An Eco-Friendly Neutralization Process by Carbon Mineralization for Ca-Rich Alkaline Wastewater Generated from Concrete Sludge
Abstract
:1. Introduction
2. Materials and Methods
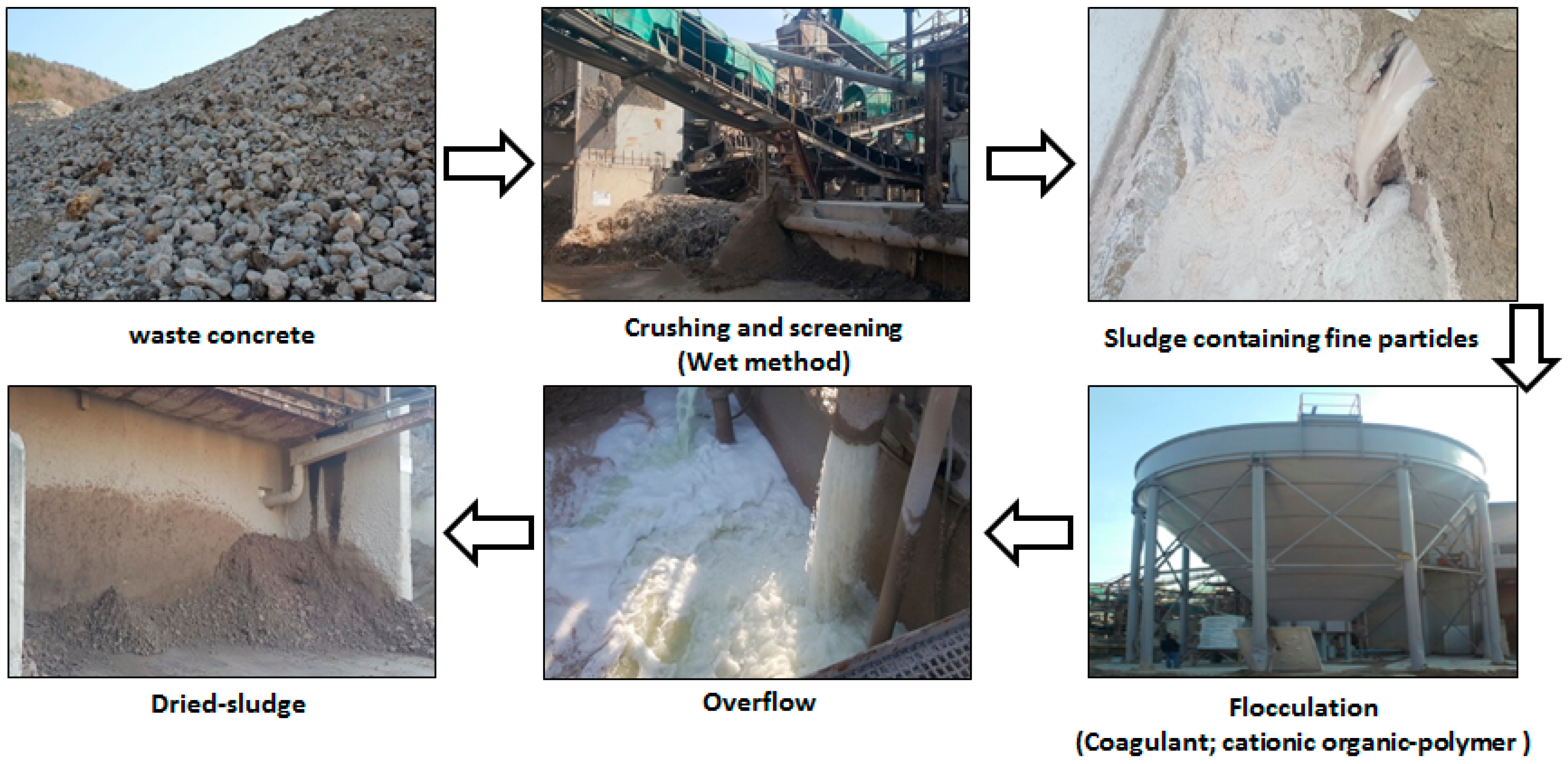
2.1. Characterization of Concrete Sludge
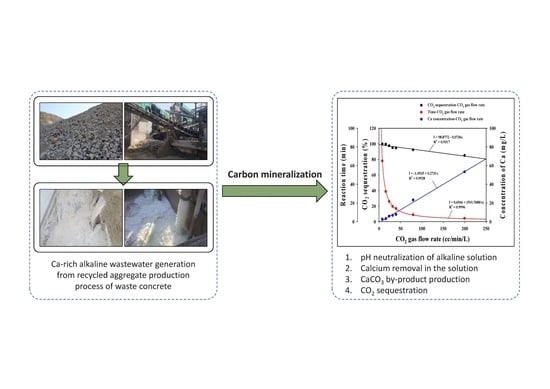
2.2. Carbon Mineralization
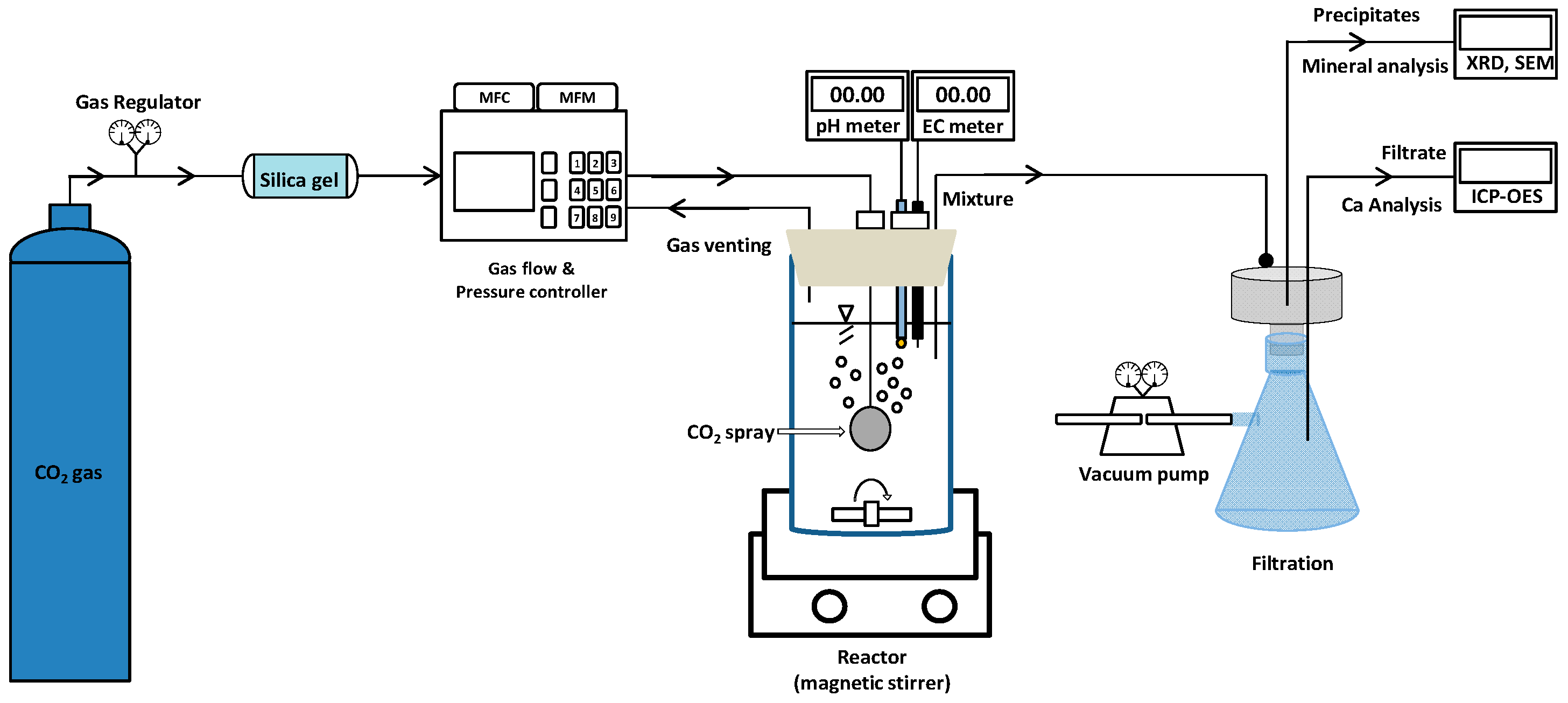
2.2.1. Apparatus of the Carbon Mineralization Processing Reactor
2.2.2. Carbon Mineralization Experiment
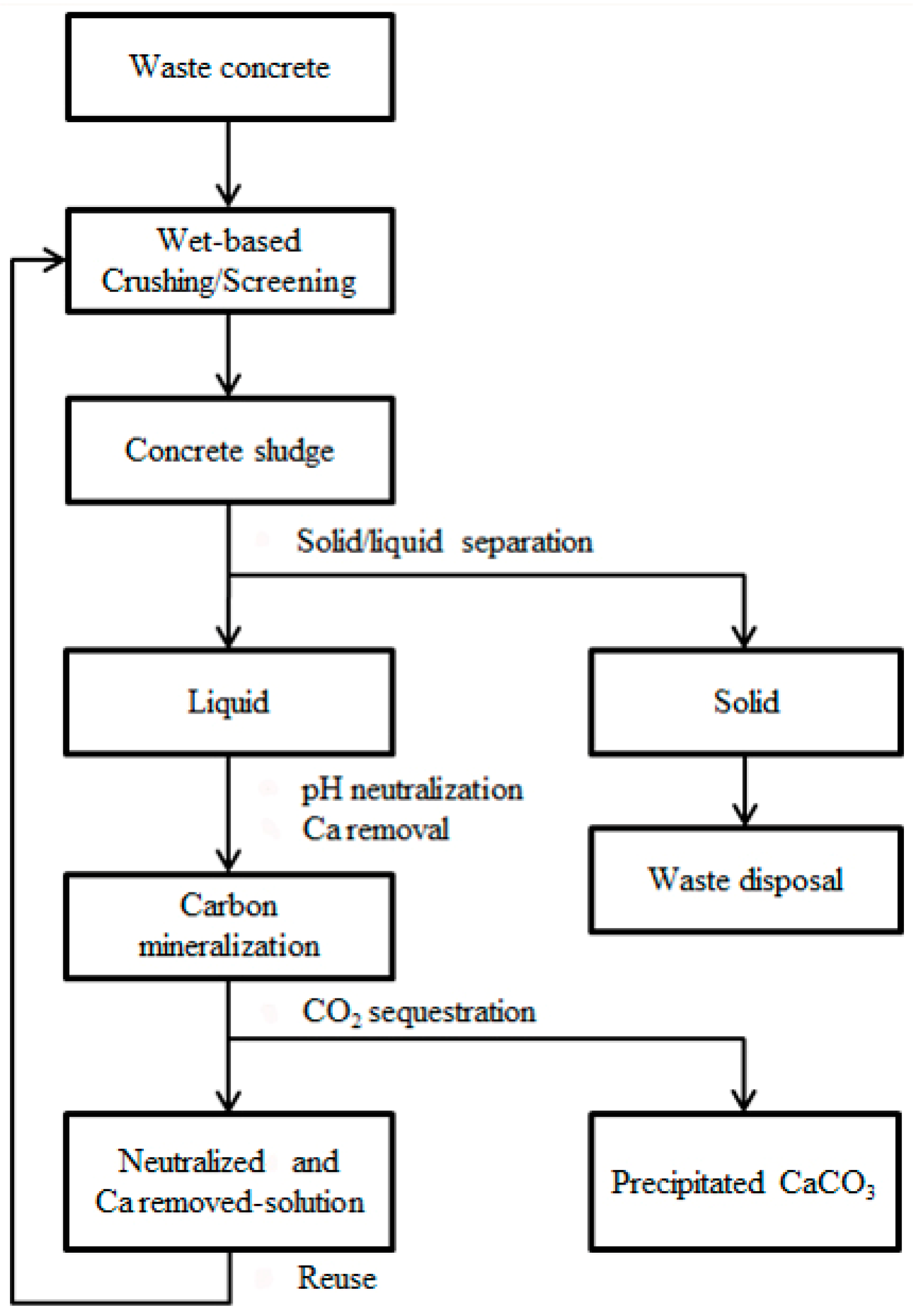
2.3. Neutralized Water Recycling
3. Results and Discussion
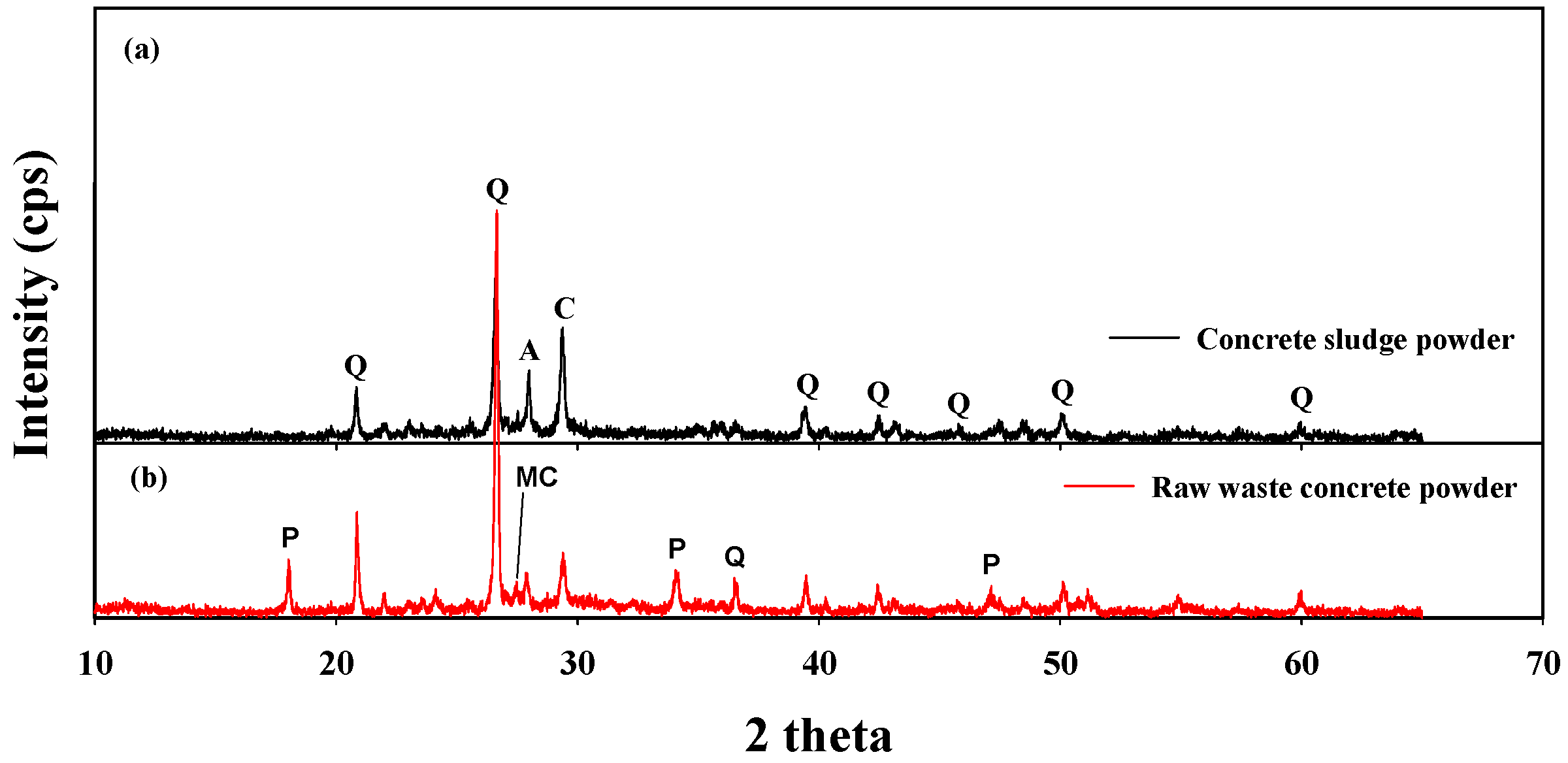
3.1. Physicochemical Characteristics of the Sample
3.2. Carbon Mineralization
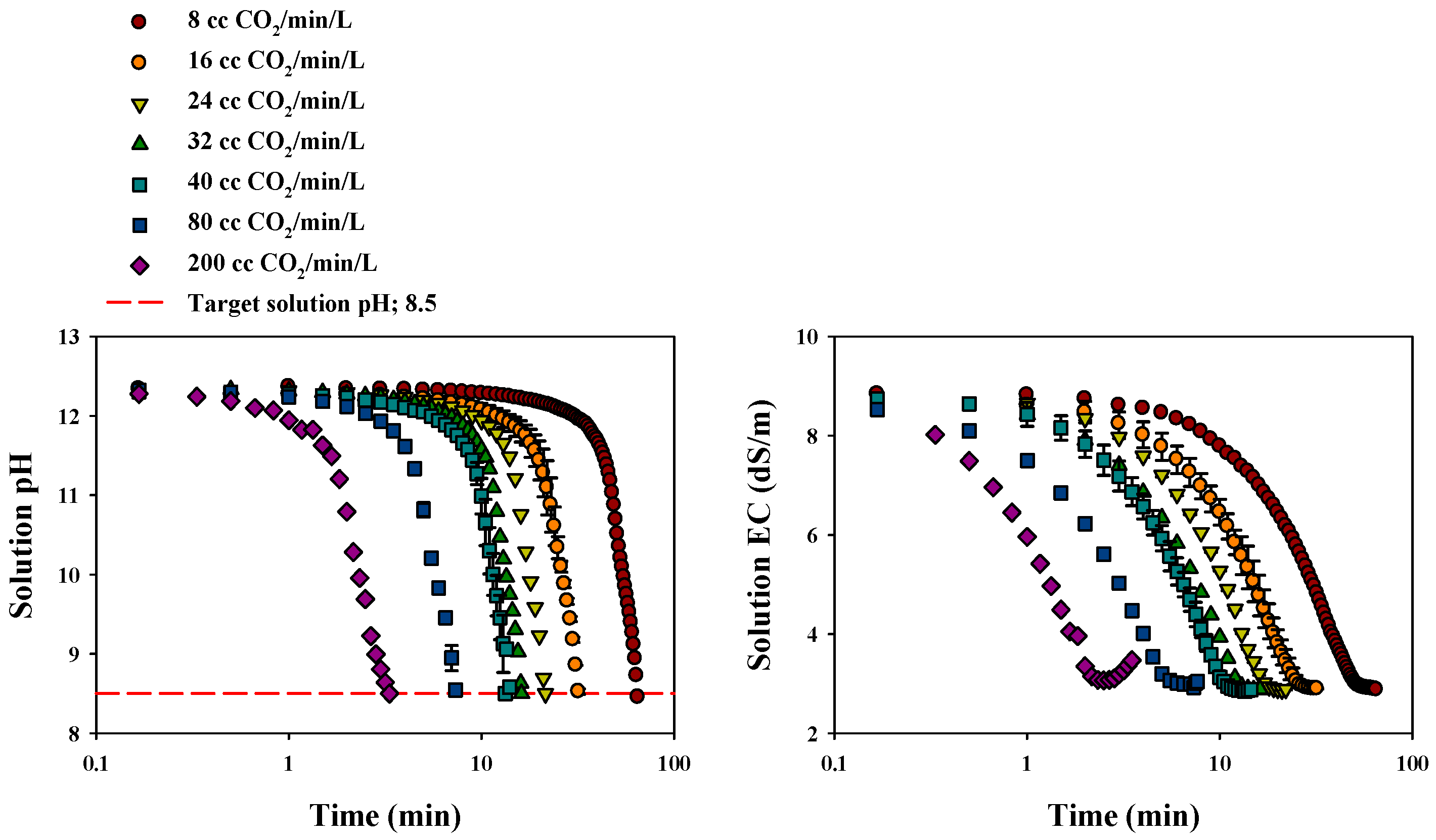
3.2.1. Changes in Solution pH and EC
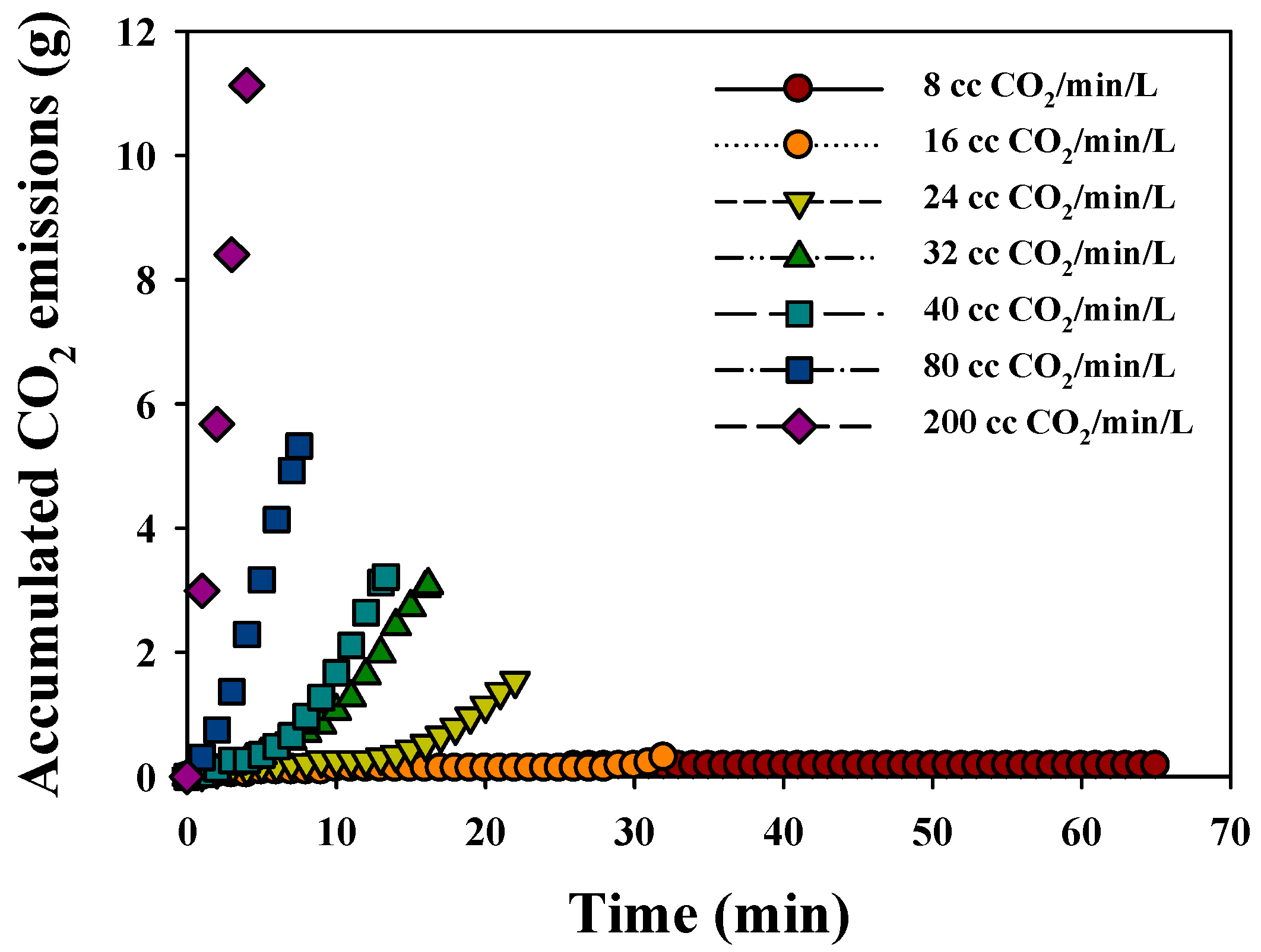
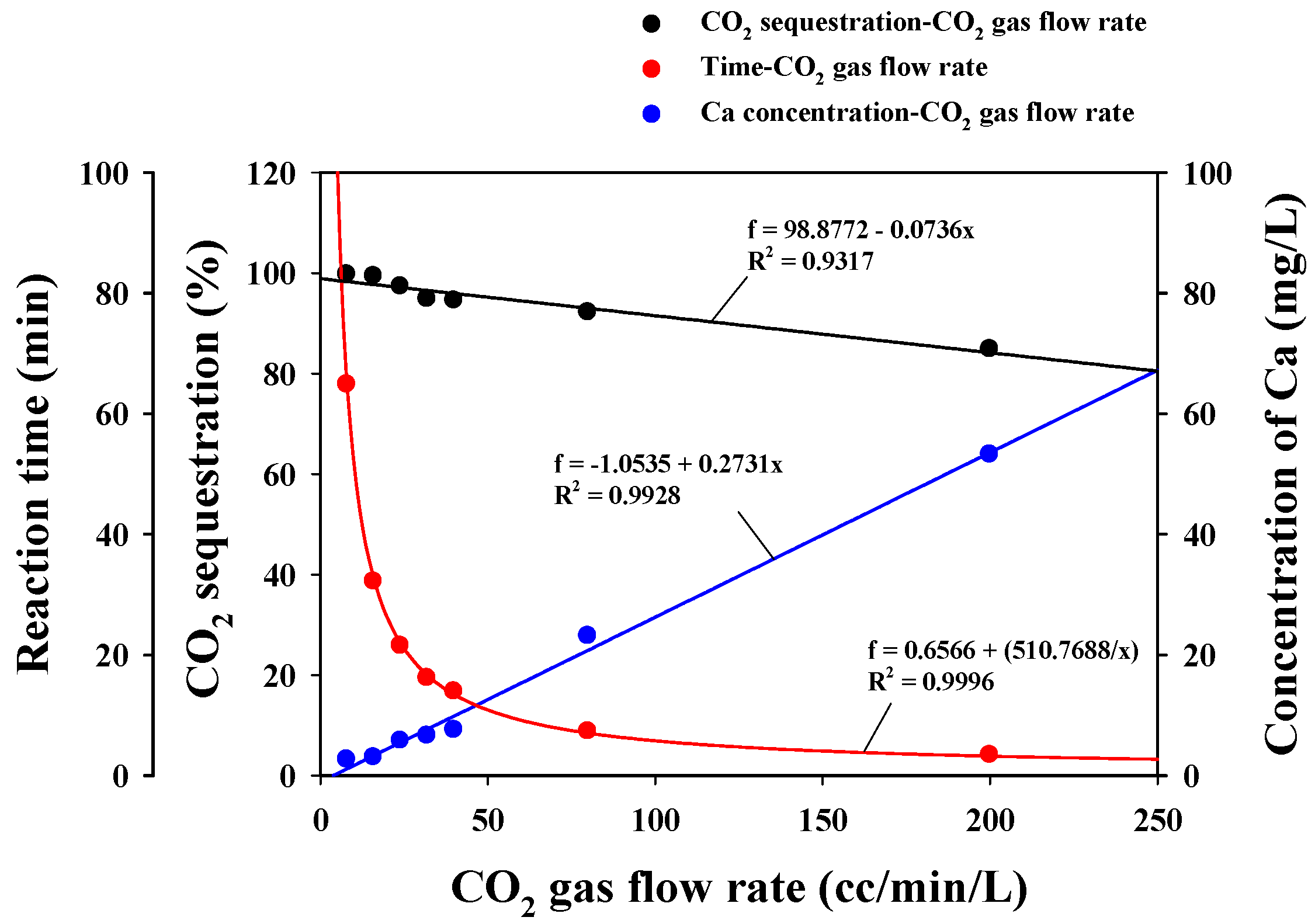
3.2.2. CO2 Sequestration
3.3. Evaluation of Neutralized Waste Water
4. Conclusions
- (1)
- Providing an eco-friendly neutralization process for the treatment of alkaline wastewater.
- (2)
- Improving the cement paste removal process through the reuse of neutralized wastewater in the wet-based crushing process.
- (3)
- Sequestering CO2 in CaCO3.
- (4)
- Producing, as a by-product, a commercially viable pure source of CaCO3.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shin, H.Y.; Ji, S.; Woo, J.Y.; Ahn, G.O.; An, S.H. A feasibility study on the utilization of by-product sludge generated from waste concrete recycling process. J. Korean Inst. Resour. Recycl. 2016, 25, 29–36. [Google Scholar] [CrossRef]
