Precipitation, Recrystallization, and Evolution of Annealing Twins in a Cu-Cr-Zr Alloy
Abstract
:1. Introduction
2. Experiments
3. Results
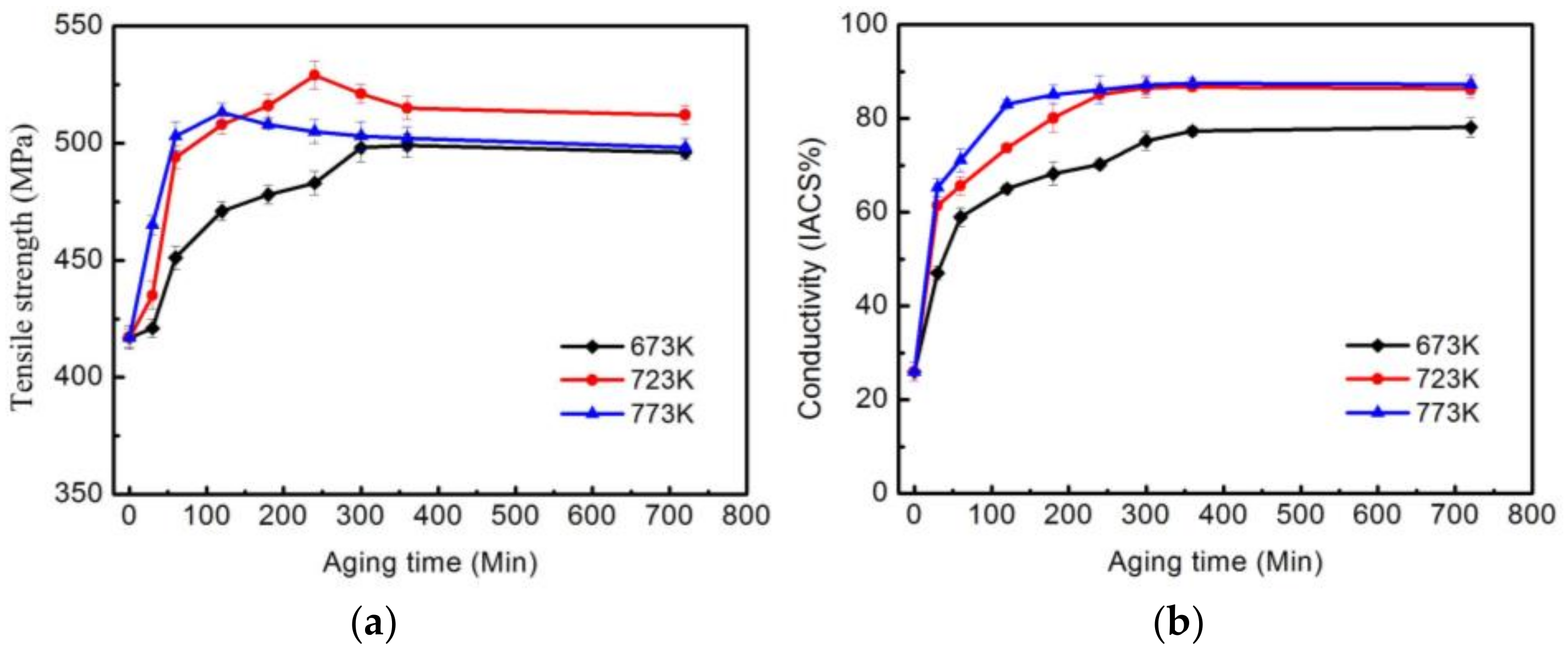
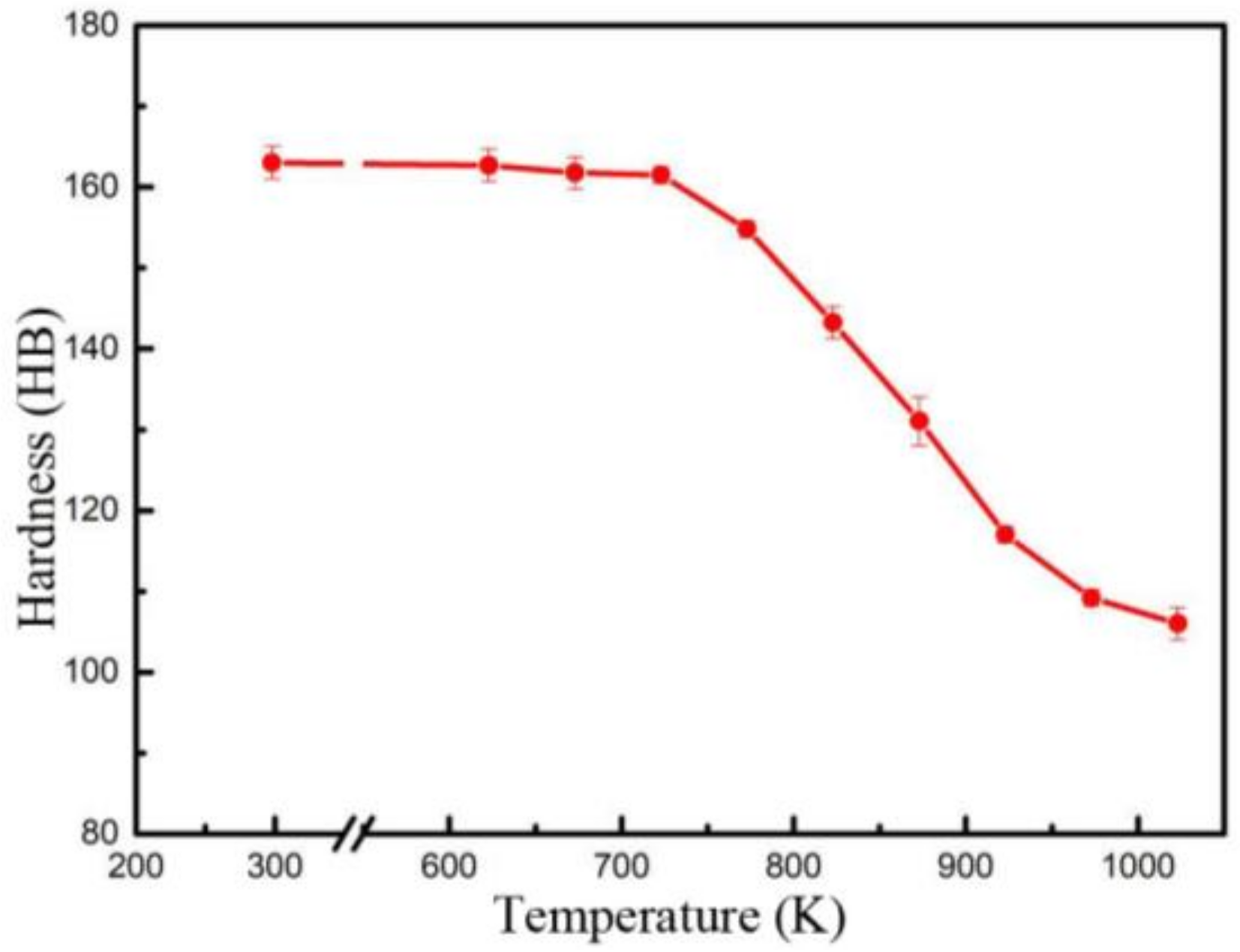
3.1. Properties
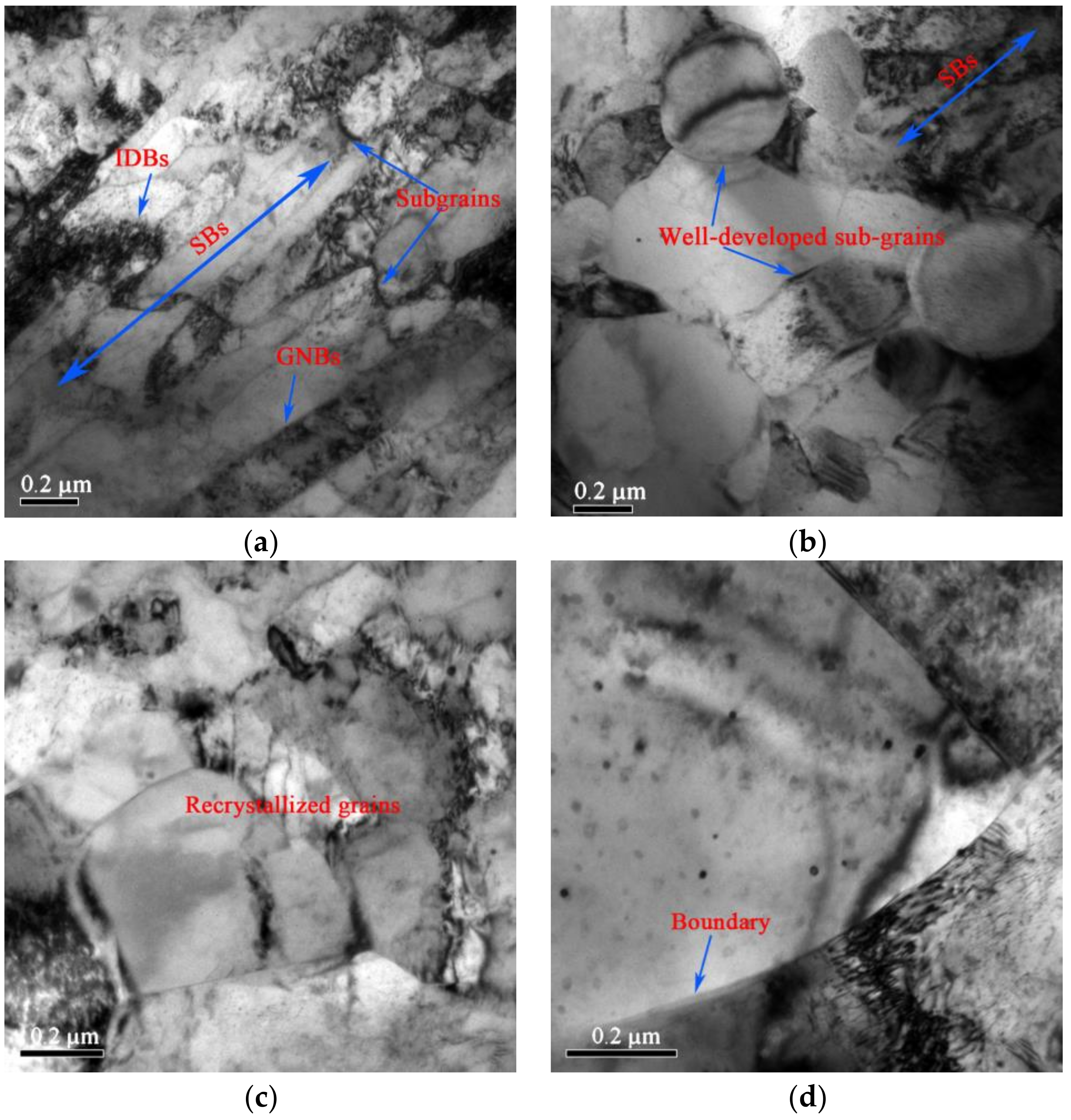
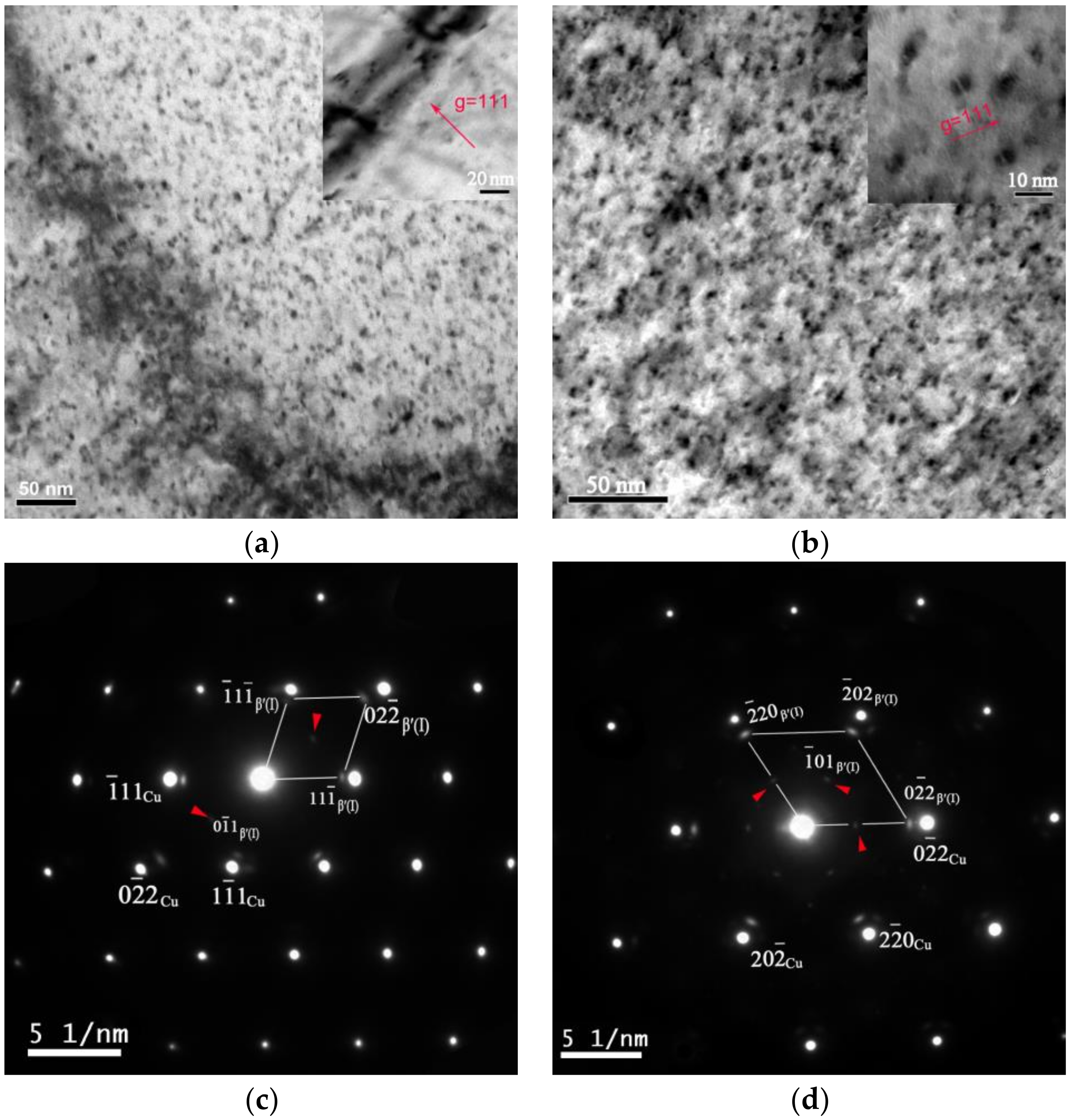
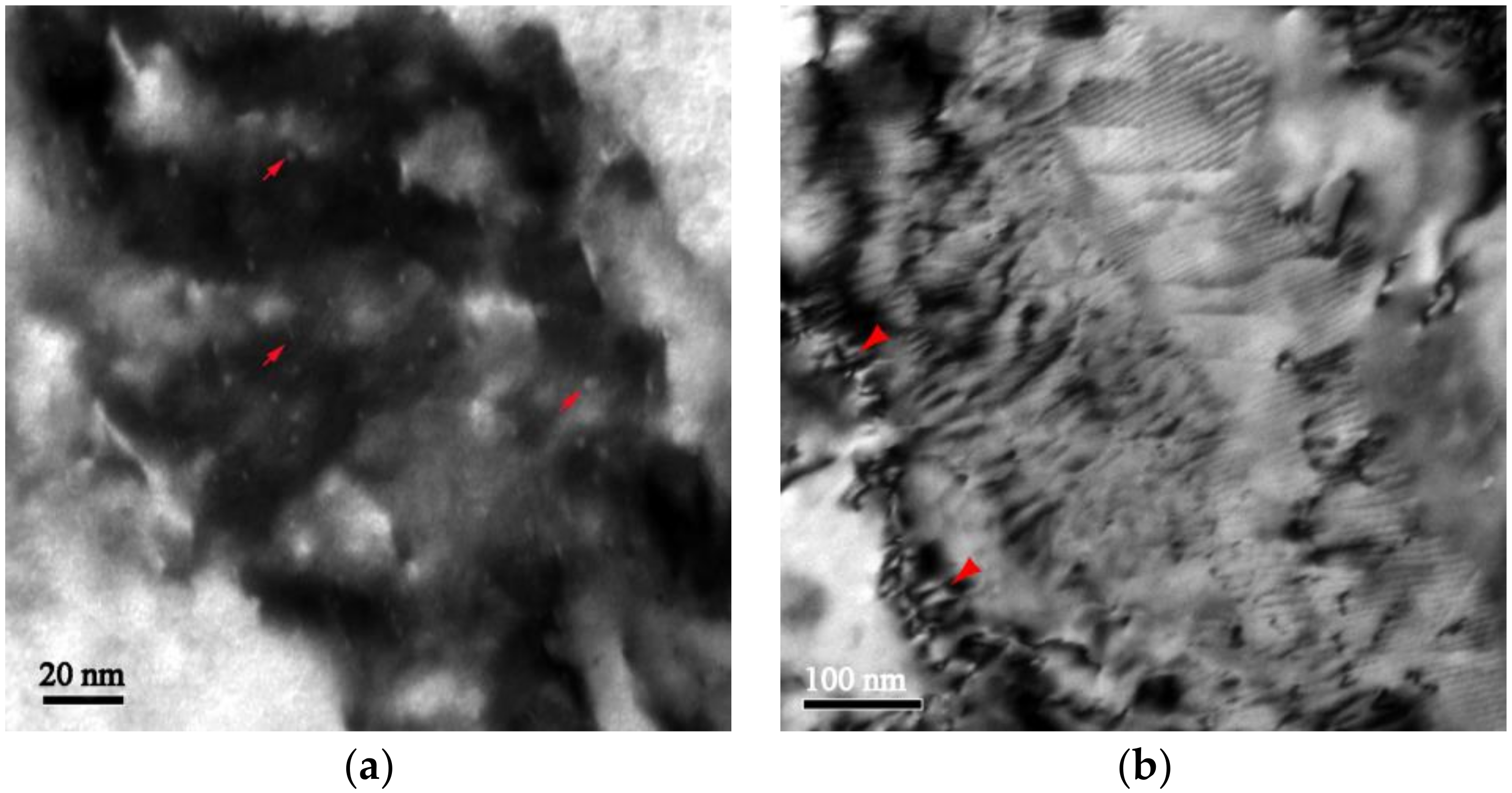
