Hydrometallurgical Approach for Leaching of Metals from Copper Rich Side Stream Originating from Base Metal Production
Abstract
:1. Introduction
- (a)
- The FeS elimination or slag making stage2FeS(s) + 3O2(g) + 2SiO2(s) = 2FeO·SiO2(s) + 2SO2(g)
- (b)
- The copper making stageCu2S(s) + 2O2(g) = 2Cu (s) + 2SO2(g)
2. Materials and Methods
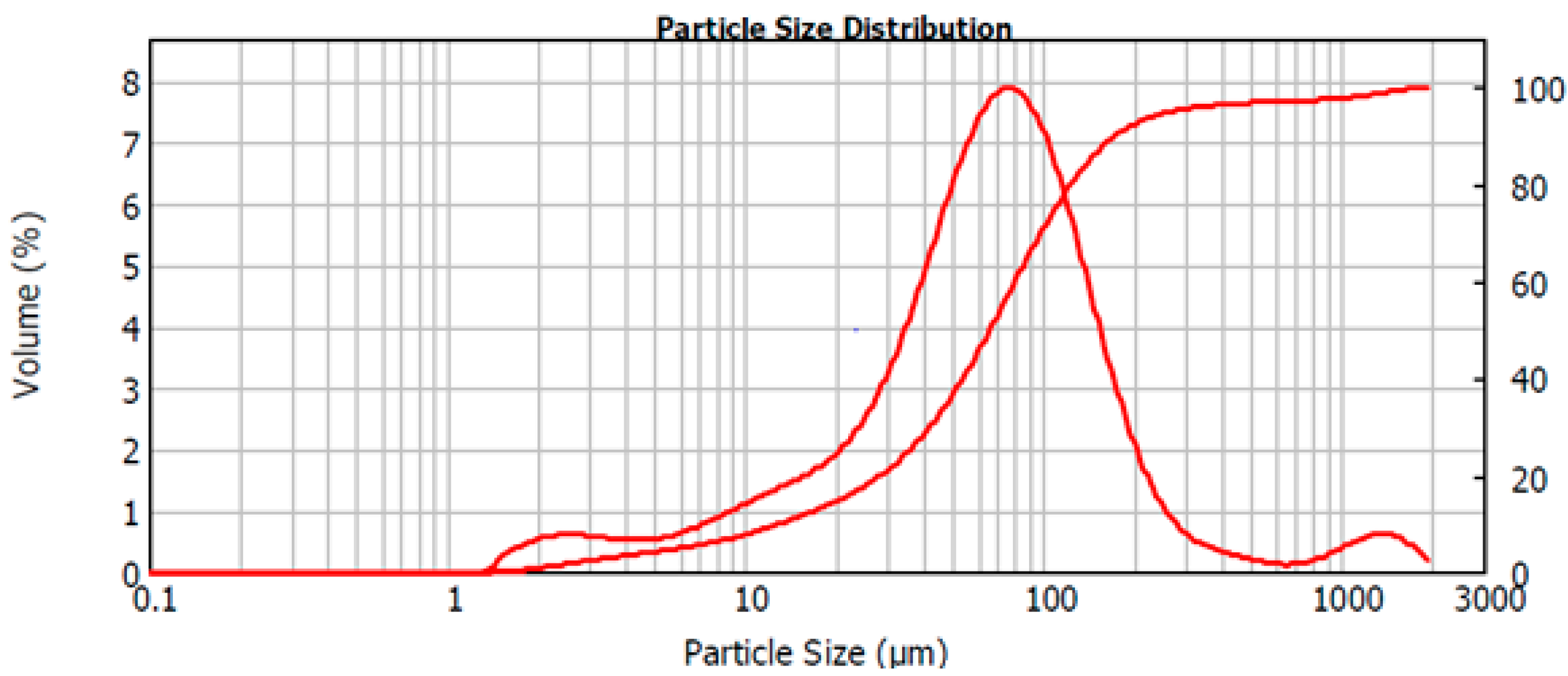
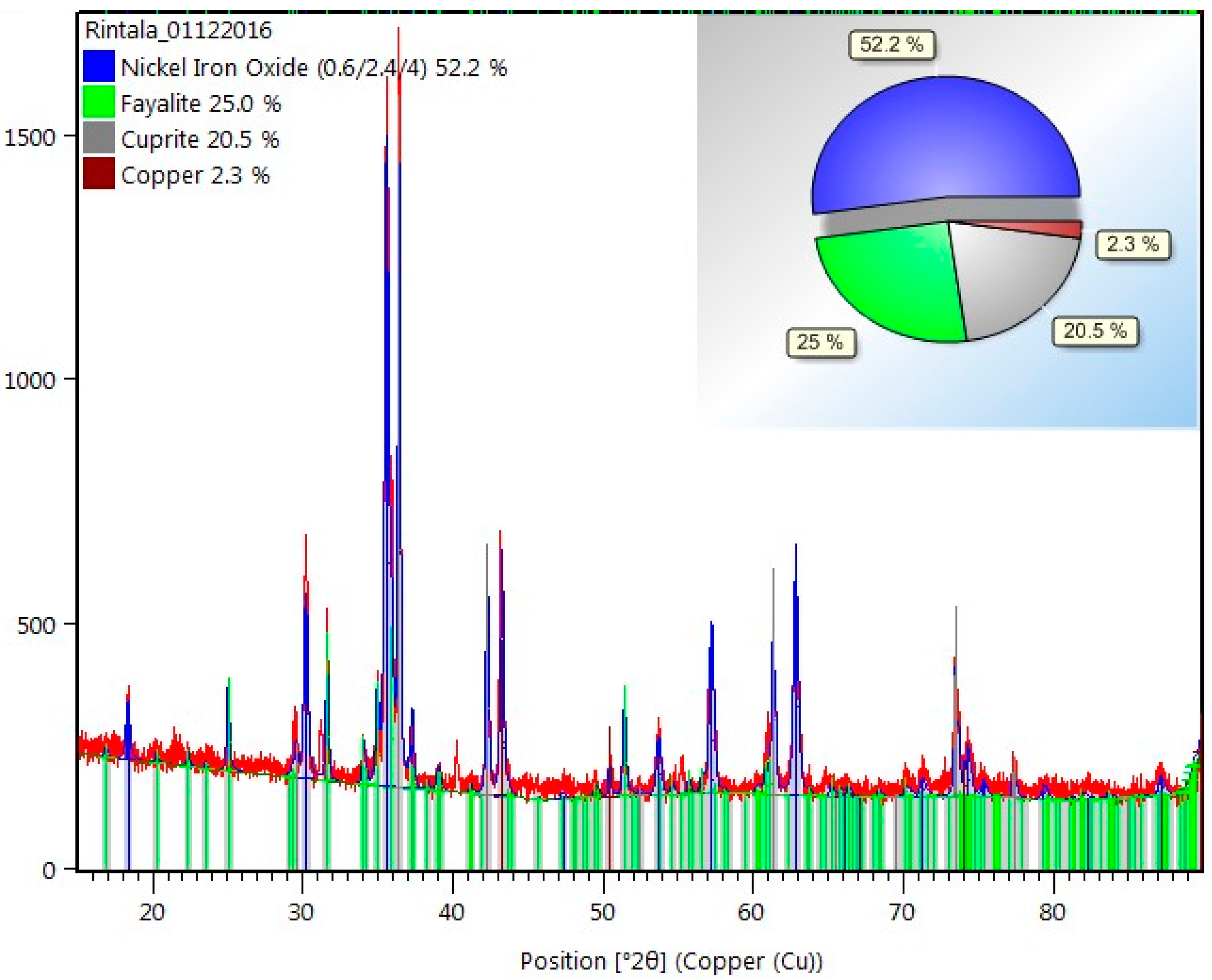
2.1. The Raw Material
2.2. Leaching Experiments
3. Results and Discussion
3.1. Leaching of Copper
3.2. Ni Leaching and Selectivity between Copper and Nickel
3.3. Leaching of Iron
3.4. Leaching of Zinc
3.5. Leaching of Cr, Pb, and Al
3.6. A Comparison of the Used Lixiviants
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Avarmaa, K.; O’ Brien, H.; Johto, H.; Taskinen, P. Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. J. Sustain. Metall. 2015, 1, 216–228. [Google Scholar] [CrossRef]
- Avarmaa, K.; Johto, H.; Taskinen, P. Distribution of precious metals (Ag, Au, Pd, Pt, and Rh) between copper matte and iron silicate slag. Metall. Mater. Trans. B 2016, 47, 244–255. [Google Scholar] [CrossRef]
- Avarmaa, K.; O’Brien, H.; Taskinen, P. Equilibria of Gold and Silver between Molten Copper and FeOx-SiO2-Al2O3 Slag in WEEE Smelting at 1300 °C. In Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 193–202. [Google Scholar]
- Jyrkonen, S.; Haavanlammi, K.; Luomala, M.; Karonen, J.; Suikkanen, P. Processing of PGM containing Ni/Cu bulk concentrates in a sustainable way by Outotec Direct Nickel Flash Smelting process. In Ni-Co 2013; Springer: Cham, Switzerland, 2013; pp. 325–334. [Google Scholar]
- Taskinen, P. Direct-to-blister smelting of copper concentrates: The slag fluxing chemistry. Miner. Process. Extr. Metall. 2011, 120, 240–246. [Google Scholar] [CrossRef]
- Taskinen, P.; Seppala, K.; Laulumaa, J.; Poijarvi, J. Oxygen pressure in the Outokumpu flash smelting furnace—Part 1: Copper flash smelting settler. Miner. Process. Extr. Metall. 2001, 110, 94–100. [Google Scholar] [CrossRef]