- Ministry of Environment. Statistics Status of waste generation and treatment. In Construction Wastes; Sejong-si, Korea. Available online: http://stat.me.go.kr (accessed on 22 May 2017).
- Ghacham, A.B.; Pasquier, L.C.; Cecchi, E.; Blais, J.F.; Mercier, G. Valorization of waste concrete through CO2 mineral carbonation: Optimizing parameters and improving reactivity using concrete separation. J. Clean. Prod. 2017, 166, 869–878. [Google Scholar] [CrossRef]
- Behera, M.; Bhattacharyya, S.; Minocha, A.; Deoliya, R.; Maiti, S. Recycled aggregate from C&D waste & its use in concrete—A breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar]
- Tsunashima, Y.; Iizuka, A.; Akimoto, J.; Hongo, T.; Yamasaki, A. Preparation of sorbents containing ettringite phase from concrete sludge and their performance in removing borate and fluoride ions from waste water. Chem. Eng. J. 2012, 200, 338–343. [Google Scholar] [CrossRef]
- Iizuka, A.; Sakai, Y.; Yamasaki, A.; Honma, M.; Hayakawa, Y.; Yanagisawa, Y. Bench-scale operation of a concrete sludge recycling plant. Ind. Eng. Chem. Res. 2012, 51, 6099–6104. [Google Scholar] [CrossRef]
