Hot Deformation Behavior and Processing Map of Mg-3Sn-2Ca-0.4Al-0.4Zn Alloy
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
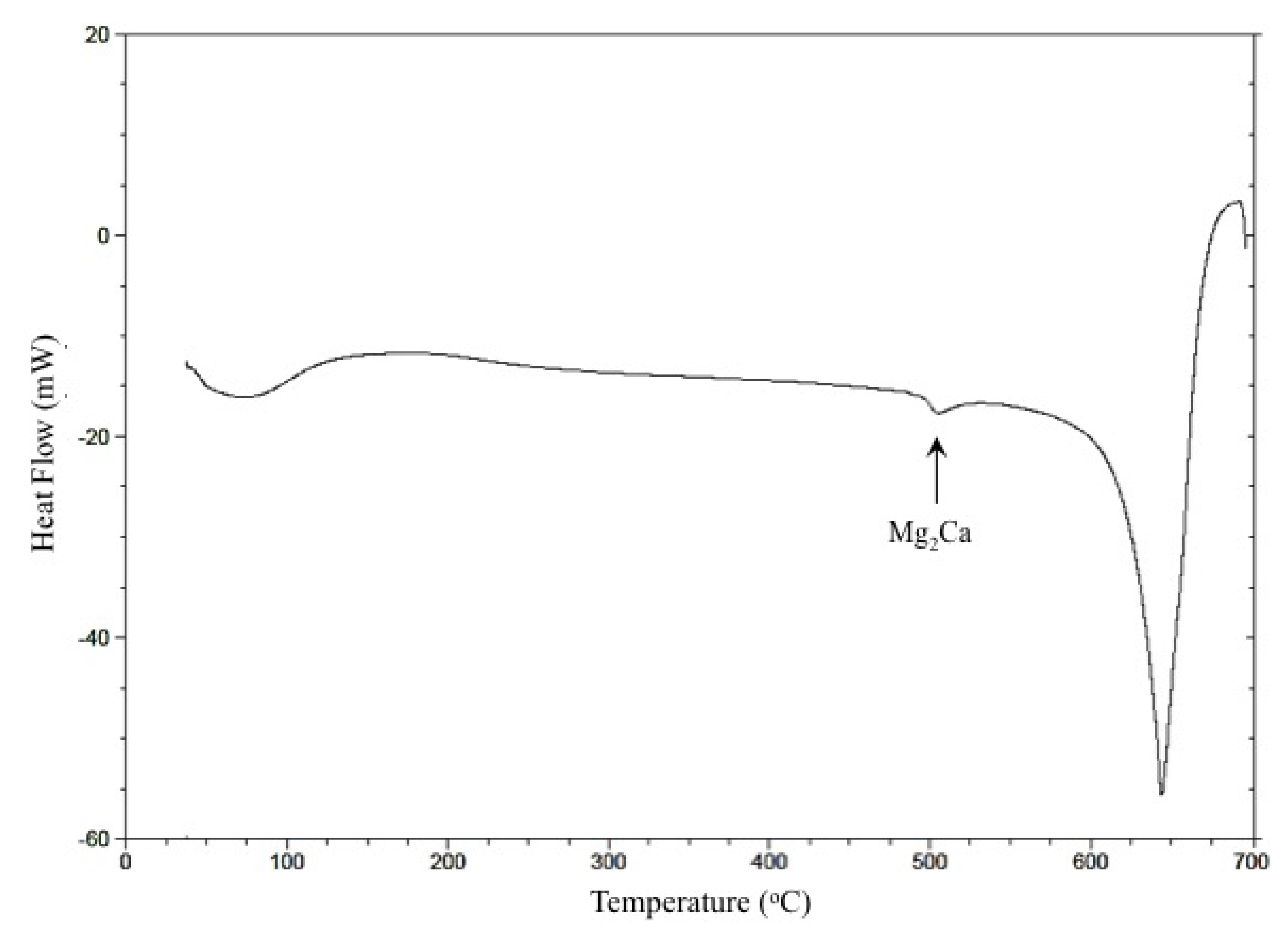
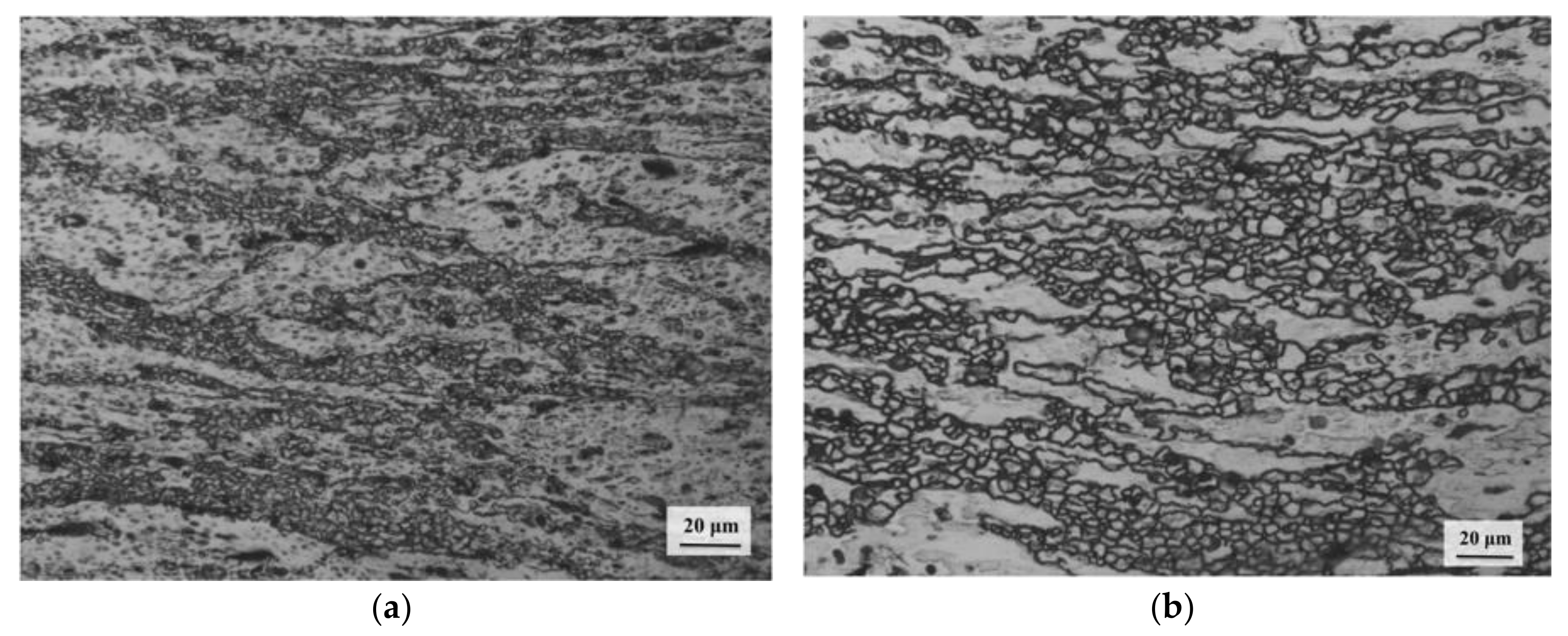