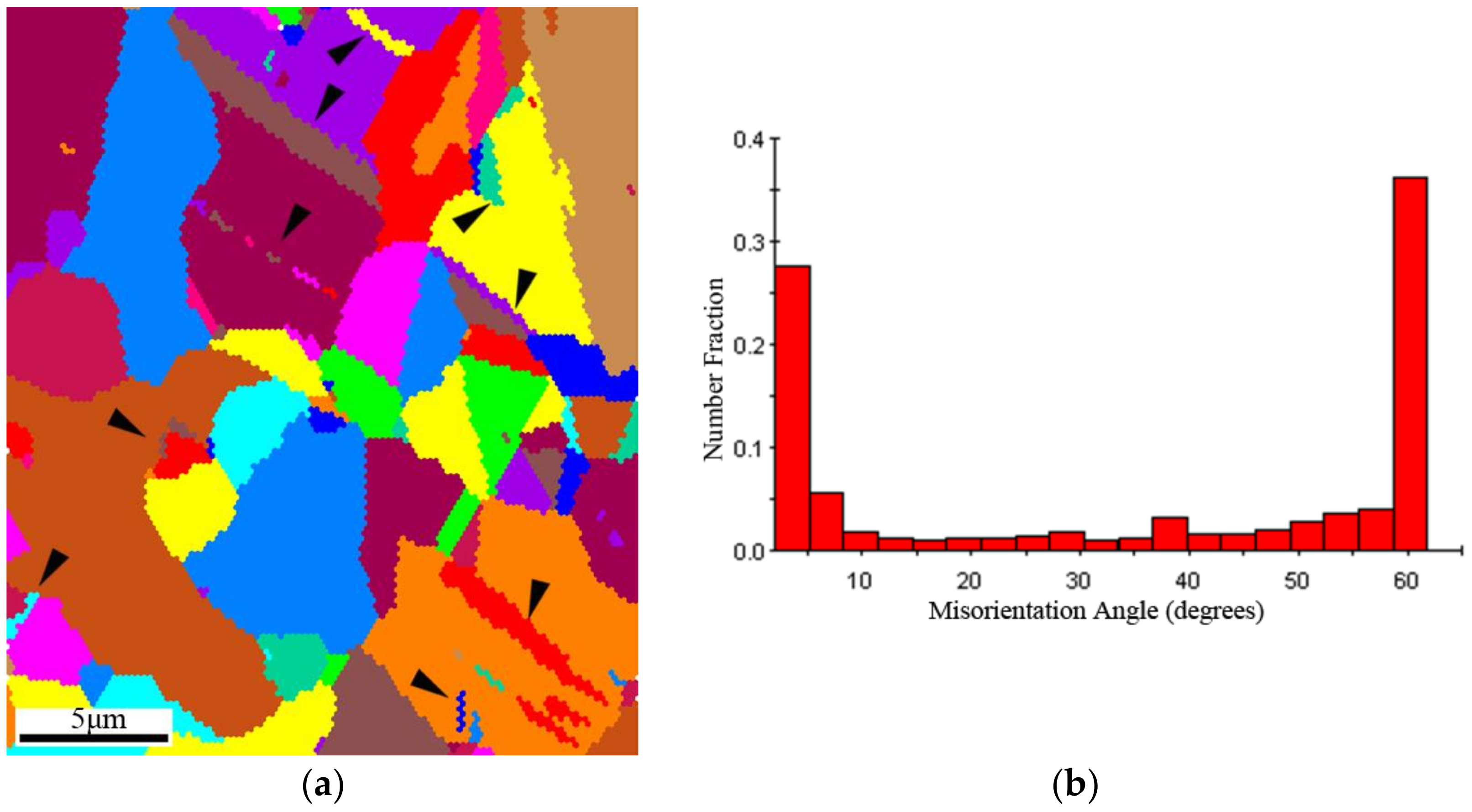
3.2. Microstructure
4. Discussion
4.1. The Influence of the Precipitation on Strength and Conductivity
4.2. The Effect of the Precipitation and Recrystallization Mechanisms on Recrystallization Resistance
5. Conclusions
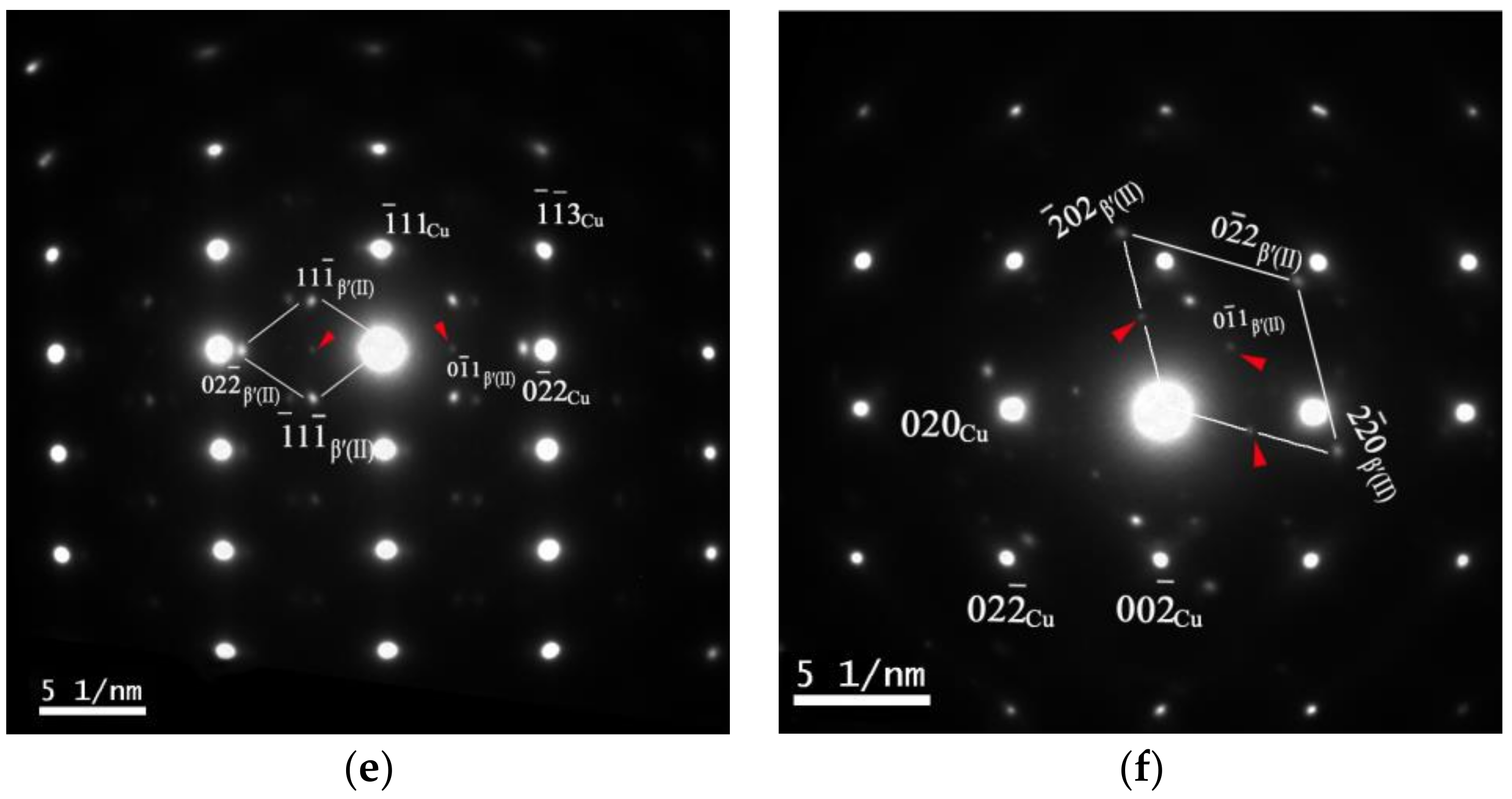
- A Cu-Cr-Zr alloy with a good combination of strength and conductivity can be obtained. The peak aged condition, with a tensile strength of 536 MPa and electron conductivity of 85.3% IACS, can be achieved after the alloy undergoes a solid solution treatment at 1253 K, before being cold-rolled with a reduction ratio of 70% and aged at 723 K for 240 min. The increasing strength and the conductivity are mainly related to the formation of the transition phase β′. A new type of fcc Cr-rich precipitate β′(II) is observed in the peak aged condition, which has an ordered structure and is coherent with the matrix, with the following OR: [011]β′(II)//[211]Cu, {200}β′(II)//{-111}Cu and [111]β′(II)//[100]Cu, {02-2}β′(II)//{02-2}Cu.