- Davenport, W.G.; King, M.J.; Schlesinger, M.E.; Biswas, A.K. Extractive Metallurgy of Copper, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2002; p. 452. [Google Scholar]
- Taskinen, P.; Dinsdale, A.; Gisby, J. Industrial slag chemistry: A case study of computational thermodynamics. Scand. J. Metall. 2005, 34, 100–107. [Google Scholar] [CrossRef]
- Mihailova, I.; Mehandjiev, D. Characterisation of fayalite from complexes. J. Chem. Technol. Metall. 2010, 45, 317–326. [Google Scholar]
- Deng, T.; Ling, Y.H. Chemical and mineralogical characterisations of a copper converter slag. Rare Met. 2002, 21, 175–178. [Google Scholar]
- Petkov, V.; Jones, P.T.; Boydens, E.; Blanpain, B.; Wollants, P. Chemical corrosion mechanisms of magnesia–chromite and chrome-free refractory bricks by copper metal and anode slag. J. Eur. Ceram. Soc. 2007, 27, 2433–2444. [Google Scholar] [CrossRef]
- Taskinen, P.; Kojo, I. Fluxing options in the direct-to-blister copper smelting. In Proceedings of the Molten 2009 Conference, Santiago, Chile, 18–21 January 2009; pp. 1140–1151. [Google Scholar]
- Li, Y.; Papangelakis, G.V.; Ilya, P. High pressure oxidative acid leaching of nickel smelter slag: Characterization of feed and residue. Hydrometallurgy 2009, 97, 185–193. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Tumen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Sanchez, M.; Parada, F.; Parra, R.; Marquez, F.; Jara, R.; Carrasco, J.; Palcios, J. Management of copper pyrometallurgical slags: Giving additional value to copper mining industry. In Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Cape Town, South Africa, 25–28 January 2004; pp. 543–550. [Google Scholar]
- Geveci, A.; Topkaya, Y.; Gerceker, E. Recovery of Copper and zinc from copper converter flue dusts. In Proceedings of the 10th International Metallurgy and Material Congress, Istanbul, Turkey, 24–28 May 2000; pp. 59–68. [Google Scholar]
- Yıldız, K.; Alp, A.; Aydın, A.O. Utilization of copper refining slags by a pyro-hydrometallurgical method. In Proceedings of the 10th International Metallurgy and Material Congress, Istanbul, Turkey, 24–28 May 2000; pp. 127–132. [Google Scholar]