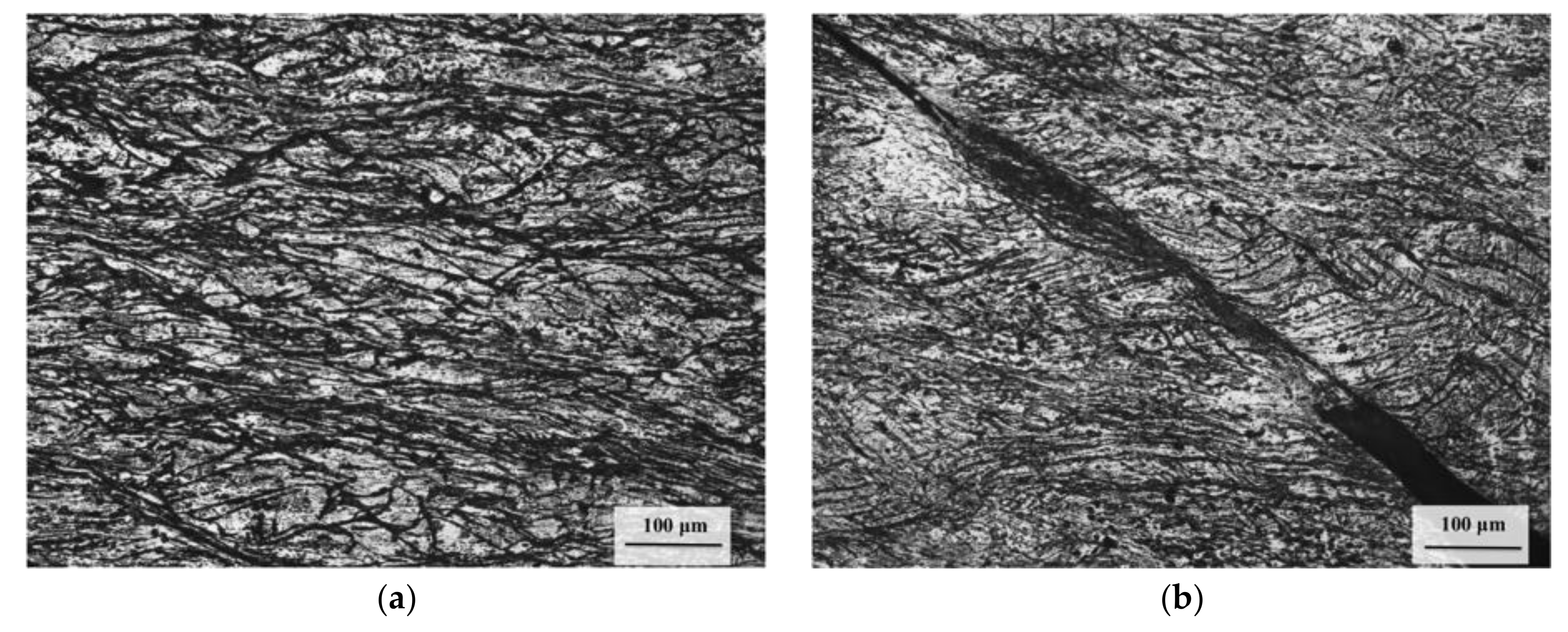
3.1. Initial Microstructure
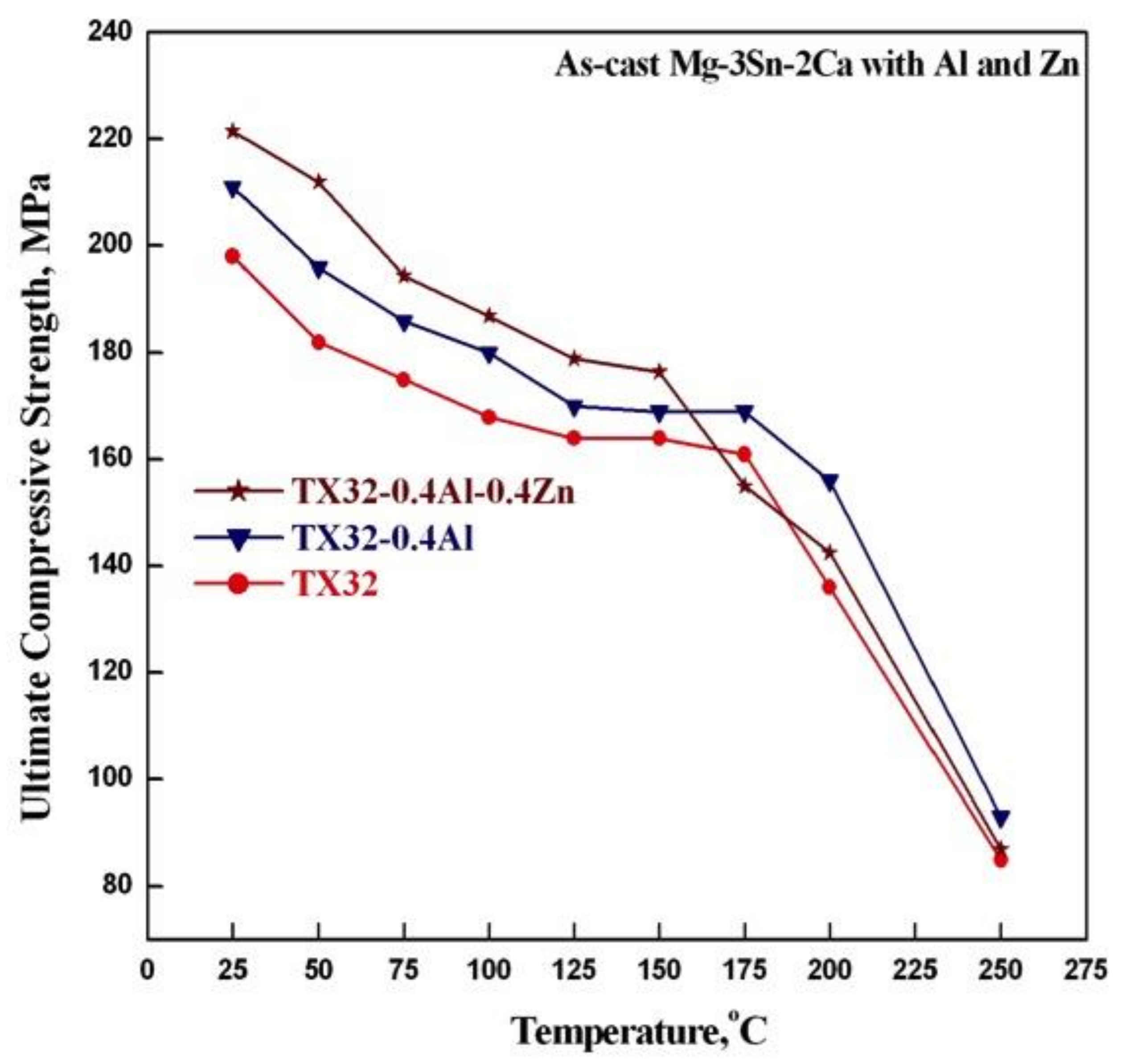
3.2. Ultimate Compressive Strength
3.3. Stress-Strain Behavior
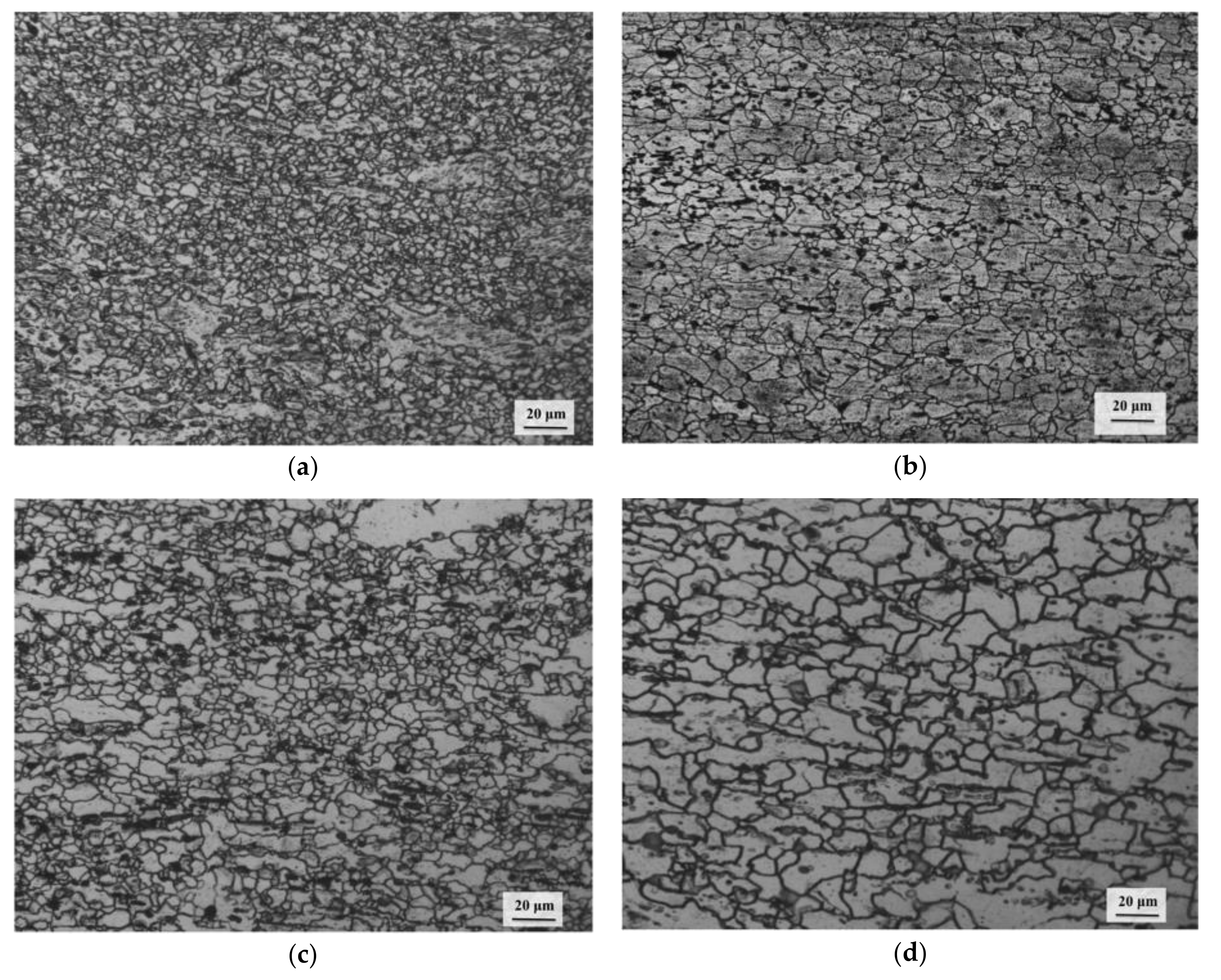