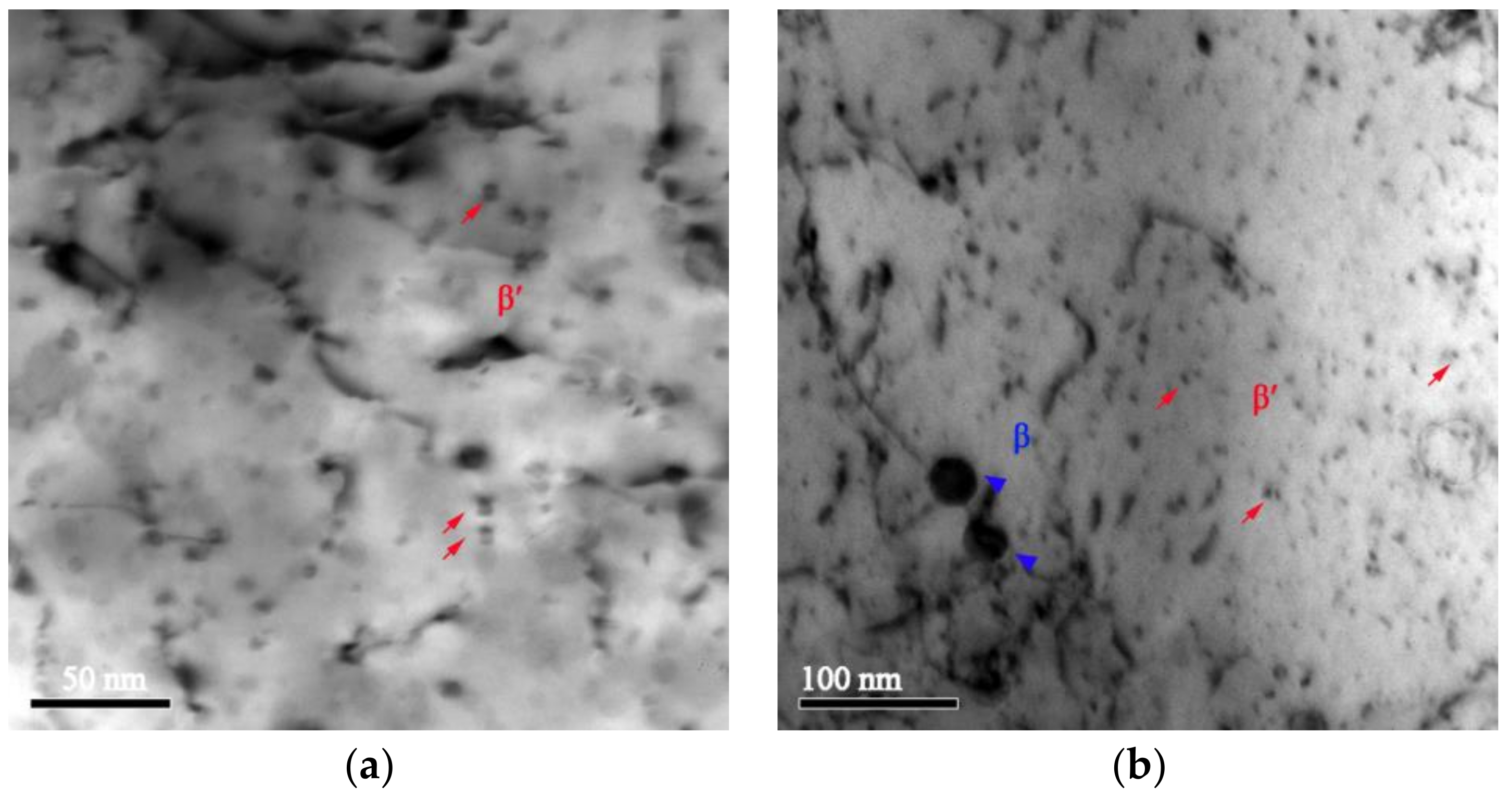
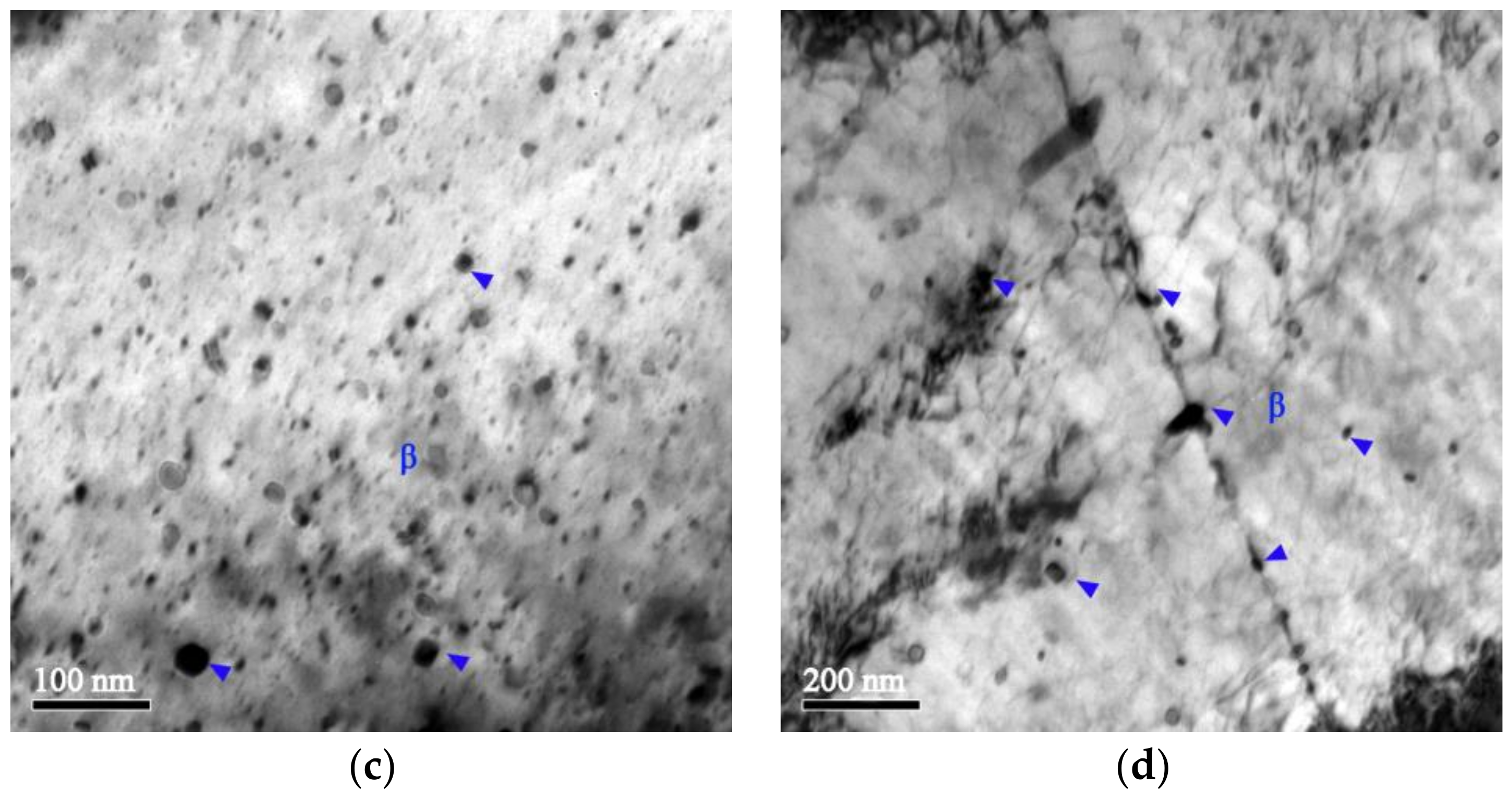
- The Cu-Cr-Zr alloy has a good recrystallization resistance. The dispersed distribution of the β′ phase shows a good thermal stability during annealing, and can pin the dislocations and grain boundaries to hinder their migration, inhibiting recrystallization and thus enhancing the recrystallization resistance. When the β′ particles are replaced by the coarsened β particles, and when there is a reduction in the density of precipitates, the pinning effect on the dislocations and grain boundaries becomes weak, and recrystallization starts to occur in the Cu-Cr-Zr alloy.
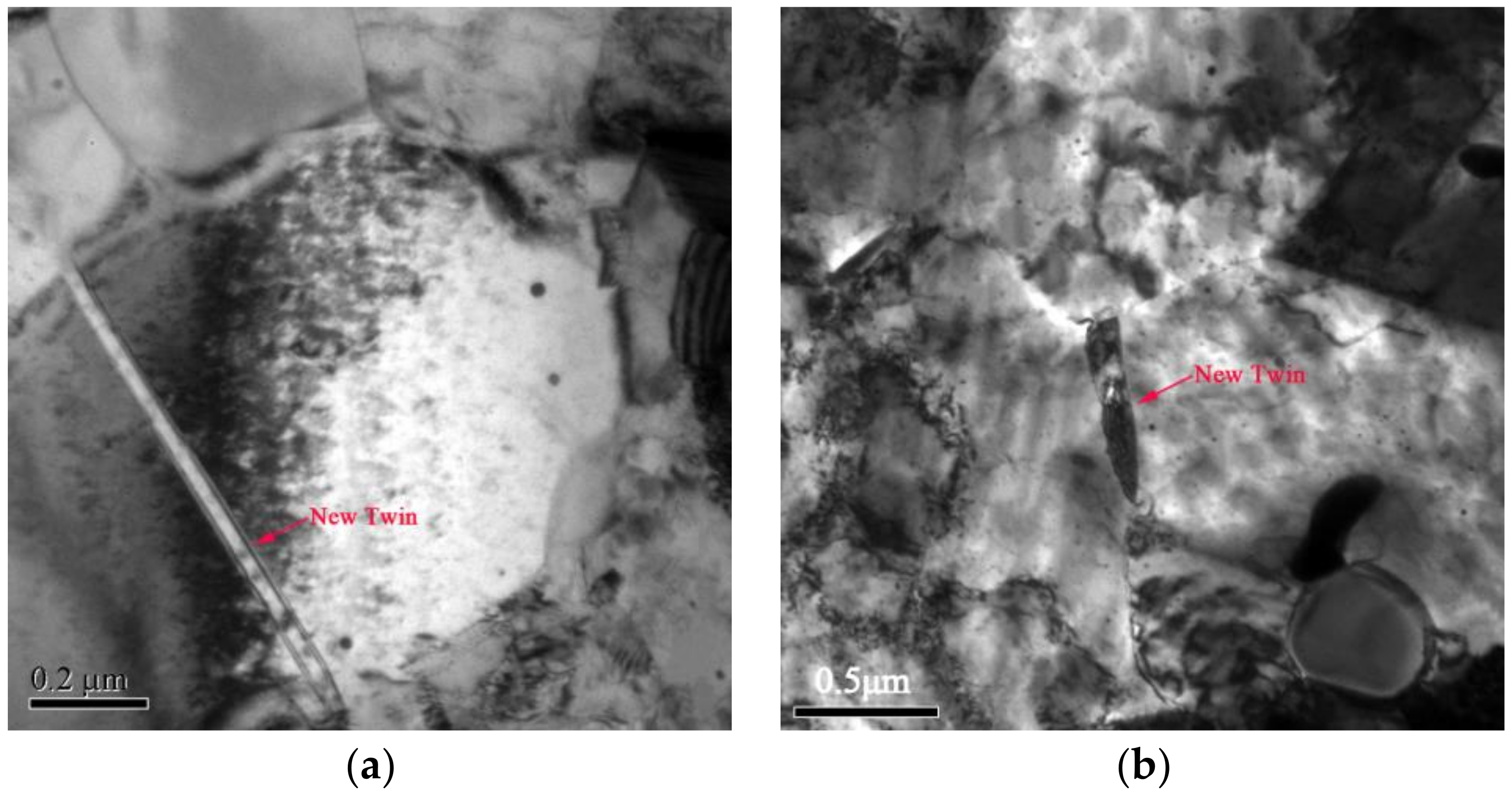
- Recrystallization nucleation and growth first occurs in the shear deformation zone due to its high deformation energy during the recrystallization process. The growth of the recrystallized grains, as well as the decrease in hardness during annealing, is hindered by the formation of annealing twins due to the release of deformation energy and the reduction of the driving force for interface migration.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Igor, A.; Hans-Achim, K.; Mozhgan, G.; Mansour, M.; Lothar, W. Ultrafine-grained precipitation hardened copper alloys by swaging or accumulative roll bonding. Metals 2015, 5, 763–776. [Google Scholar]
- Deng, L.P.; Han, K.; Wang, B.S.; Yang, X.F.; Liu, Q. Thermal stability of Cu–Nb microcomposite wires. Acta Mater. 2015, 101, 181–188. [Google Scholar] [CrossRef]
- Lei, R.S.; Wang, M.P.; Xu, S.Q.; Wang, H.P.; Chen, G.R. Microstructure, hardness evolution, and thermal stability mechanism of mechanical alloyed Cu-Nb alloy during heat treatment. Metals 2016, 6, 194. [Google Scholar] [CrossRef]
- Zhang, X.; Beach, J.A.; Wang, M.; Bellon, P.; Averback, R.S. Precipitation kinetics of dilute Cu-W alloys during low-temperature ion irradiation. Acta Mater. 2016, 120, 46–55. [Google Scholar] [CrossRef]
- Zhang, D.D.; Bai, F.; Wang, Y.; Wang, J.G.; Wang, W.Q. Grain Refinement and Mechanical Properties of Cu-Cr-Zr Alloys with Different Nano-Sized TiCp Addition. Materials 2017, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Alessandro, F.A.; Carlo, A.B.; Ausonio, T. Synthesis and structural analysis of Copper-Zirconium oxide. Metals 2016, 6, 195. [Google Scholar] [CrossRef]