- Arslan, F.; Giray, K.; Onal, G.; Gurkan, V. Development of a Flowsheet for Recovering Copper and Tin from Copper Refining Slags. Eur. J. Miner. Process. Environ. Prot. 2002, 2, 94–102. [Google Scholar]
- Anand, S.; Das, R.P.; Jena, P.K. Reduction—Roasting and ferric chloride leaching of copper converter slag for extracting copper, nickel and cobalt. Hydrometallurgy 1981, 7, 243–252. [Google Scholar] [CrossRef]
- Sukla, L.B.; Panda, S.C.; Jean, P.K. Recovery of cobalt, nickel, and copper from converter slag through roasting with ammonium sulphate and sulfuric acid. Hydrometallurgy 1986, 16, 153–165. [Google Scholar] [CrossRef]
- Tumen, F.; Bailey, N.T. Recovery of metal values from copper smelter slags by roasting with pyrite. Hydrometallurgy 1990, 25, 317–328. [Google Scholar] [CrossRef]
- Herreros, O.; Quiroza, R.; Manzanob, E.; Bou, C.; Vinalsb, J. Copper extraction from reverberatory and flash furnace slags by chlorine leaching. Hydrometallurgy 1998, 49, 87–101. [Google Scholar] [CrossRef]
- Tumen, F. Metal recovery from secondary copper slag by roasting with ammonium sulphate. Turkish J. Eng. Environ. Sci. 1994, 18, 1–5. [Google Scholar]
- Anand, S.; Rao, K.S.; Jena, P.K. Pressure leaching of copper converter slag using dilute sulphuric acid for the extraction of cobalt, nickel and copper values. Hydrometallurgy 1983, 10, 305–312. [Google Scholar] [CrossRef]
- Gbor, P.K.; Ahmed, I.B.; Jia, C.Q. Behaviour of Co and Ni during aqueous sulphur dioxide leaching of nickel slag. Hydrometallurgy 2000, 57, 13–22. [Google Scholar] [CrossRef]
- Niemela, A.; Pitkaaho, S.; Ojala, S.; Keiski, R.L.; Peramaki, P. Microwave-assisted aqua regia digestion for determining platinum, palladium, rhodium and lead in catalyst materials. Microchem. J. 2012, 101, 75–79. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; Oxford University Press: Oxford, UK, 1995; 308p, ISBN 9780198559122. [Google Scholar]
