 h at 190 °C. The tensile strength was shown to be similar to that of the air furnace heat treated specimens, and the non-equiaxed grains remained aligned in the remaining metal flows, which enhanced the elongation.
- Using an IR heat treatment can curtail the duration of the heat treatment, which showed a striking benefit, according to the Young’s modulus. The Young’s modulus values for the IR heat treatment specimens were higher than those for the air furnace heat treatment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Das, S.K. Designing aluminium alloys for a recycling friendly world. Mater. Sci. Forum 2006, 519, 1239–1244. [Google Scholar] [CrossRef]
- Birol, Y.; Ilgaz, O. Effect of cast and extruded stock on grain structure of EN AW 6082 alloy forgings. Mater. Sci. Technol. 2014, 30, 860–866. [Google Scholar] [CrossRef]
- Birol, Y.; Gokcil, E. Processing of high strength EN AW 6082 forgings without a solution heat treatment. Mater. Sci. Eng. A-Struct. Mater. 2016, 674, 25–32. [Google Scholar] [CrossRef]
- Birol, Y. Effect of extrusion press exit temperature and chromium on grain structure of EN AW 6082 alloy forgings. Mater. Sci. Technol. 2015, 31, 207–211. [Google Scholar] [CrossRef]
- Mrówka-Nowotnik, G.; Sieniawski, J. Influence of heat treatment on the microstructure and mechanical properties of 6005 and 6082 aluminium alloys. J. Mater. Process. Technol. 2005, 162, 367–372. [Google Scholar] [CrossRef]
- Vamadevan, G.; Kraft, F. Application of Rapid Infrared Heating for Processing of Aluminum Forgings. In The James Morris Honorary Symposium on Aluminum Wrought Products for Automotive, Packing, and Other Applications; The Minerals, Metals & Materials Society: Santa Barbara, CA, USA, 2006. [Google Scholar]
- Kervick, R.; Blue, C. Enhancement of Aluminum Alloy Forgings through Rapid Billet Heating; KomTeK: Worcester, MA, USA, 2006. [Google Scholar]
