3. Results
A microstructure is a relevant property that influences the tensile strength, corrosion and fatigue resistance of titanium alloys.
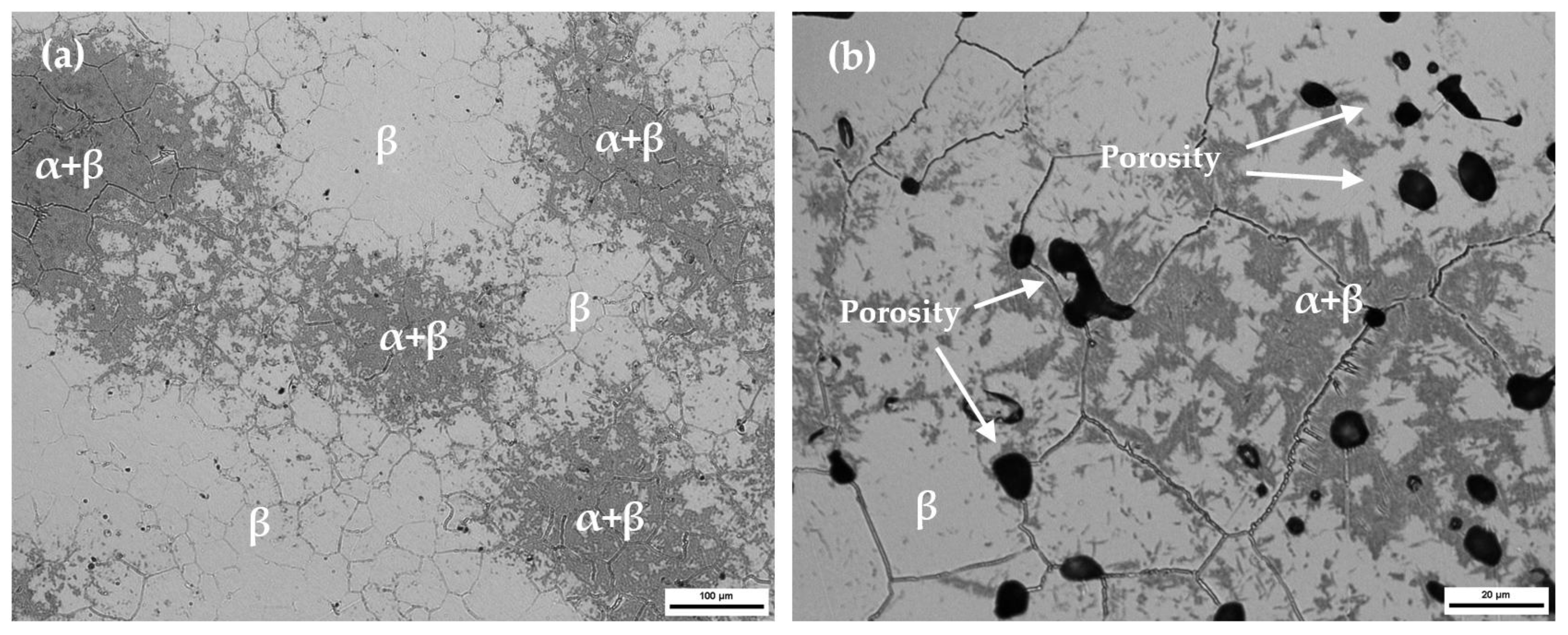
Figure 1 presents the tested alloy’s microstructures. The microstructure showed a heterogeneous phase composition, which was composed predominantly of a β equiaxial grain with a remaining α + β phase due to poor diffusion during the sintering process. The α + β regions with lower contents of refractory elements presented high chemical heterogeneity. As the studied alloys presented a retained β phase of more than 20%, they were classified at room temperature as β alloys [
18].