- Correia, J.B.; Davies, H.A.; Sellars, C.M. Strengthening in rapidly solidified age hardened Cu-Cr and Cu-Cr-Zr alloys. Acta Mater. 1997, 45, 177–190. [Google Scholar] [CrossRef]
- Morozova, A.; Borodin, E.; Bratov, V.; Zherebtsov, S.; Belyakov, A.; Kaibyshev, R. Grain Refinement Kinetics in a Low Alloyed Cu-Cr-Zr Alloy Subjected to Large Strain Deformation. Materials 2017, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Hauf, U.; Kauffmann, A.; Kauffmann-Weiss, S.; Feilbach, A.; Boening, M.; Mueller, F.E.H.; Hinrichsen, V.; Heilmaier, M. Microstructure Formation and Resistivity Change in CuCr during Rapid Solidification. Metals 2017, 7, 478. [Google Scholar] [CrossRef]
- Chbihi, A.; Sauvage, X.; Blavette, D. Atomic scale investigation of Cr precipitation in copper. Acta Mater. 2012, 60, 4575–4585. [Google Scholar] [CrossRef]
- Dobatkin, S.V.; Gubicza, J.; Shangina, D.V.; Bochvar, N.R.; Tabachkova, N.Y. High strength and good electrical conductivity in Cu–Cr alloys processed by severe plastic deformation. Mater. Lett. 2015, 153, 5–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Volinsky, A.A.; Hai, T.; Chai, Z.; Liu, P.; Tian, B.H.; Liu, Y. Aging behavior and precipitates analysis of the Cu–Cr–Zr–Ce alloy. Mater. Sci. Eng. A 2016, 650, 248–253. [Google Scholar] [CrossRef]
- Lai, R.L.; He, D.Q.; He, G.A.; Lin, J.Y.; Sun, Y.Q. Study of the microstructure evolution and properties response of a Friction-Stir-Welded Copper-Chromium-Zirconium alloy. Metals 2017, 7, 381. [Google Scholar] [CrossRef]
- Luo, C.P.; Dahmen, U. Morphology and crystallography of Cr precipitates in a Cu-0.33 wt % Cr alloy. Acta Mater. 1994, 42, 1923–1932. [Google Scholar] [CrossRef]
- Fujii, T.; Nakazawa, H.; Kato, M.; Dahmen, U. Crystallography and morphology of nanosized Cr particles in a Cu–0.2% Cr alloy. Acta Mater. 2000, 48, 1033–1045. [Google Scholar] [CrossRef]
- Tang, N.Y.; Taplin, N.M.; Dunlop, G.L. Precipitation and aging in high-conductivity Cu-Cr alloys with additions of zirconium and magnesium. Mater. Sci. Technol. 1985, 1, 270–275. [Google Scholar] [CrossRef]
- Batra, I.S.; Dey, G.K.; Kulkarni, U.D.; Banerjee, S. Microstructure and properties of a Cu–Cr–Zr alloy. J. Nucl Mater. 2001, 299, 91–100. [Google Scholar] [CrossRef]
- Liu, P.; Kang, B.X.; Cao, X.G.; Huang, J.L.; Yen, B.; Gu, H.C. Aging precipitation and recrystallization of rapidly solidified Cu–Cr–Zr–Mg alloy. Mater. Sci. Eng. A 1999, 265, 262–267. [Google Scholar] [CrossRef]