- Lim, M.; Han, G.C.; Ahn, J.W.; You, K.S. Environmental remediation and conversion of carbon dioxide (CO2) into useful green products by accelerated carbonation technology. Int. J. Environ. Res. Public Health 2010, 7, 203–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Kang, D.; Yoo, Y.; Jo, H.; Song, H.J.; Park, J. Continuous and simultaneous CO2 absorption, calcium extraction, and production of calcium carbonate using ammonium nitrate. Ind. Eng. Chem. Res. 2016, 55, 11795–11800. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chang, E.; Chiang, P.C. CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Ghacham, A.B.; Cecchi, E.; Pasquier, L.C.; Blais, J.F.; Mercier, G. CO2 sequestration using waste concrete and anorthosite tailings by direct mineral carbonation in gas–solid–liquid and gas–solid routes. J. Environ. Manag. 2015, 163, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Bertos, M.F.; Simons, S.; Hills, C.; Carey, P. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar]
- Montes-Hernandez, G.; Renard, F.; Geoffroy, N.; Charlet, L.; Pironon, J. Calcite precipitation from CO2–H2 O–Ca (OH)2 slurry under high pressure of CO2. J. Cryst. Growth 2007, 308, 228–236. [Google Scholar] [CrossRef]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Effect of flow rate and CO2 content on the phase and morphology of CaCO3 prepared by bubbling method. J. Cryst. Growth 2005, 276, 541–548. [Google Scholar] [CrossRef]