The mechanical characteristics of the Ti35Nb10Ta alloy have been published in a previous article and are summarized in
Table 2 [
19]. The P/M Ti6Al4V and Ti CP Grade 2 properties are also reported in this table for comparison purposes. A large amount of β stabilizing elements decreases diffusion during sintering. This effect implies lack of diffusion, which results in high residual porosity (
Figure 1b) that enhances the stress concentration effect and decreases mechanical properties. A higher content of beta stabilizer elements on the studied titanium alloy, compared with Ti6Al4V and Ti CP, had a significant effect by lowering Young’s Modulus. Lower Young’s Modulus values could minimize bone atrophy due to the stress shielding effect, which increases the implant durability [
2,
3,
4].
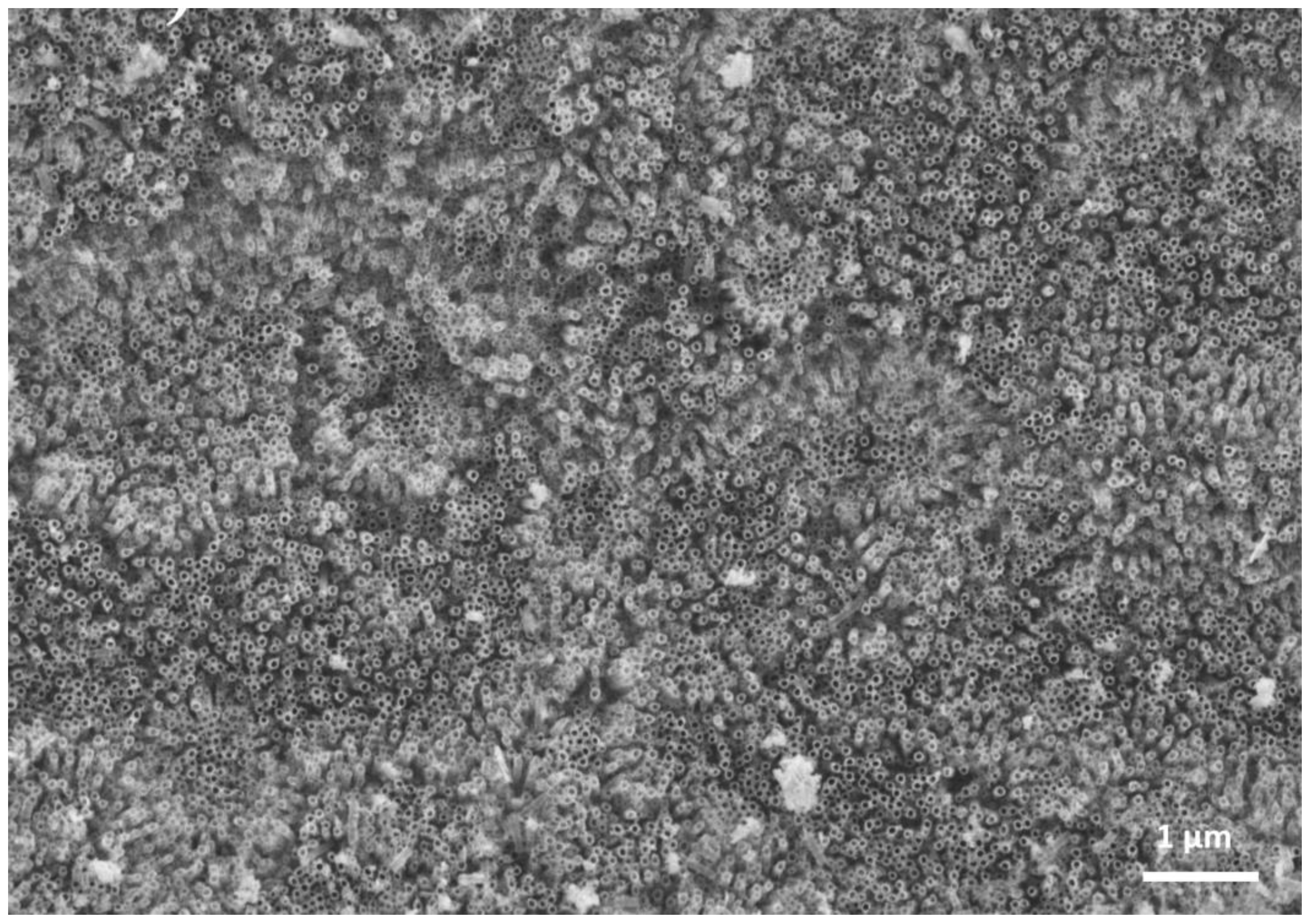
The FESEM results confirmed the presence of nanotubes on the Ti35Nb10Ta substrates fabricated by the anodic oxidation process (
Figure 2). Based on these FESEM images, the inner diameter was estimated. The nanotubes inner diameter increased with the higher voltages employed during anodization process [
20]. Nanotubes were uniformly distributed over the entire surface. The nanotubes average diameter was 38 nm for 15 V and 55 nm for 35 V employed on the anodization process [
20].