- Lu, H.; Kadolkar, P.B. Control of Grain Size and Age Hardening in AA2618 Forgings Processed by Rapid Infrared Radiant Heating. TMS Lett. 2004, 1, 47–48. [Google Scholar]
- Chang, Y.L.; Hung, F.Y. Study of microstructure and tensile properties of infrared-heat-treated cast-forged 6082 aluminum alloy. JMRT 2017, in press. [Google Scholar] [CrossRef]
- Xie, C.; Schaller, R. High damping capacity after precipitation in some commercial aluminum alloys. Mater. Sci. Eng. A-Struct. Mater. 1998, 252, 78–84. [Google Scholar] [CrossRef]
- Reeb, A.; Merzkirch, M. Heat treatment during composite extruded spring steel wire reinforced EN AW-6082. J. Mater. Process. Technol. 2016, 229, 1–8. [Google Scholar] [CrossRef]
- Chang, Y.L.; Hung, F.Y. Enhancing the tensile yield strength of A6082 aluminum alloy with rapid heat solutionizing. Mater. Sci. Eng. A-Struct. Mater. 2017, 702, 438–445. [Google Scholar] [CrossRef]
- Birol, Y. The effect of processing and Mn content on the T5 and T6 properties of AA6082 profiles. J. Mater. Process. Technol. 2006, 173, 84–91. [Google Scholar] [CrossRef]
- Birol, Y.; Ilgaz, O. Comparison of cast and extruded stock for the forging of AA6082 alloy suspension parts. Adv. Mater. Res. 2014, 939, 299–304. [Google Scholar] [CrossRef]
- Mrówka-Nowotnik, G.; Sieniawski, J. Effect of heat treatment on tensile and fracture toughness properties of 6082 alloy. J. Achieve Mater. Manuf. Eng. 2009, 32, 162–170. [Google Scholar]
- Meredith, M.; Worth, J. Intermetallic phase selection during solidification of Al-Fe-Si (-Mg) alloys. In Mater Sci Forum; Trans Tech Publications: Zürich, Switzerland, 2002; Volume 107–112. [Google Scholar]
- Brito, C.; Costa, T.A. Characterization of dendritic microstructure, intermetallic phases, and hardness of directionally solidified Al-Mg and Al-Mg-Si alloys. Metall. Mater. Trans. A 2015, 46, 3342–3355. [Google Scholar] [CrossRef]