- Iizuka, A.; Sasaki, T.; Honma, M.; Yoshida, H.; Hayakawa, Y.; Yanagisawa, Y.; Yamasaki, A. Pilot-Scale Operation of a Concrete Sludge Recycling Plant and Simultaneous Production of Calcium Carbonate. Chem. Eng. Commun. 2017, 204, 79–85. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Lee, M.G.; Kang, D.; Jo, H.; Park, J. Carbon dioxide utilization with carbonation using industrial waste-desulfurization gypsum and waste concrete. J. Mater. Cycles Waste 2016, 18, 407–412. [Google Scholar] [CrossRef]
- Palaniandy, S.; Kadir, N.A.; Jaafar, M. Value adding limestone to filler grade through an ultra-fine grinding process in jet mill for use in plastic industries. Miner. Eng. 2009, 22, 695–703. [Google Scholar] [CrossRef]
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Chuajiw, W.; Takatori, K.; Igarashi, T.; Hara, H.; Fukushima, Y. The influence of aliphatic amines, diamines, and amino acids on the polymorph of calcium carbonate precipitated by the introduction of carbon dioxide gas into calcium hydroxide aqueous suspensions. J. Cryst. Growth 2014, 386, 119–127. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, S.V.; Waghmare, S.A.; Sabale, P. Aragonite–vaterite–calcite: Polymorphs of CaCO3 in 7th century CE lime plasters of Alampur group of temples, India. Constr. Build. Mater. 