Chemical composition and topographical features are some of the most relevant surface properties for implants because they influence the osteoblastic response and enhance the osteoconductive process [
21,
22]. For this reason, the elemental composition of the oxide layer and the surface topography of the anodic oxidized samples were studied. The energy dispersive X-ray analysis results of the studied titanium alloys are summarized in
Table 3.
After performing the anodic oxidation treatment, the EDS analysis detected a thicker stable oxide formation on all the titanium alloys surfaces. The amount of detected oxygen was around 30% wt. for the anodic oxidized samples. The anodic oxidation process did not only increase the amount of oxygen on the surface of the studied alloys, but also added a fluorine element to its chemical composition. Macak et al. (2007) [
23] and Puckett et al. (2010) [
24] have reported the presence of fluorine on the titanium nanotubes, which thus corroborates the results obtained herein. Electrolyte fluorine ions react with titanium to form a water-soluble (TiF
6)
2− complex [
23]. This element may affect the osteoblast proliferation and differentiation [
25], and could enhance the hydrophobic nature of the surface due to the low surface energy of the Ti-F bond. The heat treatment carried out at 320 °C for 30 min in a vacuum reduced the nanotube fluorine weight content from 5% to 1% (
Table 3). The main nanotube structure was preserved after heat treatment, and no visible changes in the diameter and shape of the TiO
2 nanotubes were observed in FESEM.
The osseointegration process depends on the interaction between the cell and the implant surface. The topography, chemical composition and surface energy plays a significant role in the adhesion, differentiation and proliferation of cells [
21]. Surface wettability depends on the interaction between the liquid and the titanium surface.
Table 4 shows the contact angles for the four liquids used on all the tested surfaces. This table also reports the acid etching surface treatment done with the Ti6Al4V casting alloy for comparison purposes. This acid etched surface treatment is currently employed in commercial implants to improve cell adhesion [
26,
27,
28]. The acid-etching samples were obtained in two separate stages; first, the samples were immersed in HF and then they were immersed in a mixture of HCl and HF. In order to study the influence of the fluorine content on the surface energy of the beta titanium alloy, heat treatment was carried out after nanotube formation by the anodic oxidation process.
Roughness and topographical features are some of the most relevant surface properties that modify the wettability behavior of the surfaces. A conventional acid-etched surface treatment is capable of modifying titanium surfaces within a microroughness range from 1 to 10 µm [
1]. The increased microroughness in the Ti6Al4V acid etched sample has increased the contact angle in the studied four liquids compared to the polished Ti35Nb10Ta surface (
Table 4).