- Birol, Y. Effect of cooling rate on precipitation during homogenization cooling in an excess silicon AlMgSi alloy. Mater. Charact. 2012, 73, 37–42. [Google Scholar] [CrossRef]
- Santos, J.; Gouveia, R.M. Designing a new sustainable approach to the change for lightweight materials in structural components used in truck industry. J. Clean. Prod. 2017, 164, 115–123. [Google Scholar] [CrossRef]
- Nakamura, H.; Nishibata, M. Globalization of Aluminum Forging Automotive Suspension Business: Establishment of Production Bases in Japan, USA and China. “R&D” Kobe Steel Eng. Rep. 2017, 66, 99–102. [Google Scholar]
- Hosoda, N.; Nakai, M. The Effect of Microstructure on Mechanical Properties of Forged 6061 Aluminum Alloy. Mater. Forum 2004, 28, 1382–1387. [Google Scholar]
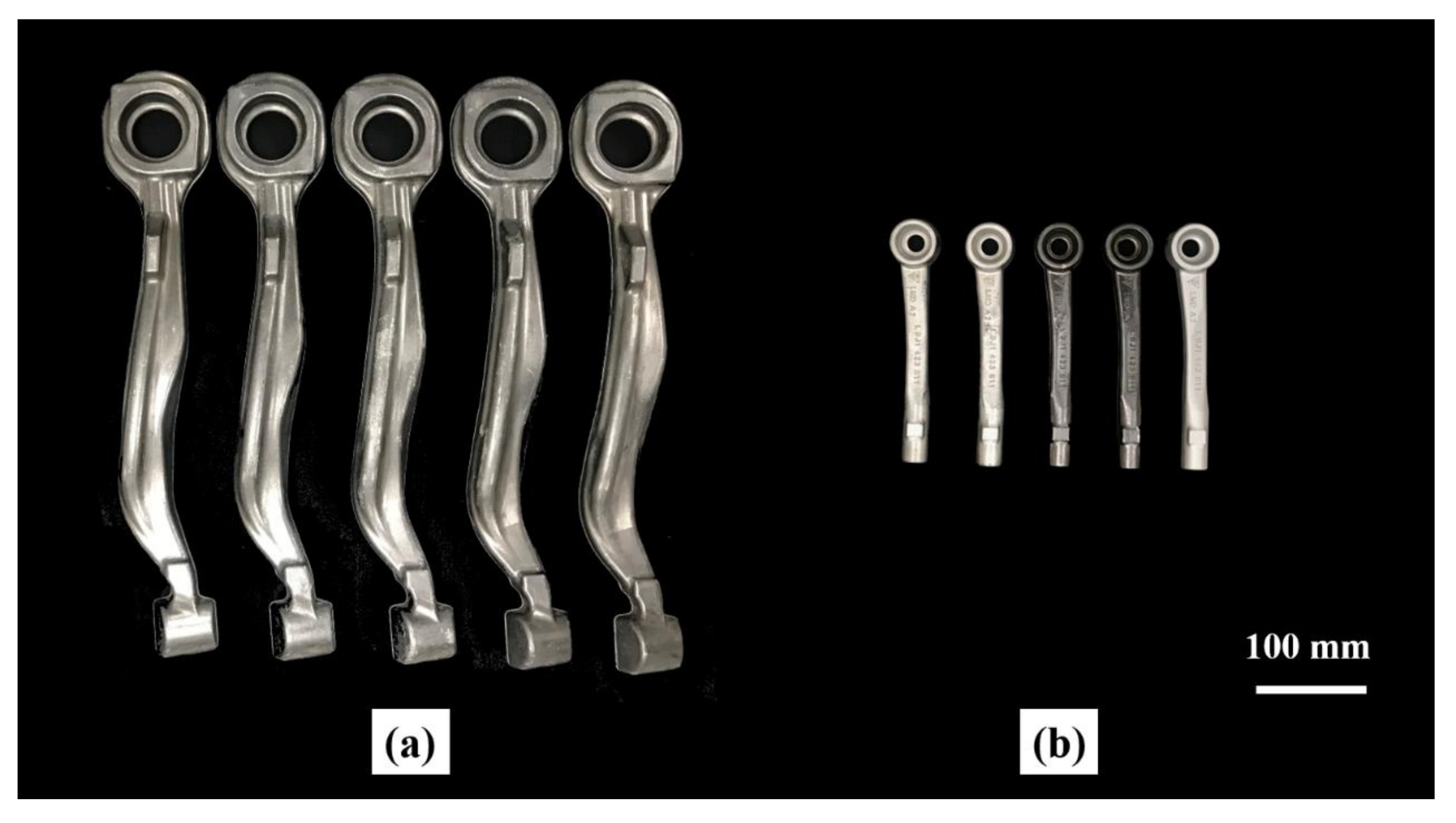

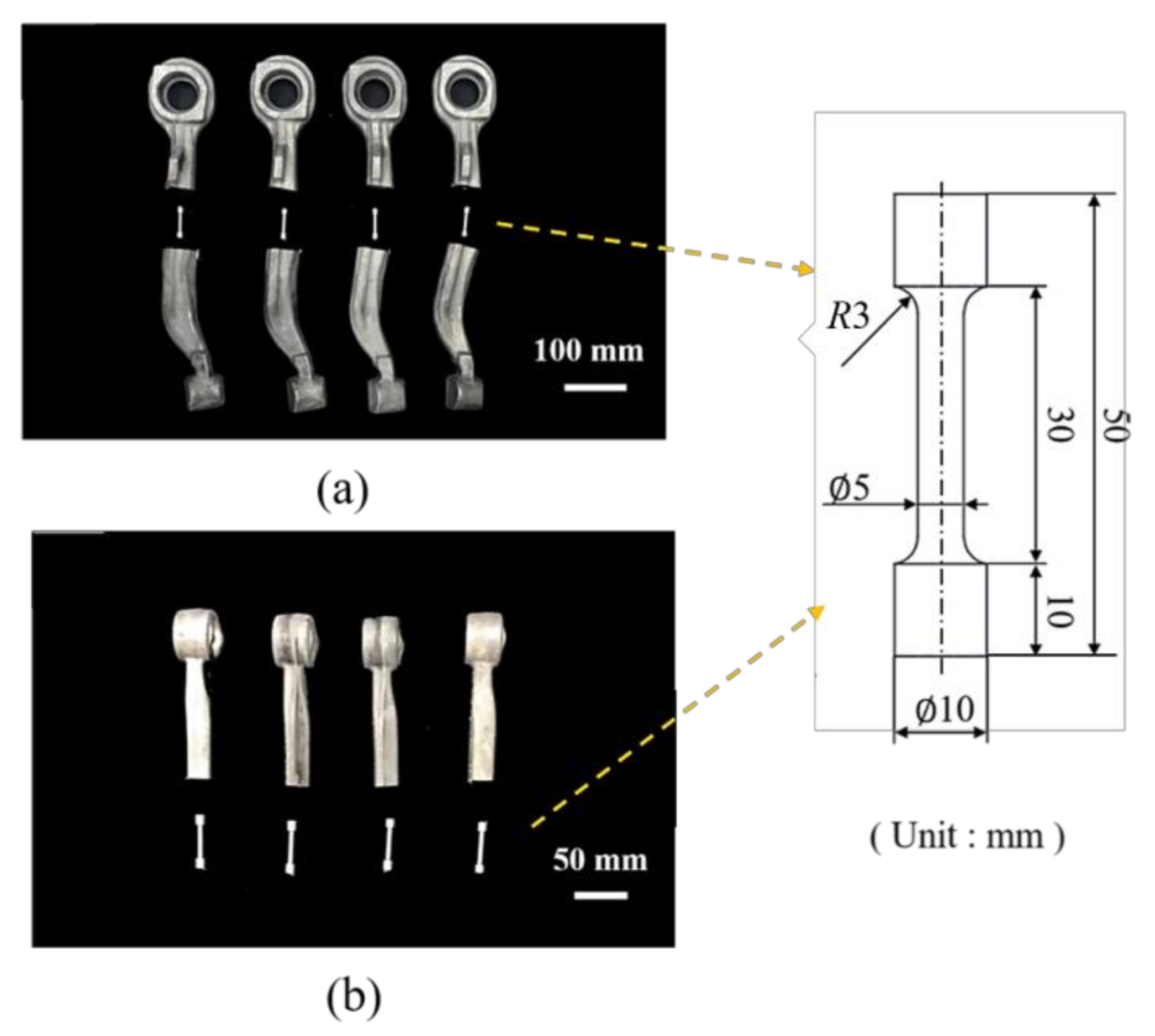
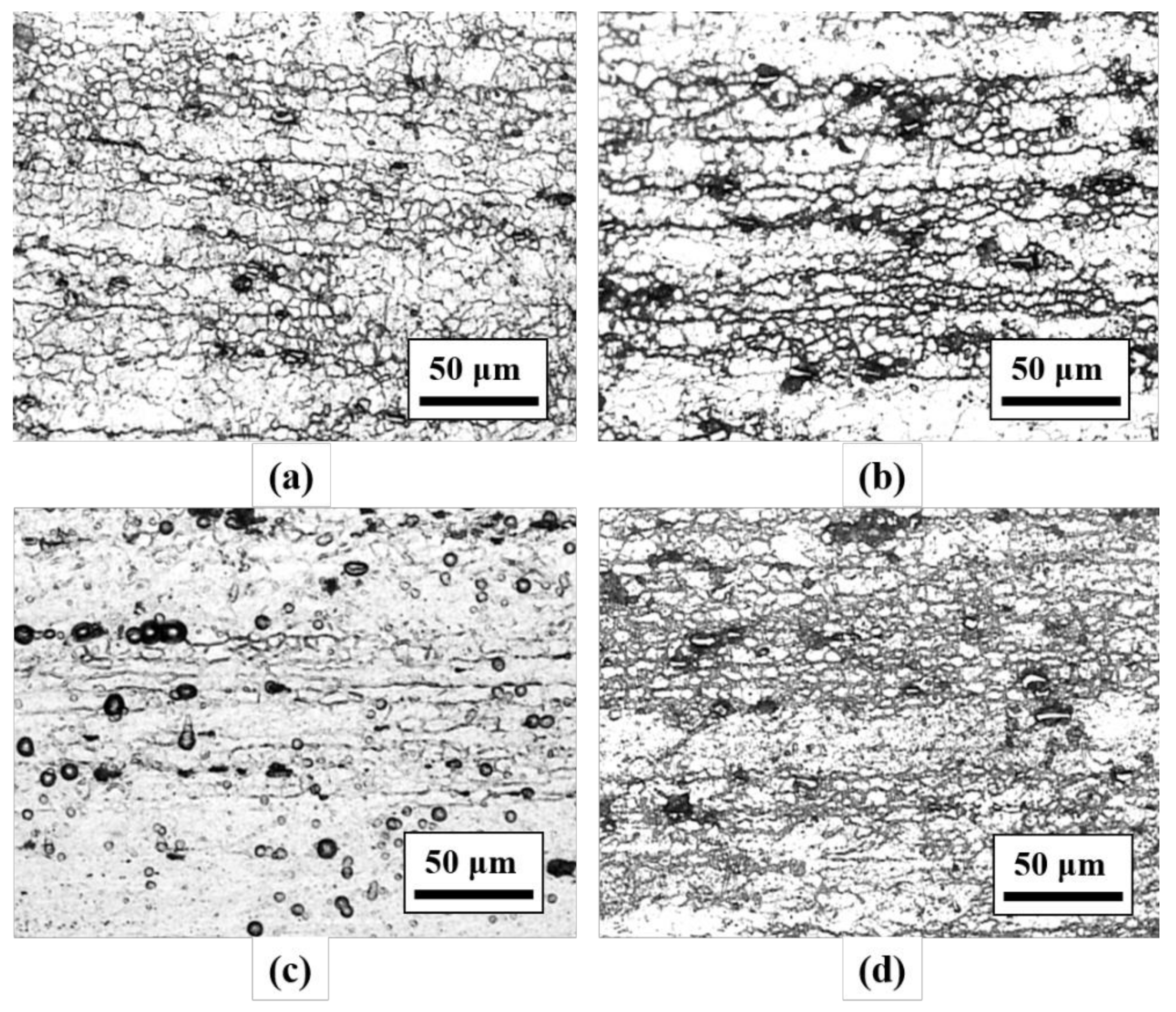
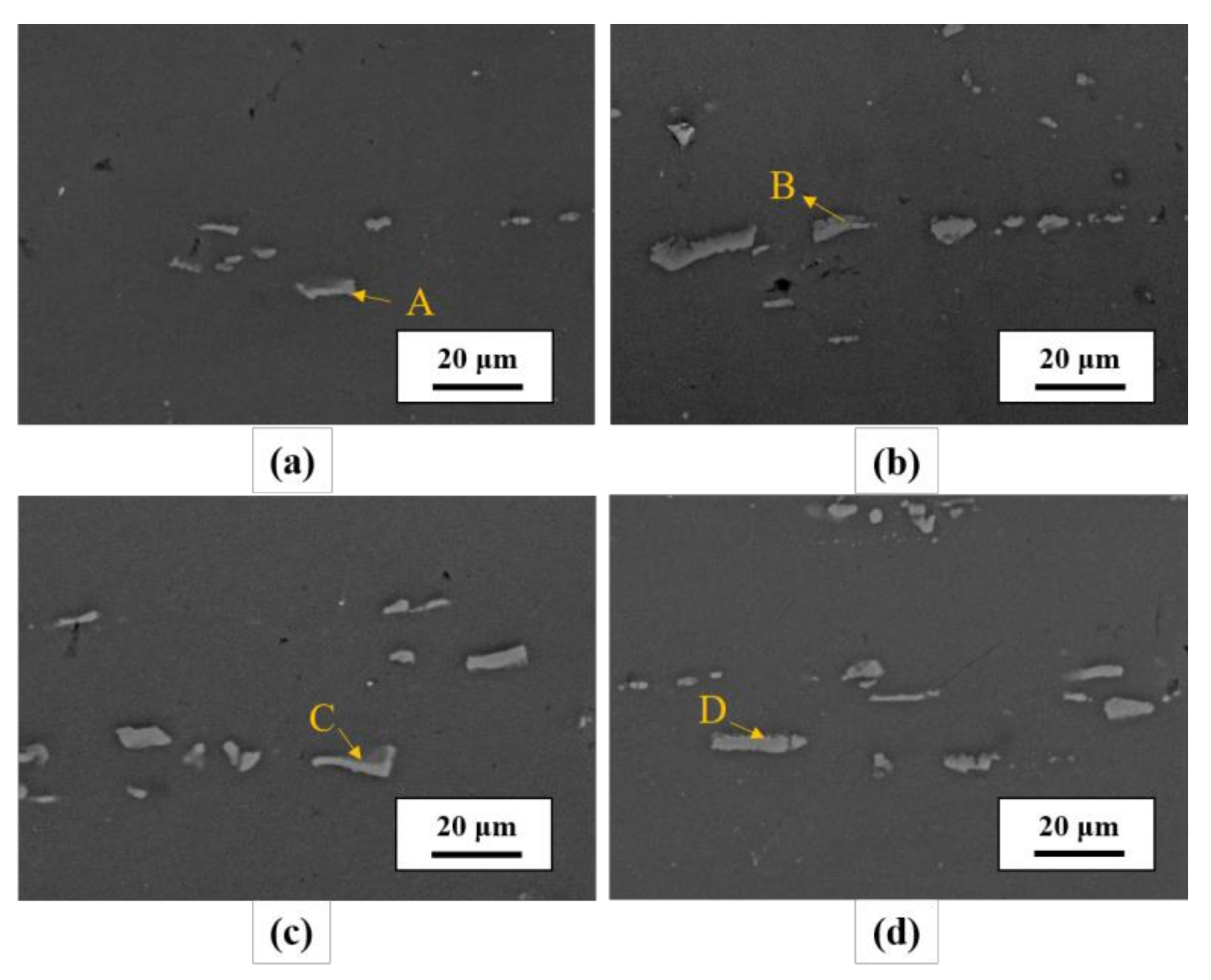
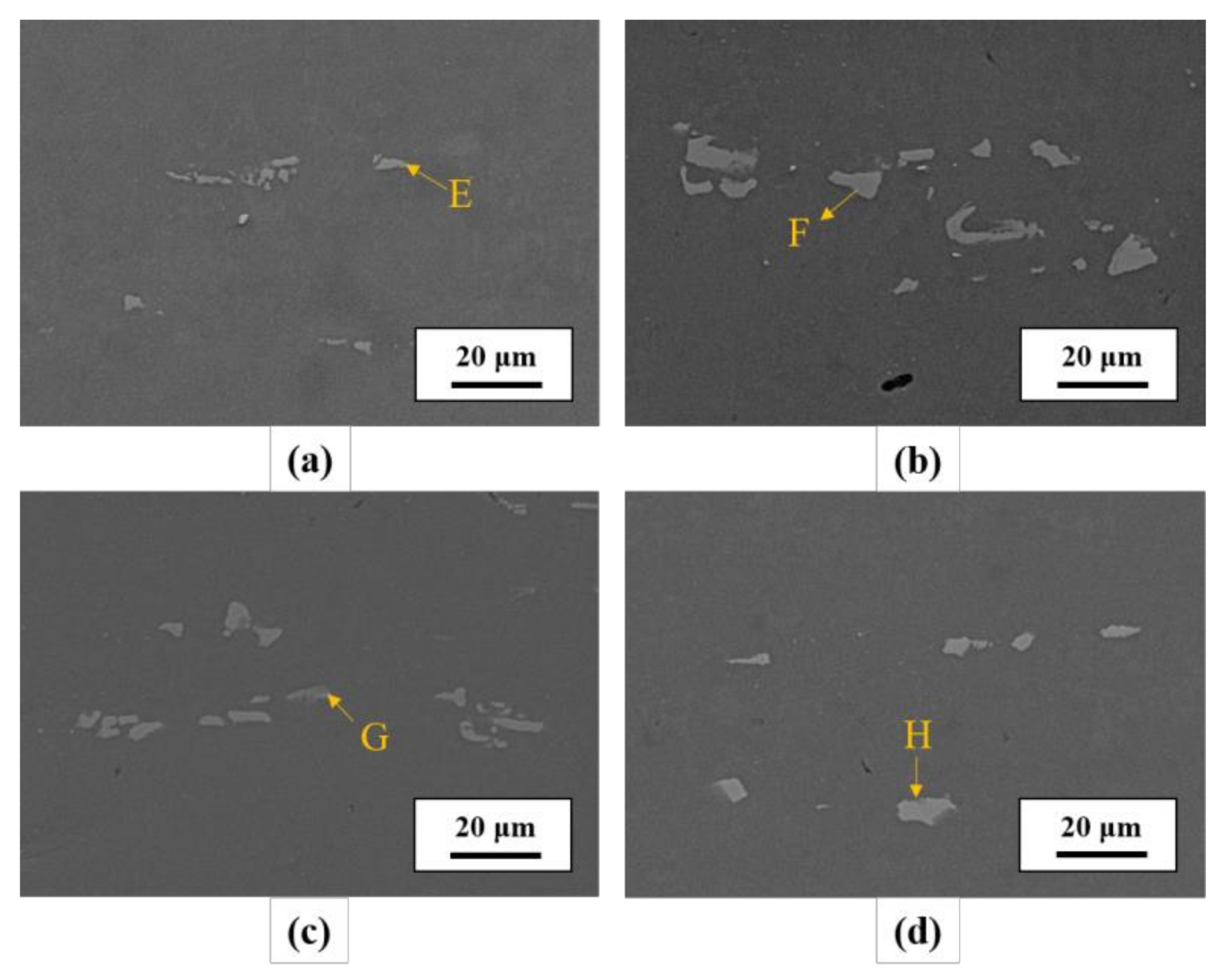