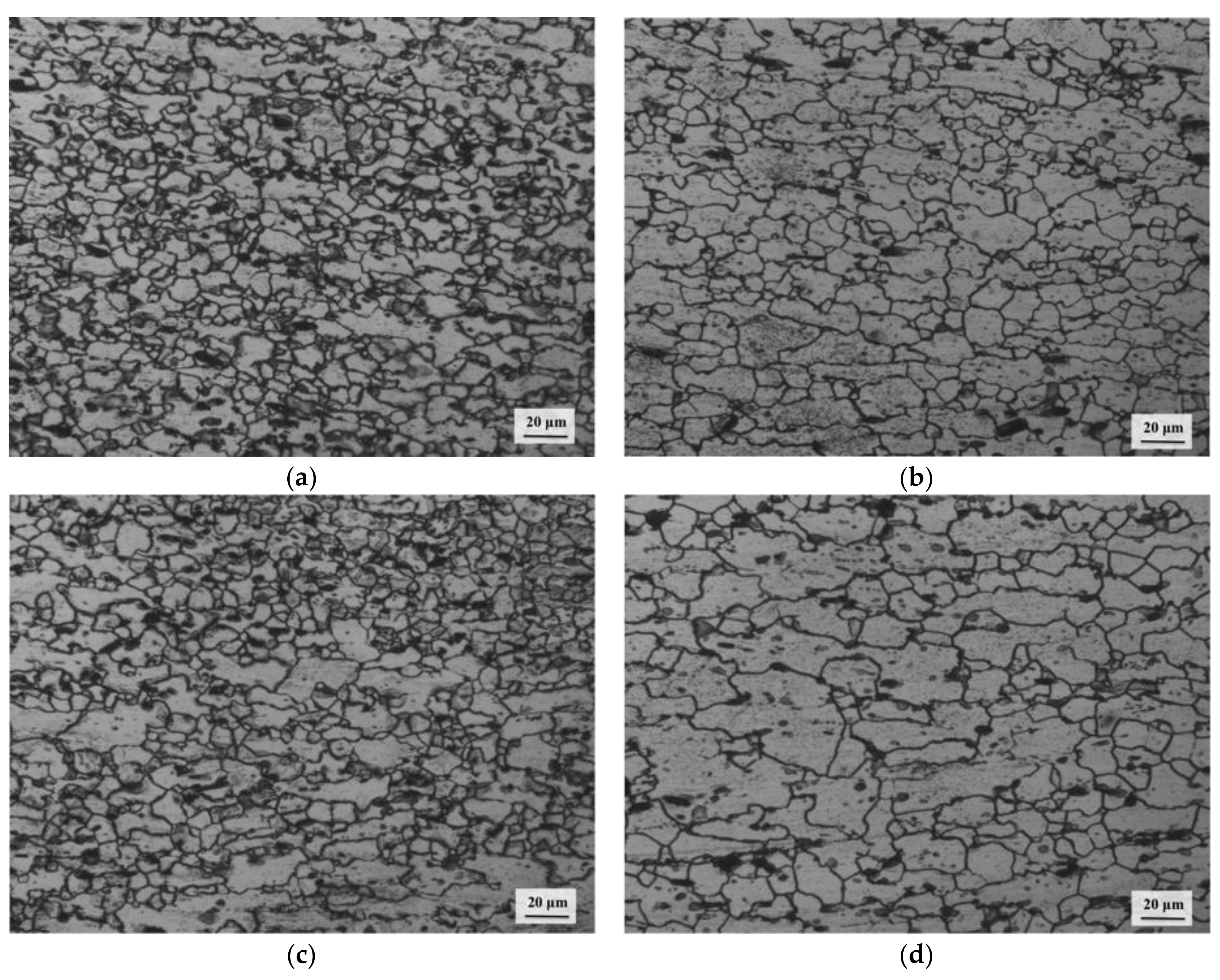
3.4. Processing Map and Microstructural Evolution
- (1)
- 300–340 °C and 0.0003–0.001 s−1; corresponding peak efficiency is 37% at 300 °C/0.0003 s−1 (Domain 1);
- (2)
- 400–480 °C and 0.01–1 s−1; corresponding peak efficiency is 38% at 450 °C/0.1 s−1 (Domain 2); and
- (3)
- 350–500 °C and 0.0003–0.01 s−1; corresponding peak efficiency is 37% at 450 °C/0.0003 s−1 (Domain 3).