2016, 112, 386–397. [Google Scholar] [CrossRef]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Mineral carbonation from metal wastes: Effect of solid to liquid ratio on the efficiency and characterization of carbonated products. Appl. Energy 2014, 113, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Phung, Q.T.; Maes, N.; Jacques, D.; De Schutter, G.; Ye, G.; Perko, J. Modelling the carbonation of cement pastes under a CO2 pressure gradient considering both diffusive and convective transport. Constr. Build. Mater. 2016, 114, 333–351. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Hand Book of Chemistry, 13th ed.; McGrawHill, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Cordeiro, G.C.; Sales, C.P. Pozzolanic activity of elephant grass ash and its influence on the mechanical properties of concrete. Cem. Concr. Compos. 2015, 55, 331–336. [Google Scholar] [CrossRef]
- Morsy, M.S. Effect of temperature on electrical conductivity of blended cement pastes. Cem. Concr. Res. 1999, 29, 603–606. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Galanos, A.; Doganis, I.; Kallithrakas-Kontos, N. Physico-chemical characterization of mortars as a tool in studying specific hydraulic components: Application to the study of ancient Naxos aqueduct. Appl. Phys. A Mater. 2011, 104, 335–348. [Google Scholar] [CrossRef]
- Gulotta, D.; Goidanich, S.; Tedeschi, C.; Nijland, T.G.; Toniolo, L. Commercial NHL-containing mortars for the preservation of historical architecture. Part 1: Compositional and mechanical characterisation. Constr. Build. Mater. 2013, 38, 31–42. [Google Scholar] [CrossRef]