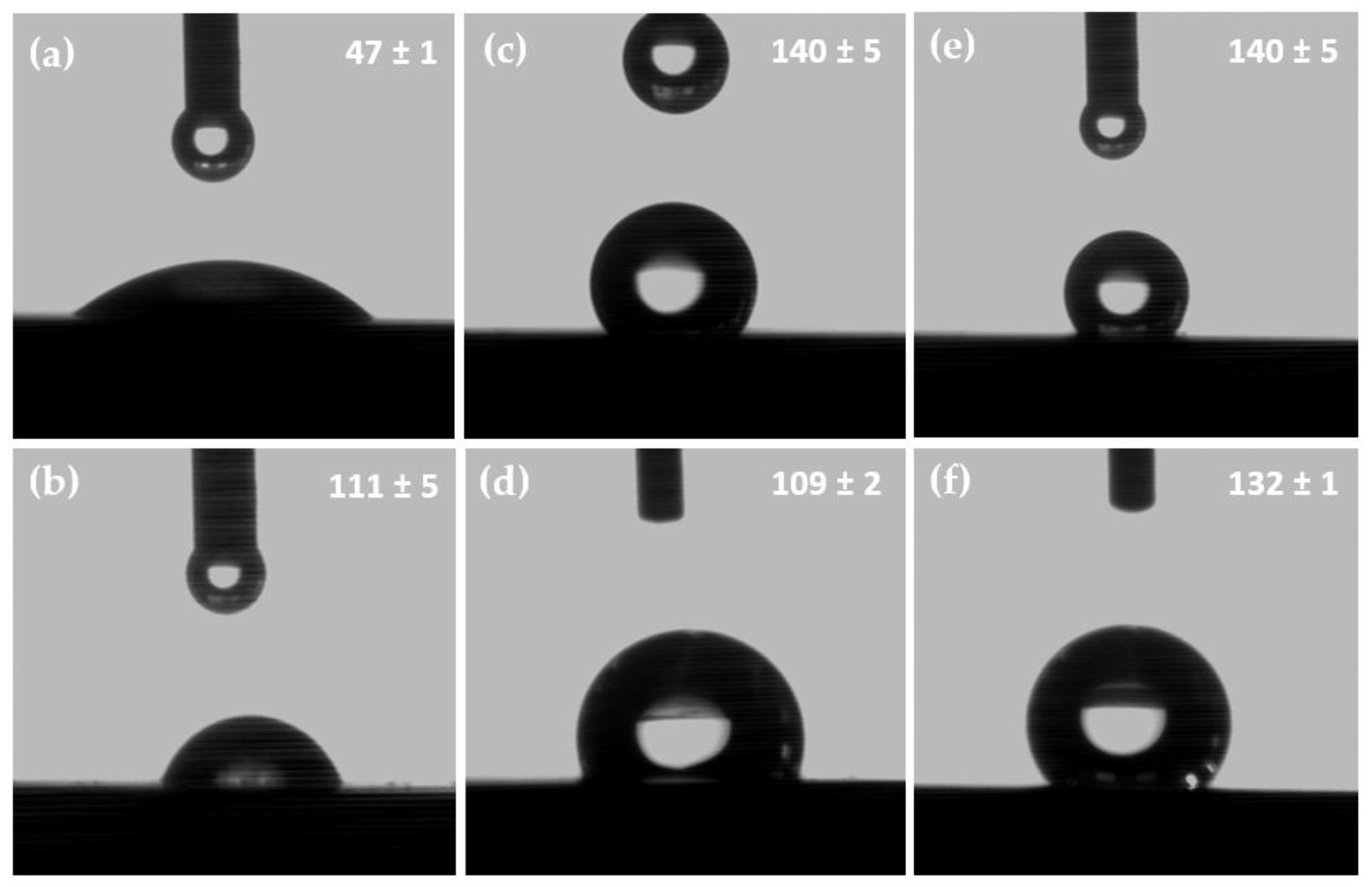
Nanotubular surfaces had much higher contact angles for each liquid used to determine the surface energy compared with the polished sample (
Table 4), which indicates the nanotubular morphology that the highest surface energy had. The nanotube that formed after running the anodic oxidation process for 45 min and without heat treatment exhibited the biggest contact angle in water (140°) compared to the 47° value obtained for the polished surface or the one of 109° for the nanotubes obtained at 15 V and with heat treatment (
Figure 3).