- Cardellicchio, N.; Buccolieri, A.; Di Leo, A.; Spada, L. Heavy metals in marine sediments from the MarPiccolo of Taranto (Ionian Sea, Southern Italy). Ann. Chim. 2006, 96, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Cardellicchio, N.; Buccolieri, A.; Di Leo, A.; Librando, V.; Minniti, Z.; Spada, L. Methodological approach for metal pollution evaluation in sediments collected from the Tarnto Gulf. Toxicol. Environ. Chem. 2009, 91, 1273–1290. [Google Scholar] [CrossRef]
- Lundstrom, M.; Liipo, J.; Karonen, J.; Aromaa, J. Dissolution of six sulfide concentrates in the hydrocopper environment. In Proceedings of the South African Institute of Mining and Metallurgy Base Metals Conference, Kasane, Botswana, 27–31 July 2009; pp. 127–138. [Google Scholar]
- Habbache, N.; Alane, N.; Djerad, S.; Tifouti, L. Leaching of copper oxide with different acid solutions. Chem. Eng. J. 2009, 152, 503–508. [Google Scholar]
- Pacović, N.V. Hydrometallurgy; ŠRIF: Bor, Serbia, 1980. Chapter 3. (In Serbian) [Google Scholar]
- Carneiro, M.F.C.; Leao, V.A. The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 2007, 87, 73–82. [Google Scholar] [CrossRef]
- Garrels, R.M.; Thompson, M.E. Oxidation of pyrite by iron sulfate solutions. Am. J. Sci. 1960, 258, 57–67. [Google Scholar]
- Roine, A. Sustainable Process Technology and Engineering, A Manual on HSC program, Continuous Research & Development. Outotec Research Centre: Finland, 8 March 2017. [Google Scholar]
- Lee, M.S.; Nam, S.H. Chemical Equilibria of Nickel chloride in HCl solution at 25 °C. Bull. Korean Chem. Soc. 2009, 30, 2203–2207. [Google Scholar]
- Ashurst, K.G. The Thermodynamics of the formation of chlorocomplexes of iron (III), cobalt (II), iron (II), manganese (II) in perchlorate medium. Nat. Inst. Metall. 1976, 1820, 1–43. [Google Scholar]
- Peek, E.M.; Van Weert, G. Chloride Metallurgy. In Proceedings of the 32nd Annual Hydrometallurgy Meeting and International Conference of the Practice and Theory of Chloride/Metal Interaction, Montréal, QC, Canada, 19–23 October 2002; pp. 760–780. [Google Scholar]