- Qi, W.X.; Tu, J.P.; Liu, F.; Yang, Y.Z.; Wang, N.Y.; Lu, H.M.; Zhang, X.B.; Guo, S.Y.; Liu, M.S. Microstructure and tribological behavior of a peak aged Cu–Cr–Zr alloy. Mater. Sci. Eng. A 2003, 343, 89–96. [Google Scholar] [CrossRef]
- Su, J.H.; Dong, Q.M.; Liu, P.; Li, H.J.; Kang, B.X. Research on aging precipitation in a Cu–Cr–Zr–Mg alloy. Mater. Sci. Eng. A 2005, 392, 422–426. [Google Scholar] [CrossRef]
- Batra, I.S.; Dey, G.K.; Kulkarni, U.D.; Banerjee, S. Precipitation in a Cu-Cr-Zr alloy. Mater. Sci. Eng. A 2003, 356, 32–36. [Google Scholar] [CrossRef]
- Xia, C.D.; Zhang, W.; Kang, Z.Y.; Jia, Y.L.; Wu, Y.F.; Zhang, R.; Xu, G.Y.; Wang, M.P. High strength and high electrical conductivity Cu–Cr system alloys manufactured by hot rolling–quenching process and thermo-mechanical treatments. Mater. Sci. Eng. A 2012, 538, 295–301. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Shen, B.; Yu, F.X. Precipitation in a Cu–Cr–Zr–Mg alloy during aging. Mater. Charact. 2013, 81, 68–75. [Google Scholar] [CrossRef]
- Huang, F.X.; Ma, J.S.; Ning, H.L.; Geng, Z.T.; Liu, C.; Guo, S.M.; Yu, X.T.; Yu, W.T.; Li, H.; Lou, H.F. Analysis of phases in a Cu–Cr–Zr alloy. Scr. Mater. 2003, 48, 97–102. [Google Scholar]
- Hatakeyama, M.; Toyama, T.; Yang, J.; Nagai, Y.; Hasegawa, M.; Ohkubo, T.; Eldrup, M.; Singh, B.N. 3D-AP and positron annihilation study of precipitation behavior in Cu–Cr–Zr alloy. J. Nucl. Mater. 2009, 386, 852–855. [Google Scholar] [CrossRef]
- Su, J.H.; Liu, P.; Dong, Q.M.; Li, H.J.; Ren, F.Z. Recrystallization and precipitation behavior of Cu-Cr-Zr Alloy. J. Mater. Eng. Perform. 2007, 16, 490–493. [Google Scholar] [CrossRef]
- Hughes, D.A.; Hansen, N.; Bammann, D.J. Geometrically necessary boundaries, incidental dislocation boundaries and geometrically necessary dislocations. Scr. Mater. 2003, 48, 147–153. [Google Scholar] [CrossRef]
- Hu, T.; Chen, J.H.; Liu, J.R.; Liu, Z.R.; Wu, C.L. The crystallographic and morphological evolution of the strengthening precipitates in Cu–Ni–Si alloys. Acta Mater. 2013, 61, 1210–1219. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Zhang, W.Z. A transmission electron microscopy investigation of crystallography of τ-Mg32 (Al, Zn)49 precipitates in a Mg–Zn–Al alloy. Scr. Mater. 2011, 64, 201–204. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Watanabe, C.; Monzen, R.; Tazaki, K. Mechanical properties of Cu–Cr system alloys with and without Zr and Ag. J. Mater. Sci. 2008, 43, 813–819. [Google Scholar] [CrossRef]
