Microstructure and Strengthening-Toughening Mechanism of Nitrogen-Alloyed 4Cr5Mo2V Hot-Working Die Steel
Abstract
:1. Introduction
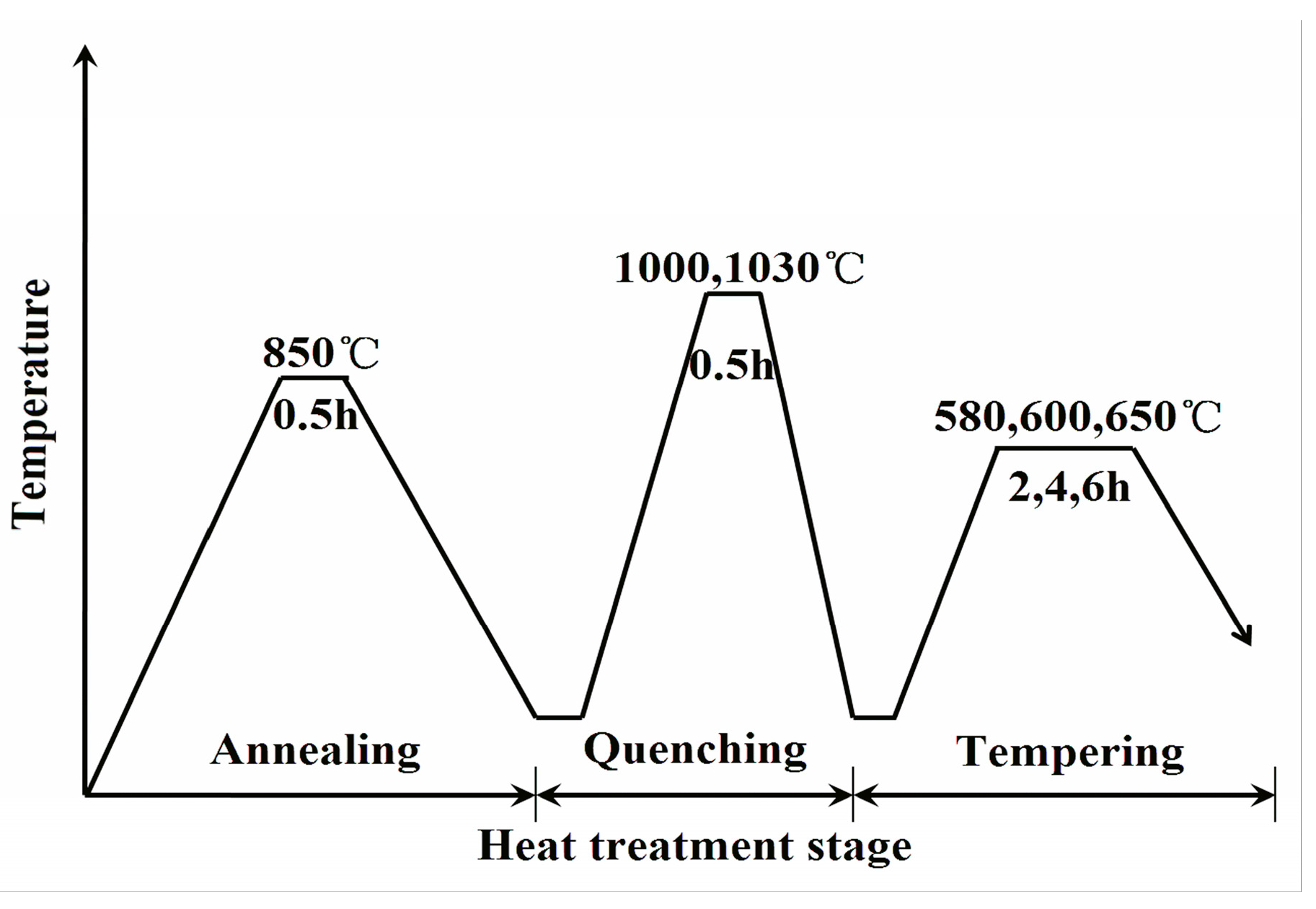