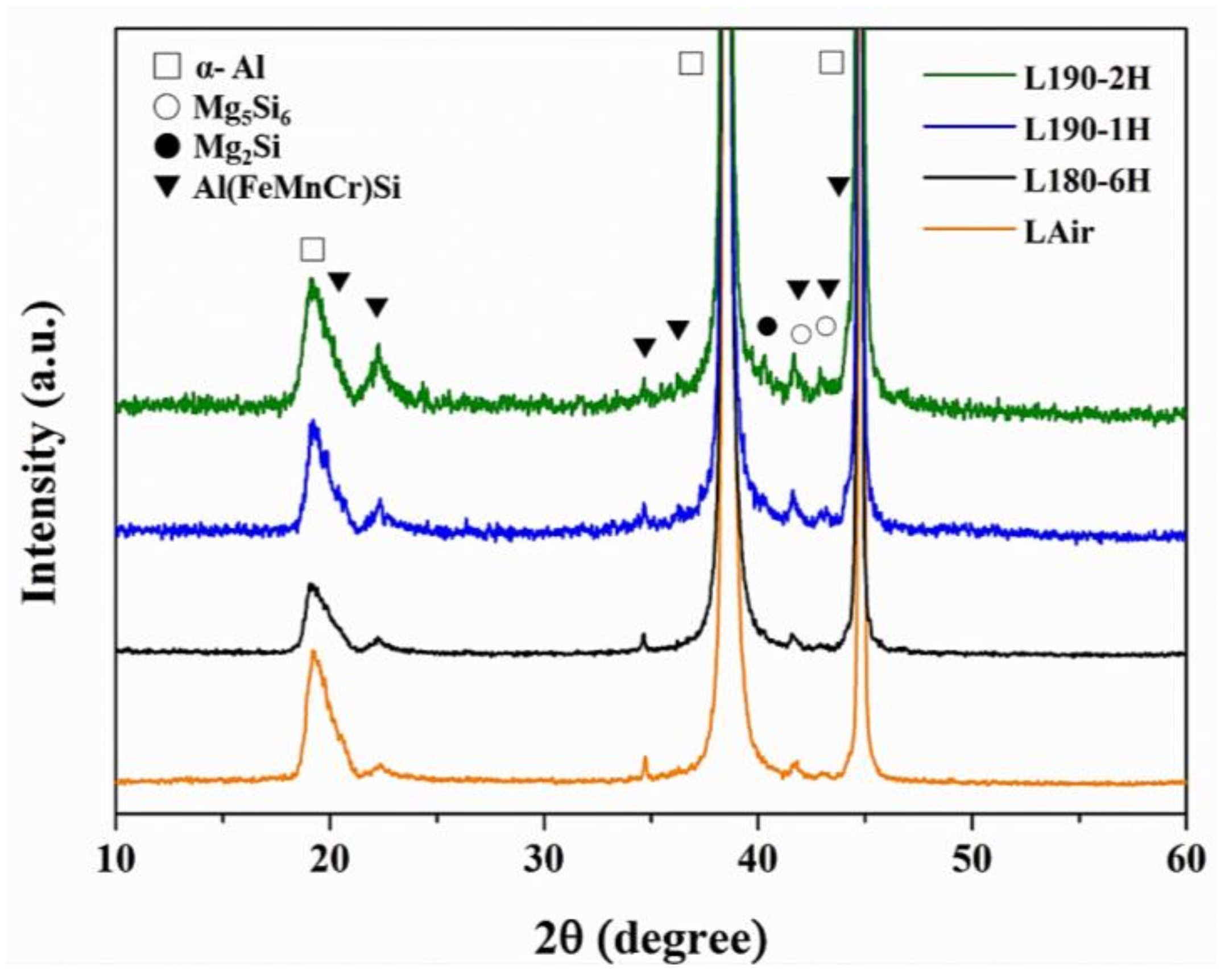

| Element | Mg | Si | Cu | Mn | Cr | Fe | Al |
|---|---|---|---|---|---|---|---|
| wt. % | 0.97 | 1.2 | 0.06 | 0.86 | 0.16 | 0.18 | Bal. |
| Sample Condition | Solution Treatment | Artificial Aging | Code |
|---|---|---|---|
| Forging L | Air, 540 °C, 2 h | Air, 175 °C, 2 h | LAir |
| IR, 560 °C, 30 min. | IR, 180 °C, 6 h | L180-6H | |
| IR, 560 °C, 30 min. | IR, 190 °C, 1 h | L190-1H | |
| IR, 560 °C, 30 min. | IR, 190 °C, 2 h | L190-2H | |
| Forging S | Air, 525 °C, 1 h | Air, 175 °C, 6 h | SAir |
| IR, 560 °C, 30 min. | IR, 180 °C, 6 h | S180-6H | |
| IR, 560 °C, 30 min. | IR, 190 °C, 1 h | S190-1H | |
| IR, 560 °C, 30 min. | IR, 190 °C, 2 h | S190-2H |
| Forgings | L Forgings | S Forgings | ||||||
|---|---|---|---|---|---|---|---|---|
| Element at. % | A | B | C | D | E | F | G | H |
| Fe | 2.52 | 4.67 | 5.86 | 5.76 | 4.79 | 7.01 | 6.62 | 8.17 |