- Misawa, T. The thermodynamic consideration for Fe-H2O system at 25 °C. Corros. Sci. 1973, 13, 659–676. [Google Scholar] [CrossRef]
- Langova, S.; Lesko, J.; Matysek, D. Selective leaching of zinc from zinc ferrite with hydrochloric acid. Hydrometallurgy 2009, 95, 179–182. [Google Scholar] [CrossRef]
- Nunez, C.; Vinals, J. Kinetics of leaching of zinc ferrite in aqueous hydrochloric acid solutions. Metall. Mater. Trans. B 1984, 15, 221–228. [Google Scholar] [CrossRef]
- Sato, T.; Nakamura, T. The stability constants of the aqueous chloro complexes of divalent zinc, cadmium and mercury determined by solvent extraction with tri-n octyl phosphine oxide. Hydrometallurgy 1980, 6, 3–12. [Google Scholar] [CrossRef]
- Winand, R. Chloride hydrometallurgy. Hydrometallurgy 1991, 27, 285–316. [Google Scholar] [CrossRef]




| Element | Concentration [wt. %] |
|---|---|
| Cu | 12.5 |
| Fe | 23.6 |
| Ni | 2.6 |
| Al | 0.5 |
| Cr | 0.1 |
| Zn | 1.6 |
| Pb | 0.1 |
| As | 0.1 |
| [wt. %] | Spectra #1–5 | Spectra #6–9, 14 | Spectra #10–12 |
|---|---|---|---|
| Cu | 88.6 | 2.2 | 3.2 |
| Fe | 1.9 | 52.4 | 3.2 |
| Ni | - | 14.7 | 1.1 |
| O | 8.8 | 23.8 | 45.8 |
| Si | 0.6 | 0.6 | 32.3 |
| Na | - | - | 0.9 |
| Mg | - | 1.0 | 3.7 |
| Al | - | 1.7 | 3.6 |
| K | - | - | 1.9 |
| Ca | - | - | 0.7 |
| Ti | - | 0.8 | 0.4 |
| Cr | - | 1.9 | - |
| Zn | - | 2.0 | - |
| Pb | - | - | 4.3 |
| Solution | Concentrations | Chemicals | Manufacturer (Grade) |
|---|---|---|---|
| HCl | 0.5 M | HCl 37% | EMPARTA ACS (for analysis) |
| 1.5 M | |||
| 2.5 M | |||
| 3.0 M | |||
| 5 M | |||
| H2SO4 | 0.51 M | H2SO4 95–97% | EMSURE ISO (for analysis) |
| 1.22 M | |||
| 1.93 M | |||
| 2.65 M | |||
| 3.06 M | |||
| HNO3 | 1 M | HNO3 65% | EMSURE (for analysis) |
| CuCl2, pH 1 | 0.5 M NaCl + 0.1 M CuCl2 | CuCl2·2H2O | VWR Chemicals (technical) |
| 4.5 M NaCl + 0.5 M CuCl2 | |||
| 4.5 M NaCl + 0.1 M CuCl2 | |||
| NaOH | 4 M | NaOH | SIGMA-ALDRICH (technical) |
| Solution | Ni | Cu | Fe | Zn | Cr | Pb | Al |
|---|---|---|---|---|---|---|---|