2. Materials and Methods
3. Results
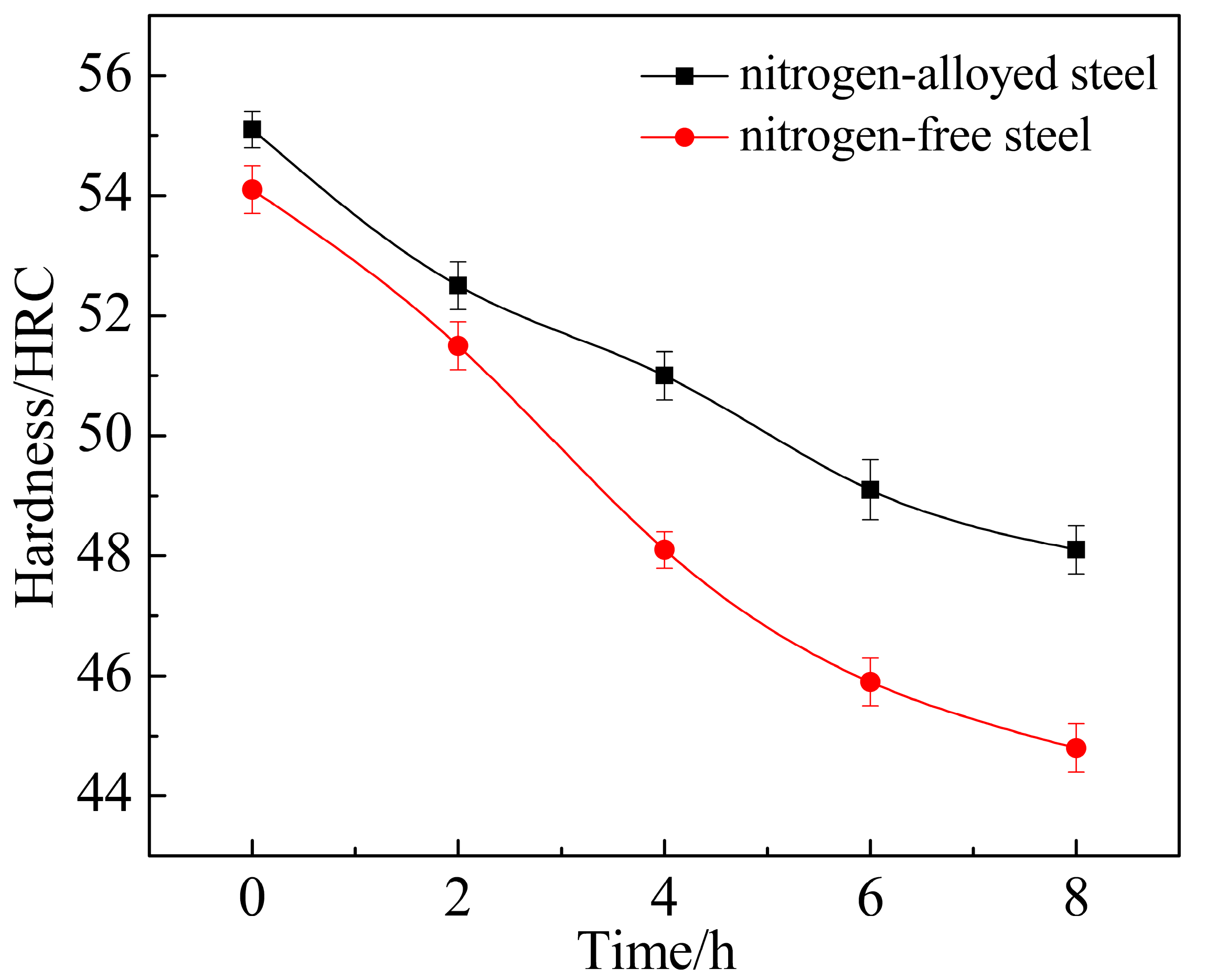
3.1. Mechanical Properties