Nanotubular morphology and the fluorine content are considered two factors that increase the surface’s hydrophobic nature [
29,
30,
31,
32,
33]. Contact angles substantially increase for the nanotube surface; the layers with large contact angles display hydrophobic properties. After heat treatment the fluorine weight content lowered (
Table 3), the contact angle in the studied four liquids reduced and surface wettability increased. The fluorine atoms displayed low polarizability and high electronegativity (3.98) compared to hydrogen (2.20) and titanium (1.54) [
33]. The titanium-fluorine bonds are polarized due to the high electronegativity of fluorine. The lack of a permanent dipole moment contributes to increase the liquid surface hydrophobicity on the nanotube surfaces. The wettability of a solid surface by a liquid is determined by the liquid’s surface tension, the solid surface’s roughness and the solid’s surface energy. The higher contact angle values found for the dark-stored nanotubes can be explained mainly by the hydrophobicity due to the Cassie-Bexter state where the liquid does not penetrate into the hollows of the nanotube surface and, consequently, faces a composite interface consisting of both solid and vapor; and also to the non-photoinduced hydrophilicity of TiO
2 [
32,
33,
34,
35,
36,
37]. The nanotube morphology allowed the air still trapped inside the nanotubes and the behavior to become substantially hydrophobic [
32,
33].
As previously described, the use of four liquids allows us to obtain the surface free energy values by the Owens-Wend method.
Table 5 shows the values for the dispersive and polar component parts of the SFE. The dispersive component of the Ti35Nb10Ta alloy increased after the anodic oxidation process was applied, which initially took values of 26 mJ/m