| 0.5 M HCl | 10 | * | 55 | 40 | 20 | 98 | 39 |
| 1.5 M HCl | 18 | * | 78 | 48 | 45 | 93 | 56 |
| 2.5 M HCl | 43 | 95 | 81 | 64 | 67 | 97 | 69 |
| 3 M HCl | 97 | 72 | 54 | 66 | 84 | 99 | 71 |
| 5 M HCl | 96 | 86 | 74 | 92 | 55 | 97 | 79 |
| 0.5 M H2SO4 | 35 | 70 | 53 | 63 | 84 | 21 | 51 |
| 1.22 M H2SO4 | 64 | 77 | 60 | 76 | 56 | 23 | 63 |
| 1.93 M H2SO4 | 77 | 81 | 82 | 80 | 62 | 23 | 69 |
| 2.65 M H2SO4 | 86 | 71 | 78 | 86 | 57 | 23 | 65 |
| 3.0 M H2SO4 | 81 | 65 | 62 | 86 | 56 | 17 | 65 |
| 4.5 M NaCl + 0.5 M Cu2+ pH 1 | 1 | 5 | - | 1 | - | 65 | 7 |
| 4.5 M NaCl + 0.1 M Cu2+ pH 1 | 1 | 3 | - | 1 | - | 62 | 5 |
| 0.5 M NaCl + 0.1 M Cu2+ pH 1 | 3 | 61 | - | 27 | - | 53 | 5 |
| 1 M HNO3 | 3 | 79 | - | 30 | - | 98 | 16 |
| 2 M HNO3 | 4 | 93 | - | 30 | - | * | 13 |
| 4 M NaOH | 0.3 | * | - | 2 | - | 59 | 22 |
| Solution | Cu Extraction (%) | Cu/Ni Selectivity | Cu/Zn Selectivity | Cu/Fe Selectivity | Pb Extraction (%) | Zn Extraction (%) |
|---|---|---|---|---|---|---|
| 1.5 M HCl | * | 7 | 3 | 1.5 | 93 | 48 |
| 0.5 M HCl | * | 10 | 3 | 1.9 | 98 | 40 |
| 4 M NaOH | * | 340 | 51 | - | 59 | 2 |
| 0.5 M NaCl, 0.1 M Cu2+ | 61 | 20 | 2 | - | 53 | 27 |
| 2 M HNO3 | 93 | 23 | 3 | - | * | 30 |
| 1 M HNO3 | 79 | 26 | 3 | - | 98 | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, U.S.; Rintala, L.; Halli, P.; Taskinen, P.; Lundström, M. Hydrometallurgical Approach for Leaching of Metals from Copper Rich Side Stream Originating from Base Metal Production. Metals 2018, 8, 40. https://doi.org/10.3390/met8010040
Mohanty US, Rintala L, Halli P, Taskinen P, Lundström M. Hydrometallurgical Approach for Leaching of Metals from Copper Rich Side Stream Originating from Base Metal Production. Metals. 2018; 8(1):40. https://doi.org/10.3390/met8010040
Chicago/Turabian StyleMohanty, Udit Surya, Lotta Rintala, Petteri Halli, Pekka Taskinen, and Mari Lundström. 2018. "Hydrometallurgical Approach for Leaching of Metals from Copper Rich Side Stream Originating from Base Metal Production" Metals 8, no. 1: 40. https://doi.org/10.3390/met8010040