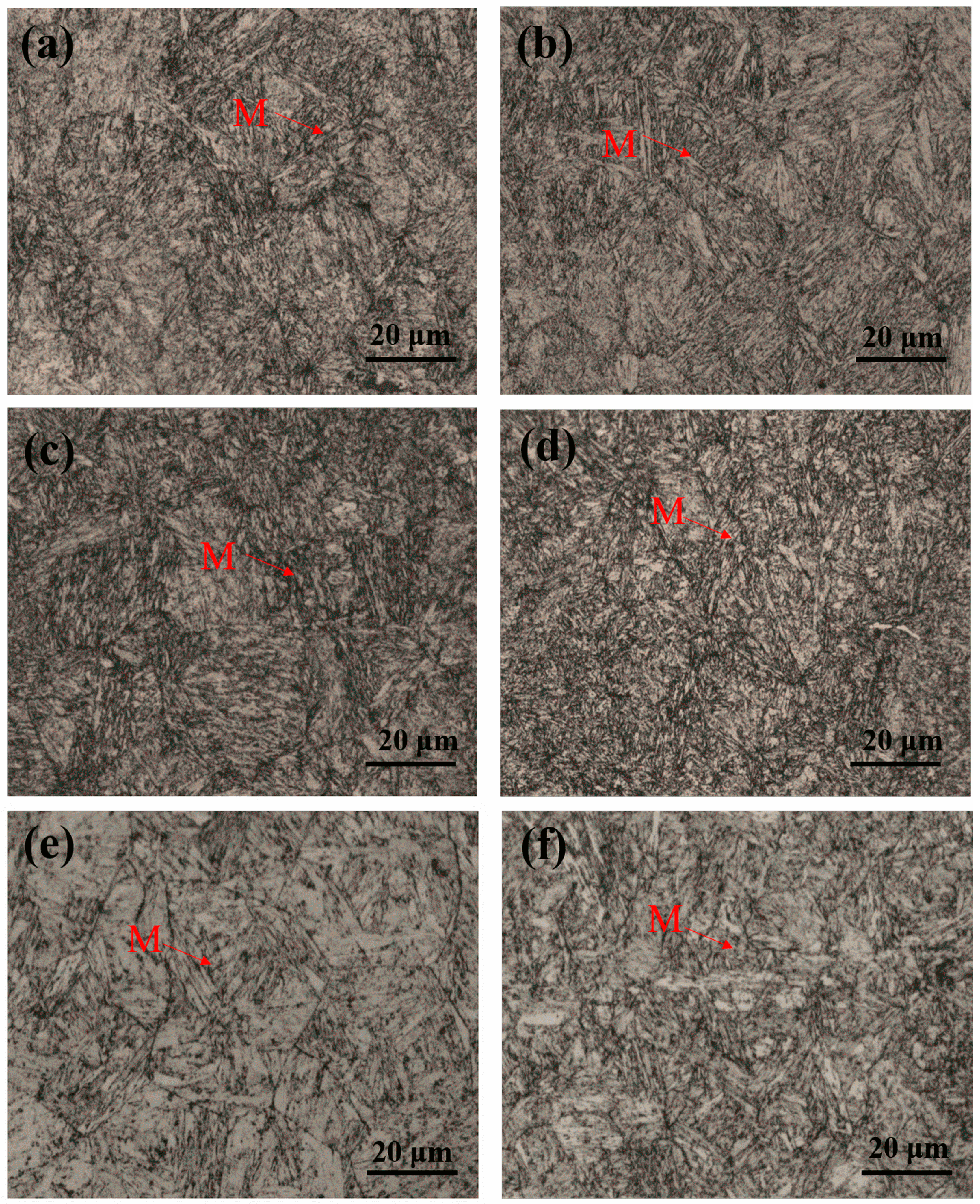
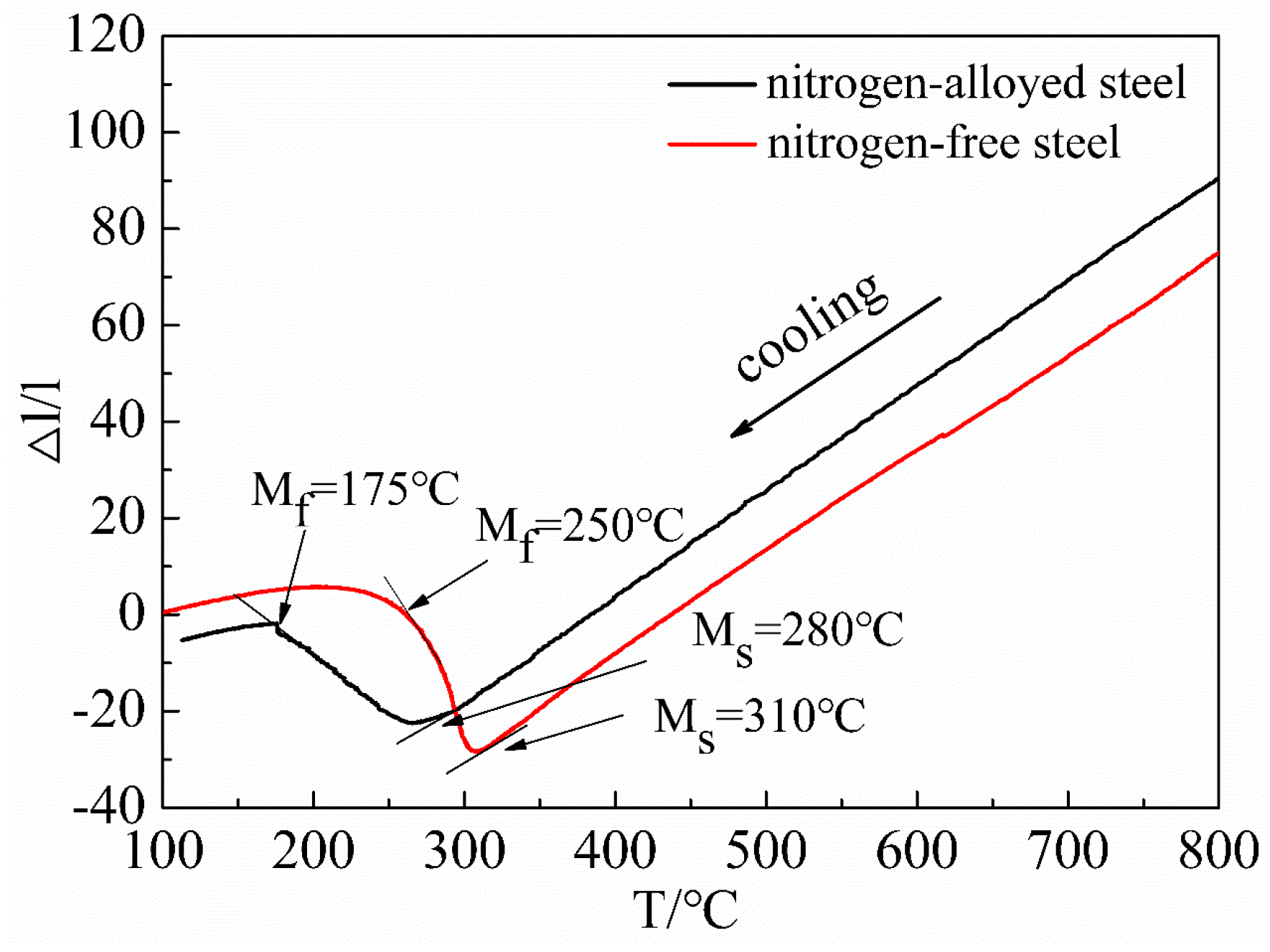