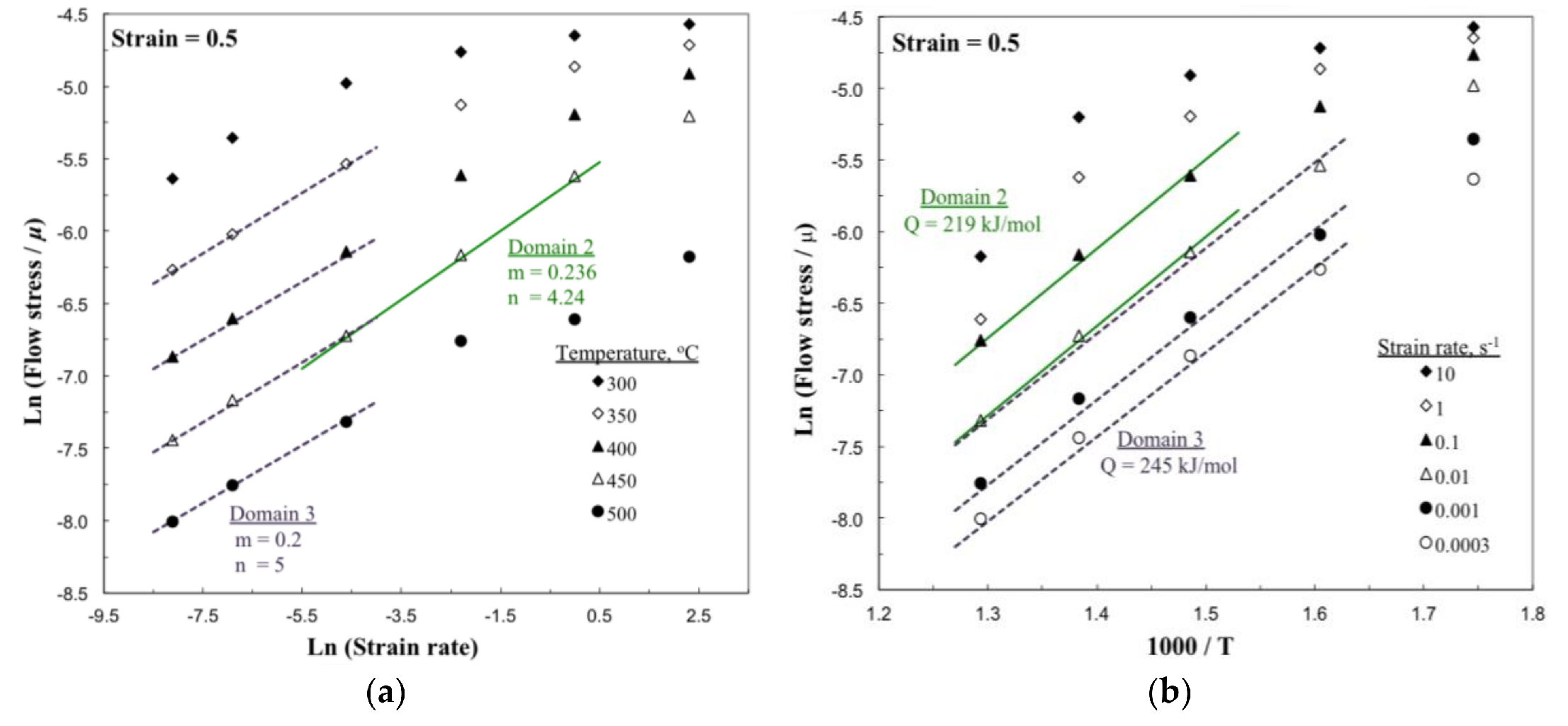
3.5. Kinetic Analysis
3.6. Deformation Mechanisms
3.7. Flow Instability
3.8. Comparison with the Processing Maps of TX32-Based Alloys
4. Conclusions
- (1)
- The ultimate compressive strength of TXAZ3200 alloy is higher than the base TX32-0.4Al alloy in the temperature range of 25–150 °C.
- (2)
- The processing map of the alloy, in the range of 300–500 °C and 0.0003–10 s−1, exhibited three domains in the ranges: (1) 300–340 °C and 0.0003–0.001 s−1; (2) 400–480 °C and 0.01–1 s−1; and (3) 350–500 °C and 0.0003–0.01 s−1.