| Cu | 0.47 | 0.68 | 0.38 | 0.49 | 1.14 | 1.42 | 1.37 | 1.63 |
| Mg | 1.60 | 0.88 | 0.55 | 1.01 | 1.71 | 1.18 | 0.91 | 1.31 |
| Al | 81.18 | 74.49 | 73.23 | 70.52 | 82.67 | 75.35 | 77.06 | 73.26 |
| Si | 6.53 | 9.55 | 11.24 | 9.65 | 5.65 | 7.91 | 7.86 | 8.46 |
| Cr | 2.42 | 2.66 | 1.98 | 3.43 | 0.75 | 0.87 | 0.70 | 1.16 |
| Mn | 5.28 | 7.07 | 6.76 | 9.14 | 3.29 | 6.25 | 5.48 | 6.01 |
| Sample | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Uniform Elongation (%) | Total Elongation (%) |
|---|---|---|---|---|
| LAir | 380 | 430 | 7 | 17 |
| L180-6H | 380 | 425 | 7 | 16 |
| L190-1H | 340 | 419 | 12 | 21 |
| L190-2H | 400 | 438 | 12 | 24 |
| SAir | 405 | 440 | 8 | 20 |
| S180-6H | 350 | 405 | 8 | 20 |
| S190-1H | 350 | 400 | 6 | 18 |
| S190-2H | 320 | 431 | 12 | 21 |
| Sample | Young’s Modulus σ/ε (GPa) | Comparison with Air Furnace Heat Treatment Specimens |
|---|---|---|
| LAir | 22.3 | - |
| L180-6H | 32.4 | ↑45.3% |
| L190-1H | 29.0 | ↑30.0% |
| L190-2H | 32.2 | ↑44.4% |
| SAir | 21.9 | - |
| S180-6H | 31.3 | ↑42.9% |
| S190-1H | 29.1 | ↑32.6% |
| S190-2H | 29.7 | ↑35.6% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-L.; Hung, F.-Y.; Lui, T.-S. Enhancement of the Young’s Modulus through Infrared Heat Treatment: A Study of the Microstructure and the Mass Effect of Real Body 6082 Aluminum Forgings. Metals 2018, 8, 239. https://doi.org/10.3390/met8040239
Chang Y-L, Hung F-Y, Lui T-S. Enhancement of the Young’s Modulus through Infrared Heat Treatment: A Study of the Microstructure and the Mass Effect of Real Body 6082 Aluminum Forgings. Metals. 2018; 8(4):239. https://doi.org/10.3390/met8040239
Chicago/Turabian StyleChang, Yi-Ling, Fei-Yi Hung, and Truan-Sheng Lui. 2018. "Enhancement of the Young’s Modulus through Infrared Heat Treatment: A Study of the Microstructure and the Mass Effect of Real Body 6082 Aluminum Forgings" Metals 8, no. 4: 239. https://doi.org/10.3390/met8040239