2 for the polished samples, and increased to exceed 60 mJ/m
2 for the nanotubular surfaces. However, the polar component values lowered for the nanotubular surfaces compared with the polished samples. All the nanotubular surfaces displayed significantly higher surface free energy than the unmodified and polished Ti35Nb10Ta surfaces, and the microrough surfaces obtained in conventional acid etched Ti6Al4V alloys.
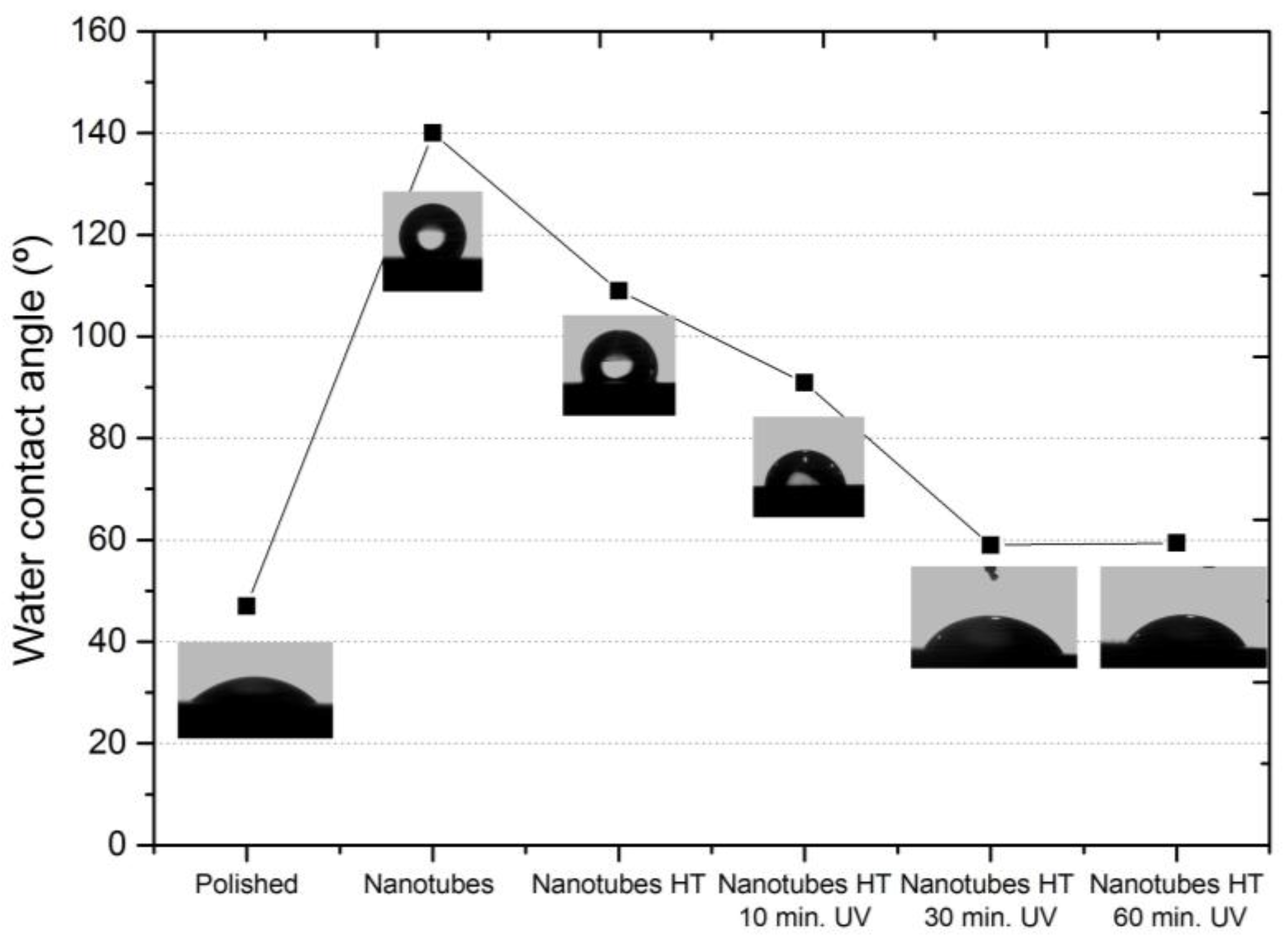
The nanotube formed in the Ti35Nb10Ta alloy had a smaller contact angle after being irradiated with UV light. The water contact angle for the polished Ti35Nb10Ta surfaces obtained an average value of 47°. After the samples had been stored in the dark for 2 weeks, the water contact angle increased to values that exceeded 100°, but the water contact angle reduced to 59° after 30 min of UV irradiation. A smaller water contact angle for the UV-irradiated samples corresponded to a much higher wettability or hydrophilic property (
Figure 4). Wang et al. [
34] reported the same behavior for compact TiO
2 surfaces. The anodic oxidized compact surfaces presented a water contact angle of 15°, the water contact angle increased to 72° after been stored in the dark, and the contact angle reduced to 0° after UV irradiation [
34]. UV irradiation creates oxygen vacancies due to the formation of two coordinated bridging sites, which converts the corresponding Ti
4+ sites to Ti
3+ sites that enhance the amount of absorbed dissociated water and increase the surface free energy [
35]. A gradual reversion of this hydrophilicity has been observed during dark storage [
36].