- (3)
- Dynamic recrystallization (DRX) occurs in all three domains and is dominated by basal slip in the first domain, pyramidal slip in the second, and prismatic slip in the third. The recovery mechanisms are climb in Domains 1 and 3 and cross-slip in Domain 2.
- (4)
- The estimated apparent activation energy (Q) values from the kinetic analysis for the as-cast TXAZ3200 alloy are 219 and 245 kJ/mole in Domains 2 and 3, respectively. These values are higher than the value of Q required for self-diffusion in Mg, which suggests the generation of significant back stress associated with the presence of intermetallic particles in the matrix and as well as on grain boundaries.
- (5)
- To maximize the workability of TXAZ3200 alloy, bulk processing may be done in Domain 2 and a completion step may be done in Domain 3 to obtain a fine grain size in the final product.
- (6)
- Compared with the processing map for TX32-0.4Al alloy, Domain 1 is slightly less wide, Domain 2 moves slightly on the temperature axis by 20 °C, and a new third domain exhibited by the Zn-containing alloy appears at higher temperatures and lower strain rates. The regime of flow instability is reduced in TXAZ3200 alloy.
- (7)
- In the processing map of TXAZ3211 alloy that has a high amount of Al and Zn (1 wt % each), Domain 1 moves to a higher temperature by 60 °C and the third domain is not present, as compared to the map of TXAZ3200 alloy. The flow instability regime extends along the entire temperature range at high strain rates, and another regime forms at high temperatures and lower strain rates.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kainer, K.U.; Dieringa, H.; Dietzel, W.; Hort, N.; Blawert, C. The use of magnesium alloys: Past, present and future. In Magnesium Technology in the Global Age, Proceedings of the International Symposium on Magnesium Technology in the Global Age, Montreal, QC, Canada, 1–4 October 2006; Pekguleryuz, M.O., Mackenzie, L.W.F., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2006; pp. 3–19. [Google Scholar]
- Hort, N.; Huang, Y.; Abu Leil, T.; Maier, P.; Kainer, K.U. Microstructural investigations of the Mg-Sn-xCa system. Adv. Eng. Mater. 2006, 8, 359–364. [Google Scholar] [CrossRef]
- Abu Leil, T.; Huang, Y.; Dieringa, H.; Hort, N.; Kainer, K.U.; Bursik, J.; Jiraskova, Y.; Rao, K.P. Effect of heat treatment on the microstructure and creep behavior of Mg-Sn-Ca alloys. Mater. Sci. Forum. 2007, 546–549, 69–72. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.D.; Abu Leil, T.; Rao, K.P.; Kainer, K.U. Properties and processing of magnesium-tin-calcium alloys. Kovove Mater. 2011, 49, 163–177. [Google Scholar] [CrossRef]