- Zhang, D.L.; Mihara, K.; Tsubokawa, S.; Suzuki, H.G. Precipitation characteristics of Cu–15Cr–0.15Zr in situ composite. Mater. Sci. Technol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Matsuda, K.; Uetani, Y.; Sato, T.; Ikeno, S. Metastable phases in an Al-Mg-Si alloy containing copper. Metall. Mater. Trans. A 2001, 32, 1293–1299. [Google Scholar] [CrossRef]
- Marioara, C.D.; Nordmark, H.; Andersen, S.J.; Holmestad, R. Post-β″phases and their influence on microstructure and hardness in 6xxx Al-Mg-Si alloys. J. Mater. Sci. 2006, 41, 471–478. [Google Scholar] [CrossRef]
- Ninive, P.H.; Strandlie, A.; Gulbrandsen-Dahl, S.; Lefebvre, W.; Marioara, C.D.; Andersen, S.J.; Friis, J.; Holmestad, R.; Lovvik, O.M. Detailed atomistic insight into the β″phase in Al-Mg-Si alloys. Acta Mater. 2014, 69, 126–134. [Google Scholar] [CrossRef]
- Lei, Q.; Xiao, Z.; Hu, W.; Derby, B.; Li, Z. Phase transformation behaviors and properties of a high strength Cu-Ni-Si alloy. Mater. Sci. Eng. A 2017, 697, 37–47. [Google Scholar] [CrossRef]
- Cuniberti, A.; Tolley, A.; Riglos, M.V.C.; Giovachini, R. Influence of natural aging on the precipitation hardening of an Al-Mg-Si alloy. Mater. Sci. Eng. A 2010, 527, 5307–5311. [Google Scholar] [CrossRef]
- Holzwarth, U.; Stamm, H. The precipitation behaviour of ITER-grade Cu-Cr-Zr alloy after simulating the thermal cycle of hot isostatic pressing. J. Nucl. Mater. 2000, 279, 31–45. [Google Scholar] [CrossRef]
- Mabuchi, M.; Higashi, K. Strengthening mechanism of Mg-Si alloy. Acta Mater. 1996, 44, 4611–4618. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation-hardening of metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Jiang, F.; Jiang, J.; Xu, P.; Tong, M.; Tang, Z. Precipitation, Recrystallization, and Evolution of Annealing Twins in a Cu-Cr-Zr Alloy. Metals 2018, 8, 227. https://doi.org/10.3390/met8040227
Chen X, Jiang F, Jiang J, Xu P, Tong M, Tang Z. Precipitation, Recrystallization, and Evolution of Annealing Twins in a Cu-Cr-Zr Alloy. Metals. 2018; 8(4):227. https://doi.org/10.3390/met8040227
Chicago/Turabian StyleChen, Xiaobo, Feng Jiang, Jingyu Jiang, Pian Xu, Mengmeng Tong, and Zhongqin Tang. 2018. "Precipitation, Recrystallization, and Evolution of Annealing Twins in a Cu-Cr-Zr Alloy" Metals 8, no. 4: 227. https://doi.org/10.3390/met8040227