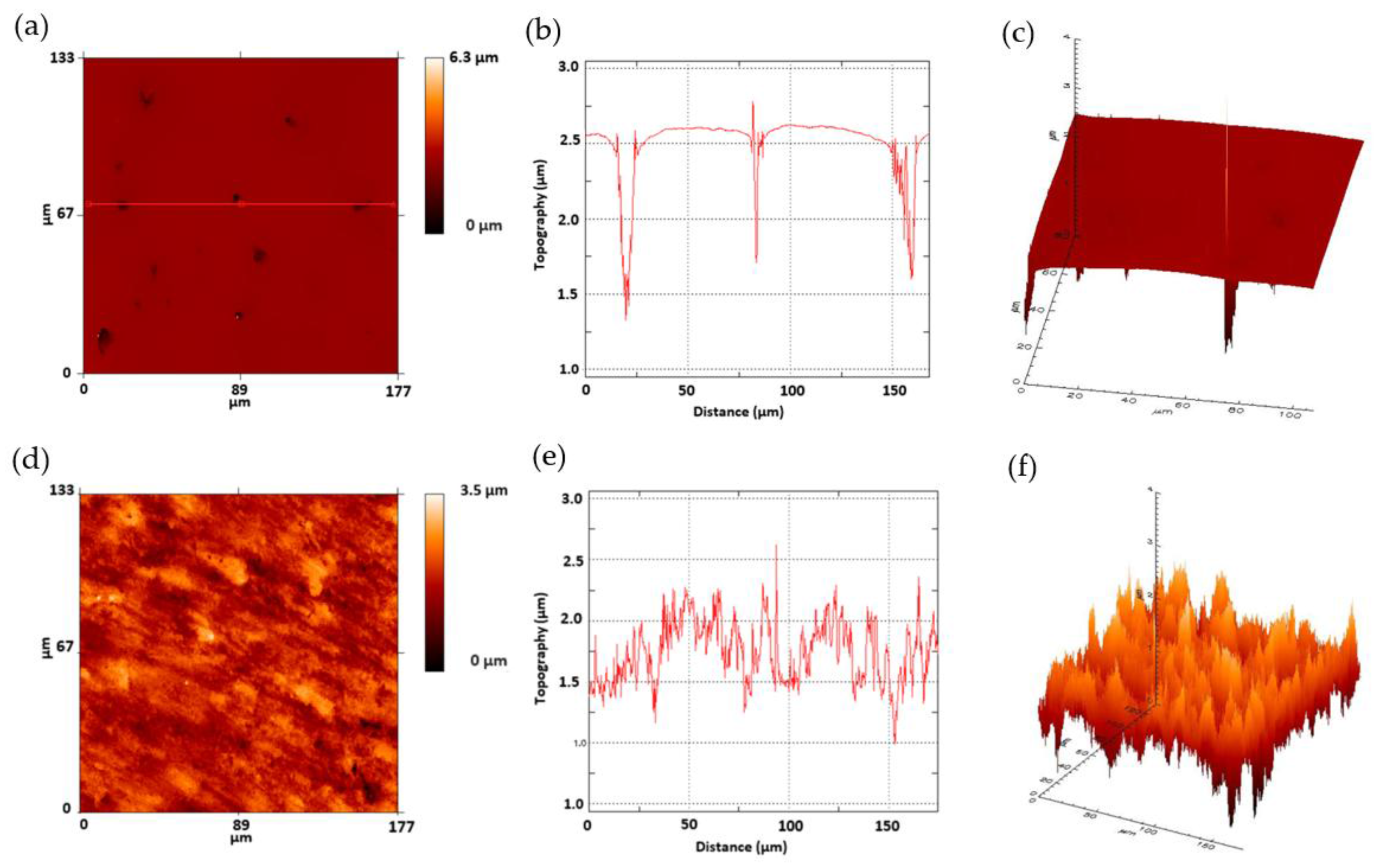
The values of the roughness parameters for the polished and nanotubes Ti35Nb10Ta alloy studied herein are shown in
Table 6. The anodic oxidation process modified the topography of the Ti35Nb10Ta surface by increasing the mean roughness and the quadratic average roughness values. These changes can be explained by the nanoscale modification of the titanium surface by the mechanism of chemical dissolution of Ti, Nb and Ta metals due to the etching effect of the fluorine ions, and by oxidation due to the difference in the applied voltage. This phenomenon was also observed in the 2D and 3D profiles (
Figure 5), obtained by employing the FRT Mark III
® software, when the nanotube surface presented a nanoroughness that increased the surface area. Similar values of peak to valley roughness (
Rz) values were obtained on the polished and nanotube surfaces. These values can be explained by the residual porosity of the powder metallurgy samples, and are clearly observed in
Figure 5b,c. Several studies have demonstrated that nanotubes topography is beneficial for the biomechanical anchorage of implants [
8,
9,
11,
12,
21].