- Abu Leil, T.; Hort, N.; Dieringa, H.; Blawert, C.; Huang, Y.; Kainer, K.U.; Rao, K.P. Development and characterization of a series of Mg-Sn-Ca alloys. In Magnesium Technology in the Global Age, Proceedings of the International Symposium on Magnesium Technology in the Global Age, Montreal, QC, Canada, 1–4 October 2006; Pekguleryuz, M.O., Mackenzie, L.W.F., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2006; pp. 739–749. [Google Scholar]
- Liu, H.; Chen, Y.; Tang, Y.; Wei, S.; Niu, G. The microstructure, tensile properties, and creep behavior of as-cast Mg-(1–10)%Sn alloys. J. Alloys Compd. 2007, 440, 122–126. [Google Scholar] [CrossRef]
- Rao, K.P.; Prasad, Y.V.R.K.; Hort, N.; Huang, Y.; Kainer, K.U. High Temperature Deformation Behaviour of a New Magnesium Alloy. Key Eng. Mater. 2007, 340–341, 89–94. [Google Scholar] [CrossRef]
- Rao, K.P.; Suresh, K.; Hort, N.; Kainer, K.U. Effect of minor additions of Al and Si on the mechanical properties of cast Mg-3Sn-2Ca alloys in low temperature range. Mater. Sci. Forum. 2010, 654–656, 635–638. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Substitutional solution hardening of magnesium single crystals. Philos. Mag. 1972, 25, 897–916. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Solid solution strengthening of magnesium single crystals-I, Alloying behaviour in basal slip. Acta Metall. 1969, 17, 1339–1349. [Google Scholar] [CrossRef]
- Akhtar, A.; Teghtsoonian, E. Solid solution strengthening of magnesium single crystals-II, The effect of solute on the ease of prismatic slip. Acta Metall. 1969, 17, 1351–1356. [Google Scholar] [CrossRef]
- Blake, A.H.; Cáceres, C.H. Solid solution effects on the tensile behavior of concentrated Mg-Zn alloys. In Essential Readings in Magnesium Technology; Mathaudhu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 263–267. [Google Scholar]
- Prasad, Y.V.R.K.; Rao, K.P.; Sasidhara, S. Hot Working Guide: A Compendium of Processing Maps, 2nd ed.; ASM International, Materials Park: Novelty, OH, USA, 2015; ISBN 978-1-62708-091-0. [Google Scholar]
- Prasad, Y.V.R.K.; Seshacharyulu, T. Modelling of hot deformation for microstructural control. Inter. Mater. Rev. 1998, 43, 243–258. [Google Scholar] [CrossRef]
- Ziegler, H. Progress in Solid Mechanics; Sneddon, I.N., Hill, R., Eds.; John Wiley: New York, NY, USA, 1965; Volume 4, pp. 91–193. [Google Scholar]
- Prasad, Y.V.R.K.; Rao, K.P. Processing maps and rate controlling mechanisms of hot deformation of electrolytic tough pitch copper in the temperature range 300–950 °C. Mater. Sci. Eng. A 2005, 391, 141–150. [Google Scholar] [CrossRef]