- Mackie, A.L.; Walsh, M.E. Bench-scale study of active mine water treatment using cement kiln dust (CKD) as a neutralization agent. Water Res. 2012, 46, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Ji, S.; Lee, P.K.; Oh, C. Bauxite residue neutralization with simultaneous mineral carbonation using atmospheric CO2. J. Hazard. Mater. 2017, 326, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Daud, A.R.M. Mineral carbonation of red gypsum via pH-swing process: Effect of CO2 pressure on the efficiency and products characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Sahu, R.C.; Patel, R.K.; Ray, B.C. Neutralization of red mud using CO2 sequestration cycle. J. Hazard. Mater. 2010, 179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.Y.; Kim, J.H.; Lee, Y.J.; Lee, M.; Choh, S.J. Evaluation of factors affecting mineral carbonation of CO2 using coal fly ash in aqueous solutions under ambient conditions. Chem. Eng. J. 2012, 183, 77–87. [Google Scholar] [CrossRef]
- Han, S.J.; Im, H.J.; Wee, J.H. Leaching and indirect mineral carbonation performance of coal fly ash-water solution system. Appl. Energy 2015, 142, 274–282. [Google Scholar] [CrossRef]
- Chang, E.E.; Chiu, A.C.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Jo, H.; Young Jo, H.; Jang, Y.N. Effect of extraction solutions on carbonation of cementitious materials in aqueous solutions. Environ. Technol. 2012, 33, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.Y.; Ahn, J.H.; Jo, H. Evaluation of the CO2 sequestration capacity for coal fly ash using a flow-through column reactor under ambient conditions. J. Hazard. Mater. 2012, 241, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Jo, H.; Lee, M.G.; Park, J. Carbon dioxide utilization using a pretreated brine solution at normal temperature and pressure. Chem. Eng. J. 2016, 284, 1270–1278. [Google Scholar] [CrossRef]
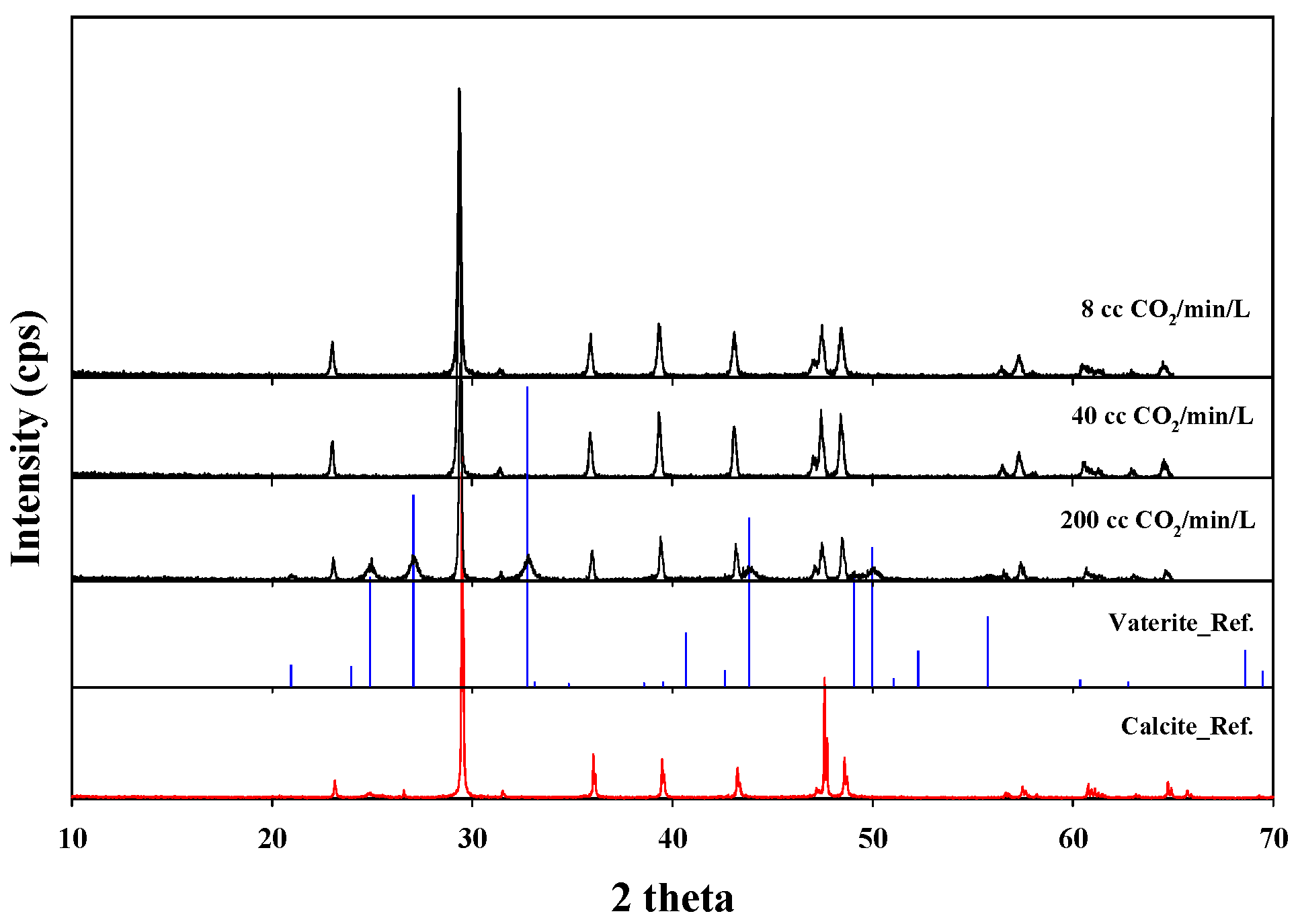
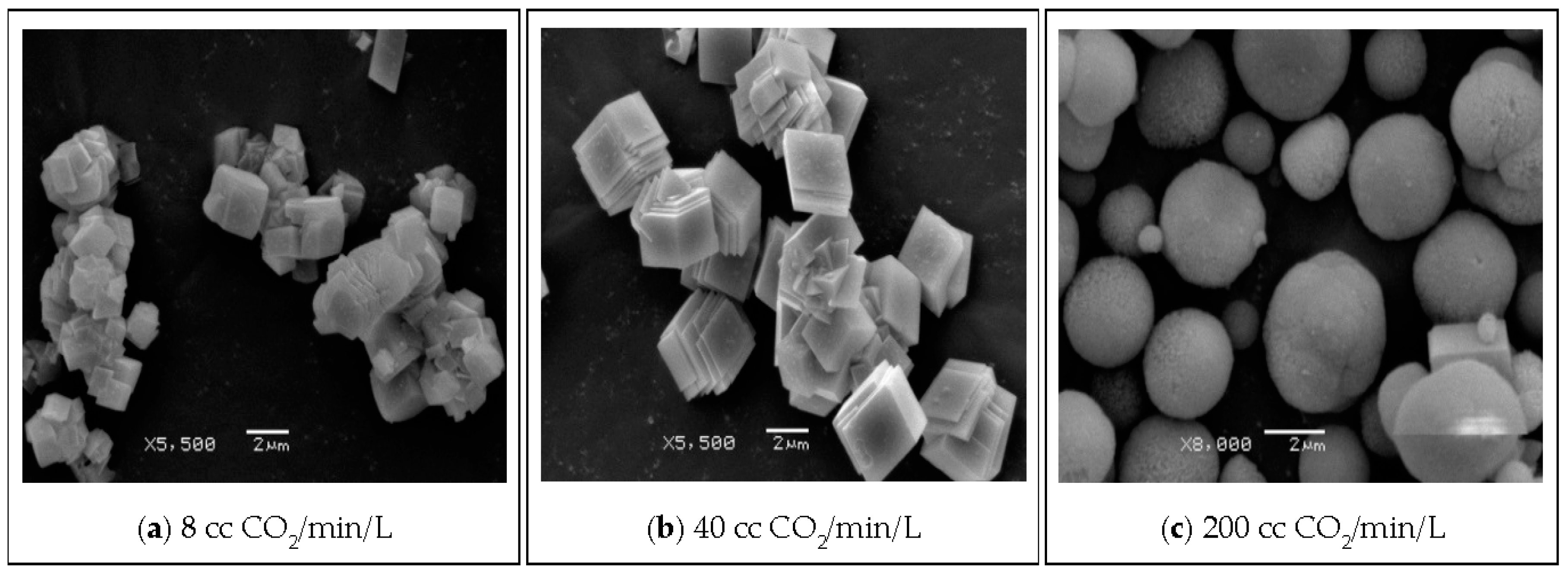
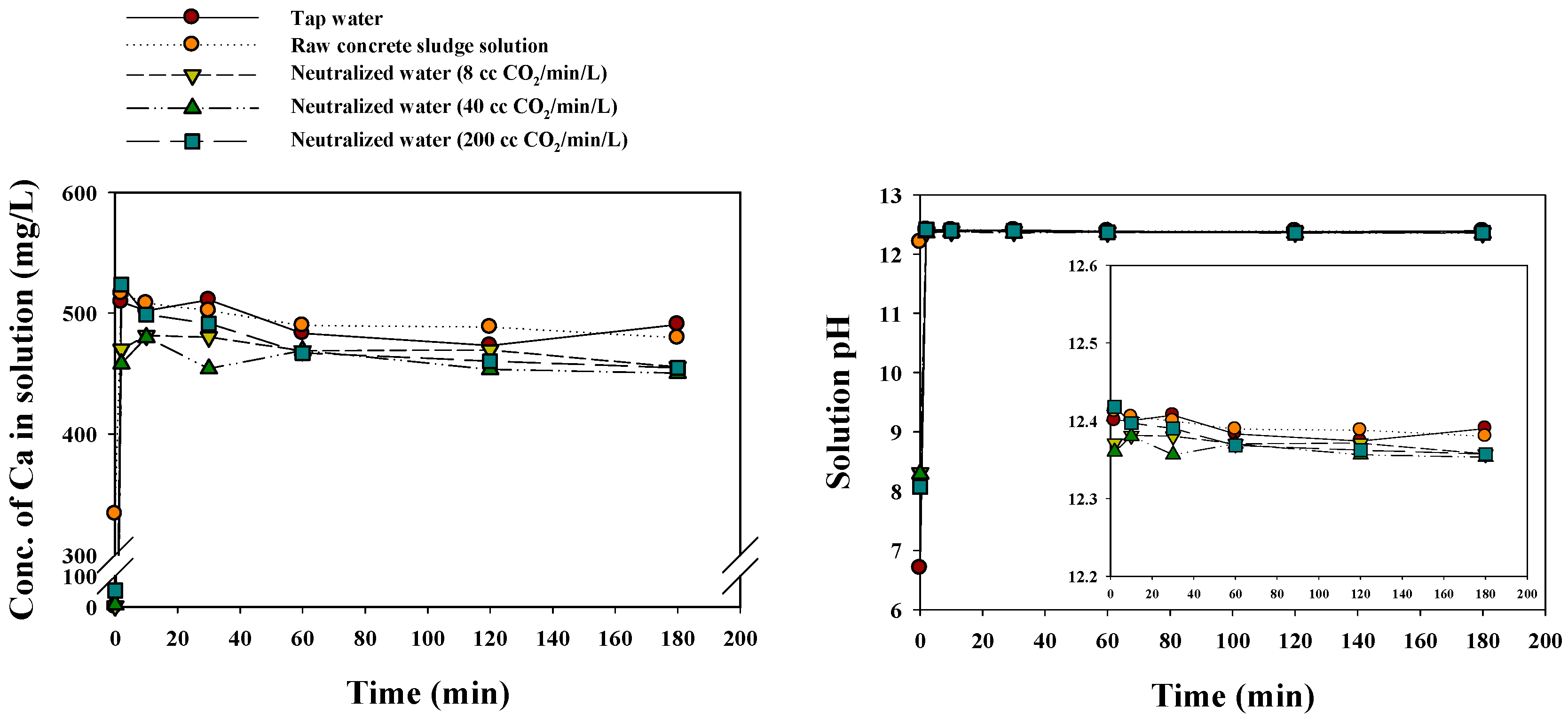
| Parameter | Value |
|---|---|
| pH | 12.2 |
| EC (dS/m) | 8.9 |
| ORP a (mV) | −108.0 |
| Moisture content (%) | 92.5 |
| Particle size distribution (wt %) (KS F 2309 b) | |
| >70 mesh (>0.212 mm) | 1.3 |
| 70–100 mesh (0.212–0.150 mm) | 0.4 |
| 100–200 mesh (0.150–0.075 mm) | 7.9 |
| 200–325 mesh (0.075–0.043 mm) | 9.5 |
| 325–400 mesh (0.043–0.038 mm) | 7.7 |
| <400 mesh (<0.038 mm) | 73.1 |
| Elements in aqueous solution (mg/L) | |
| Ca | 333.9 ± 2.0 |
| K | 257.1 ± 1.4 |
| Na | 317.5 ± 0.0 |
| Oxide | Composition (wt %) | |
|---|---|---|
| Dried Concrete Sludge | Raw Waste Concrete | |
| SiO2 | 45.9 | 38.5 |
| CaO | 20.2 | 30.2 |
| Al2O3 | 9.4 | 6.9 |
| Fe2O3 | 3.0 | 4.1 |
| MgO | 1.5 | 1.8 |
| K2O | 2.3 | 1.9 |
| LOI a | 15.4 | 14.5 |
| Others b | 1.8 | 1.6 |
| CO2 Gas Flow Rate (cc/min/L) | Time (min) | Final EC (dS/m) | Total CO2 Injection (g) | Total CO2 Venting (mg) | CO2 Sequestration (1 − (Vout/Vin)) | Conc. of Ca (mg/L) | Precipitated CaCO3 (g) |
|---|---|---|---|---|---|---|---|
| 8 | 65.0 | 2.9 | 2.38 | 7.33 | 1.00 | 2.64 | 2.69 |
| 16 | 32.0 | 2.9 | 2.09 | 13.74 | 0.99 | 3.00 | 2.60 |
| 24 | 22.0 | 2.9 | 2.23 | 59.52 | 0.97 | 5.76 | 2.64 |
| 32 | 16.2 | 2.9 | 2.33 | 120.87 | 0.95 | 6.58 | 2.58 |
| 40 | 13.3 | 2.9 | 2.35 | 129.11 | 0.95 | 7.55 | 2.66 |
| 80 | 7.5 | 3.0 | 2.72 | 212.44 | 0.92 | 23.13 | 2.50 |
| 200 | 3.4 | 3.5 | 3.05 | 463.33 | 0.85 | 53.21 | 1.78 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Shin, H.; Ji, S. An Eco-Friendly Neutralization Process by Carbon Mineralization for Ca-Rich Alkaline Wastewater Generated from Concrete Sludge. Metals 2017, 7, 371. https://doi.org/10.3390/met7090371
Yoo J, Shin H, Ji S. An Eco-Friendly Neutralization Process by Carbon Mineralization for Ca-Rich Alkaline Wastewater Generated from Concrete Sludge. Metals. 2017; 7(9):371. https://doi.org/10.3390/met7090371
Chicago/Turabian StyleYoo, Jongchan, Heeyoung Shin, and Sangwoo Ji. 2017. "An Eco-Friendly Neutralization Process by Carbon Mineralization for Ca-Rich Alkaline Wastewater Generated from Concrete Sludge" Metals 7, no. 9: 371. https://doi.org/10.3390/met7090371