- Dharmendra, C.; Rao, K.P.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Hot workability analysis with processing maps and texture characteristics of as-cast TX32 magnesium alloy. J. Mater. Sci. 2013, 48, 5236–5246. [Google Scholar] [CrossRef]
- Dharmendra, C.; Rao, K.P.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Hot working mechanisms and texture development in Mg-3Sn-2Ca-0.4Al alloy. Mater. Chem. Phys. 2012, 136, 1081–1091. [Google Scholar] [CrossRef]
- Dharmendra, C.; Rao, K.P.; Zhao, F.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Effect of aluminum on microstructural evolution during hot deformation of TX32 magnesium alloy. J. Mater. Sci. 2014, 49, 5885–5898. [Google Scholar] [CrossRef]
- Dharmendra, C.; Rao, K.P.; Zhao, F.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Effect of silicon content on hot working, processing maps, and microstructural evolution of cast TX32-0.4Al magnesium alloy. Mater. Sci. Eng. A 2014, 606, 11–23. [Google Scholar] [CrossRef]
- Suresh, K.; Rao, K.P.; Prasad, Y.V.R.K.; Hort, N.; Kainer, K.U. Microstructure and mechanical properties of as-cast Mg-Sn-Ca alloys and effect of alloying elements. Trans. Nonferrous Met. Soc. China 2013, 23, 3604–3610. [Google Scholar] [CrossRef]
- Jonas, J.J.; Sellars, C.M.; Tegart, W.J.M.G. Strength and structure under hot working conditions. Metall. Rev. 1969, 14, 1–24. [Google Scholar] [CrossRef]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps; Pergamon Press: Oxford, UK, 1982; p. 44. [Google Scholar]
- Obara, T.; Yoshinga, H.; Morozumi, S. {112} <1123> slip system in magnesium. Acta Metall. 1973, 21, 845–853. [Google Scholar] [CrossRef]
- Morris, J.R.; Schraff, J.; Ho, K.M.; Turner, D.E.; Ye, Y.Y.; Yoo, M.H. Prediction of a {1122} hcp stacking fault using a modified generalized stacking-fault calculation. Philos. Mag. A 1997, 76, 1065–1077. [Google Scholar] [CrossRef]


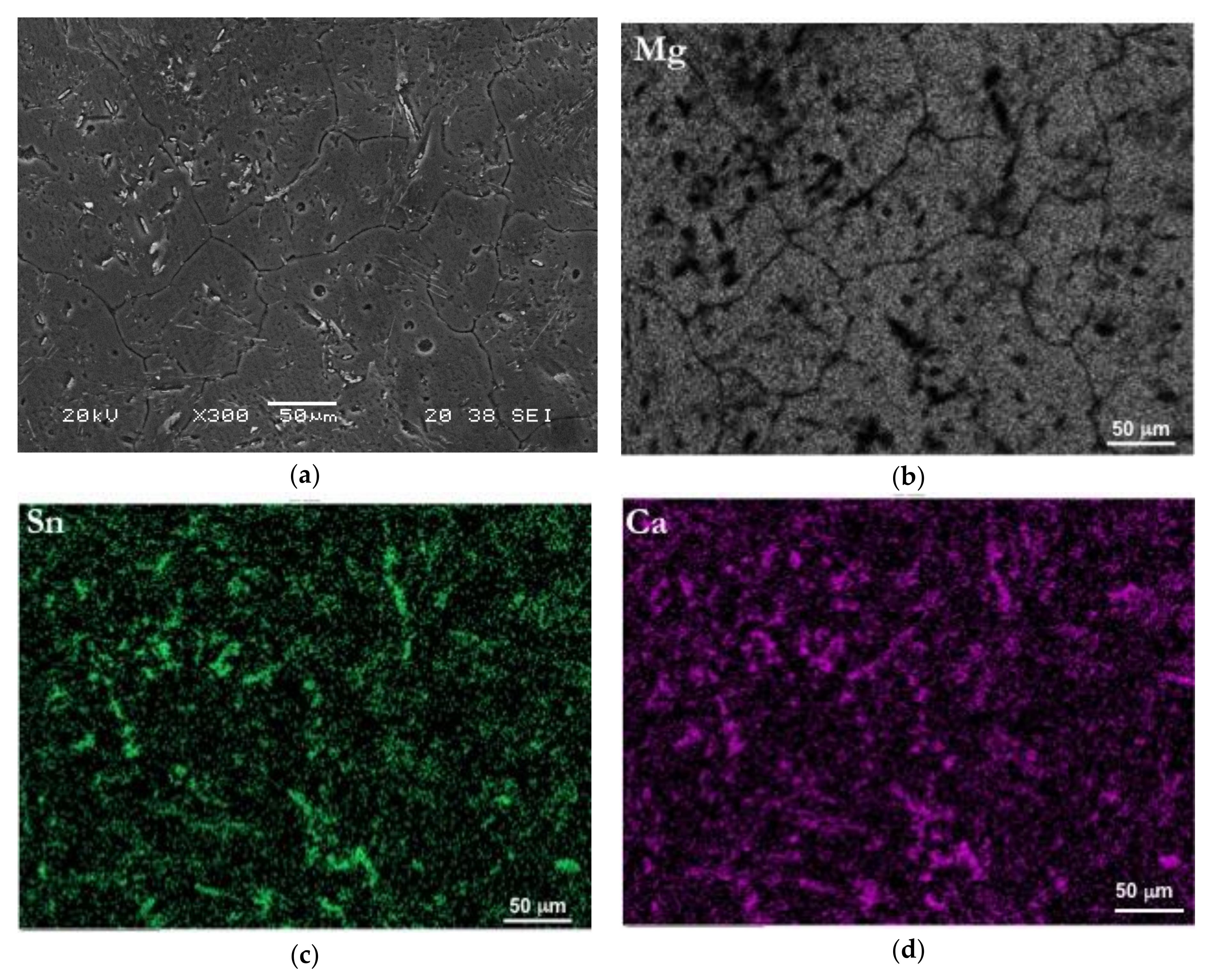
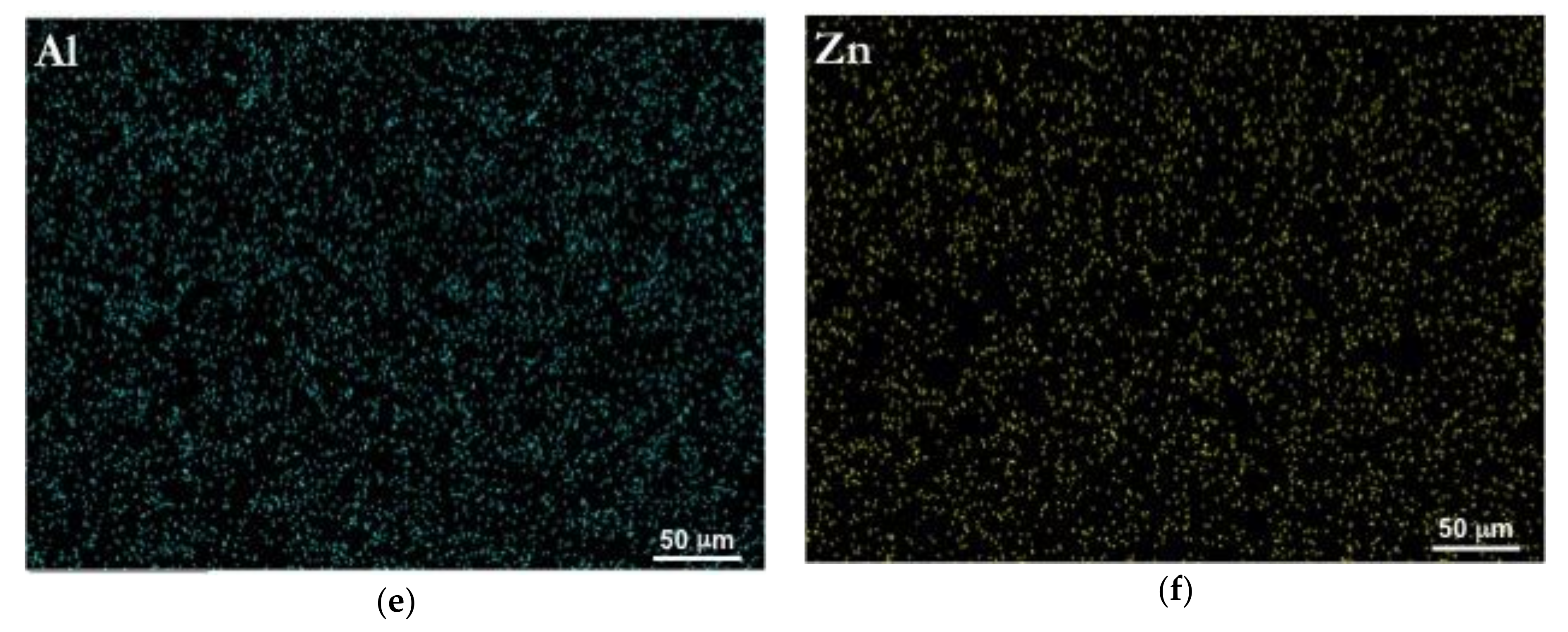




| Sn | Ca | Al | Zn | Mg |
|---|---|---|---|---|
| 2.844 | 1.857 | 0.384 | 0.38 | Balance (94.53) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmendra, C.; Rao, K.P.; Suresh, K.; Hort, N. Hot Deformation Behavior and Processing Map of Mg-3Sn-2Ca-0.4Al-0.4Zn Alloy. Metals 2018, 8, 216. https://doi.org/10.3390/met8040216
Dharmendra C, Rao KP, Suresh K, Hort N. Hot Deformation Behavior and Processing Map of Mg-3Sn-2Ca-0.4Al-0.4Zn Alloy. Metals. 2018; 8(4):216. https://doi.org/10.3390/met8040216
Chicago/Turabian StyleDharmendra, Chalasani, Kamineni Pitcheswara Rao, Kalidass Suresh, and Norbert Hort. 2018. "Hot Deformation Behavior and Processing Map of Mg-3Sn-2Ca-0.4Al-0.4Zn Alloy" Metals 8, no. 4: 216. https://doi.org/10.3390/met8040216