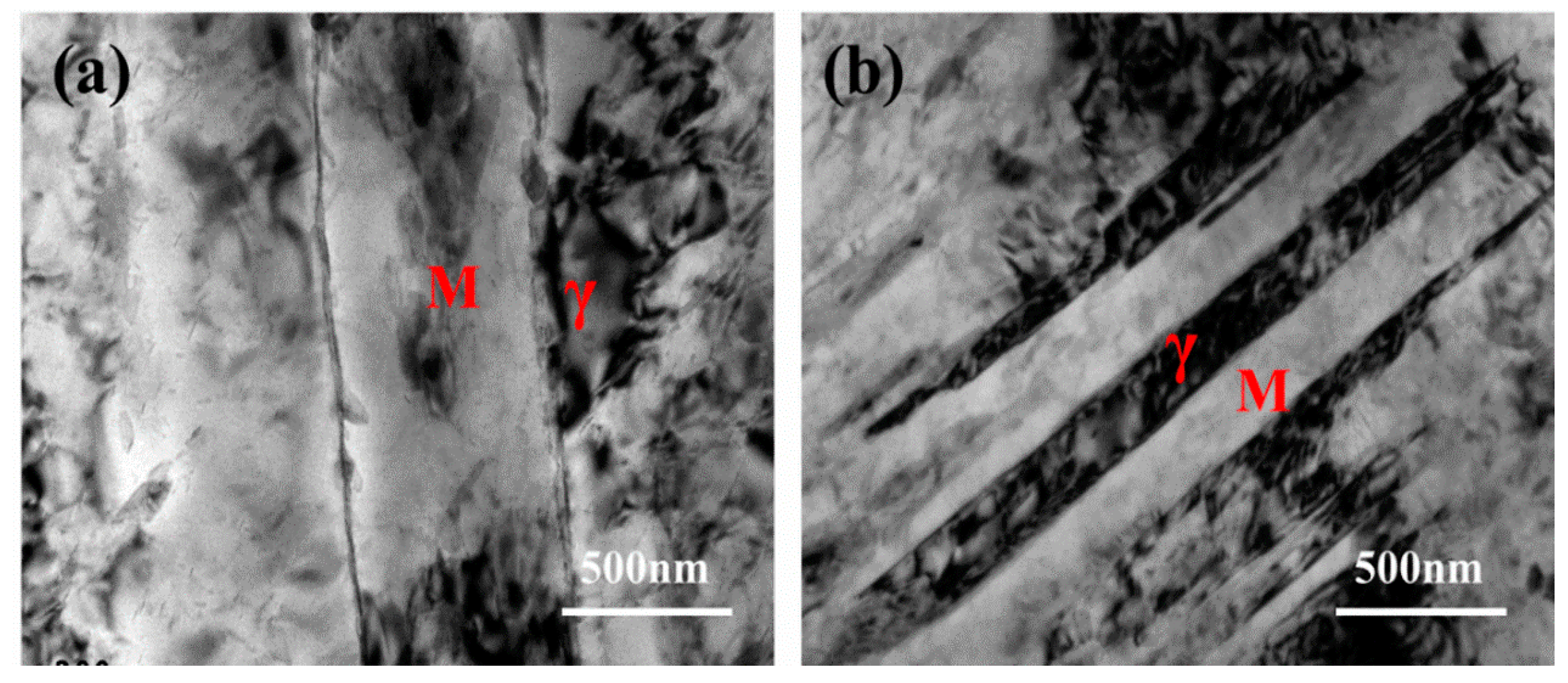
3.2. Martensite
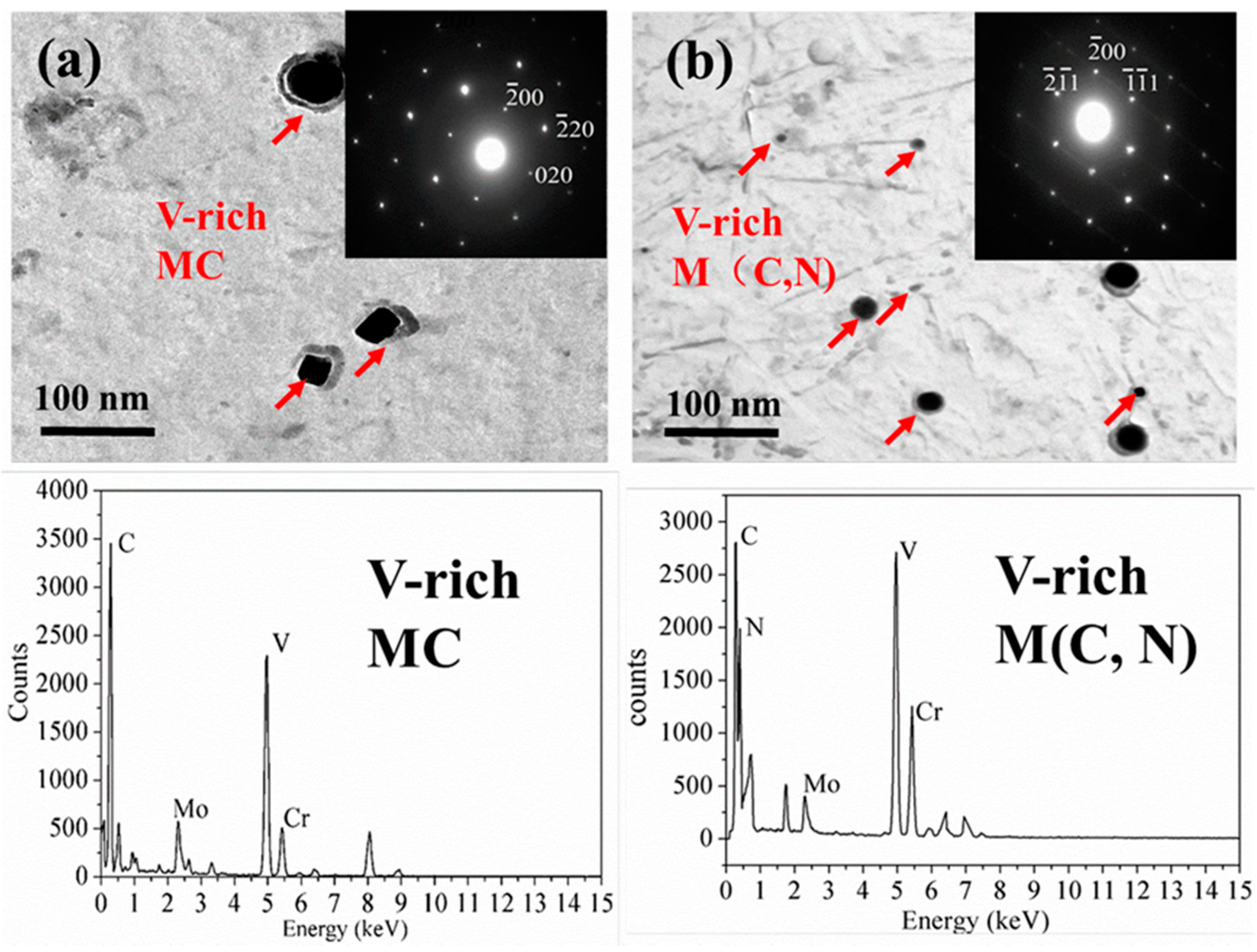
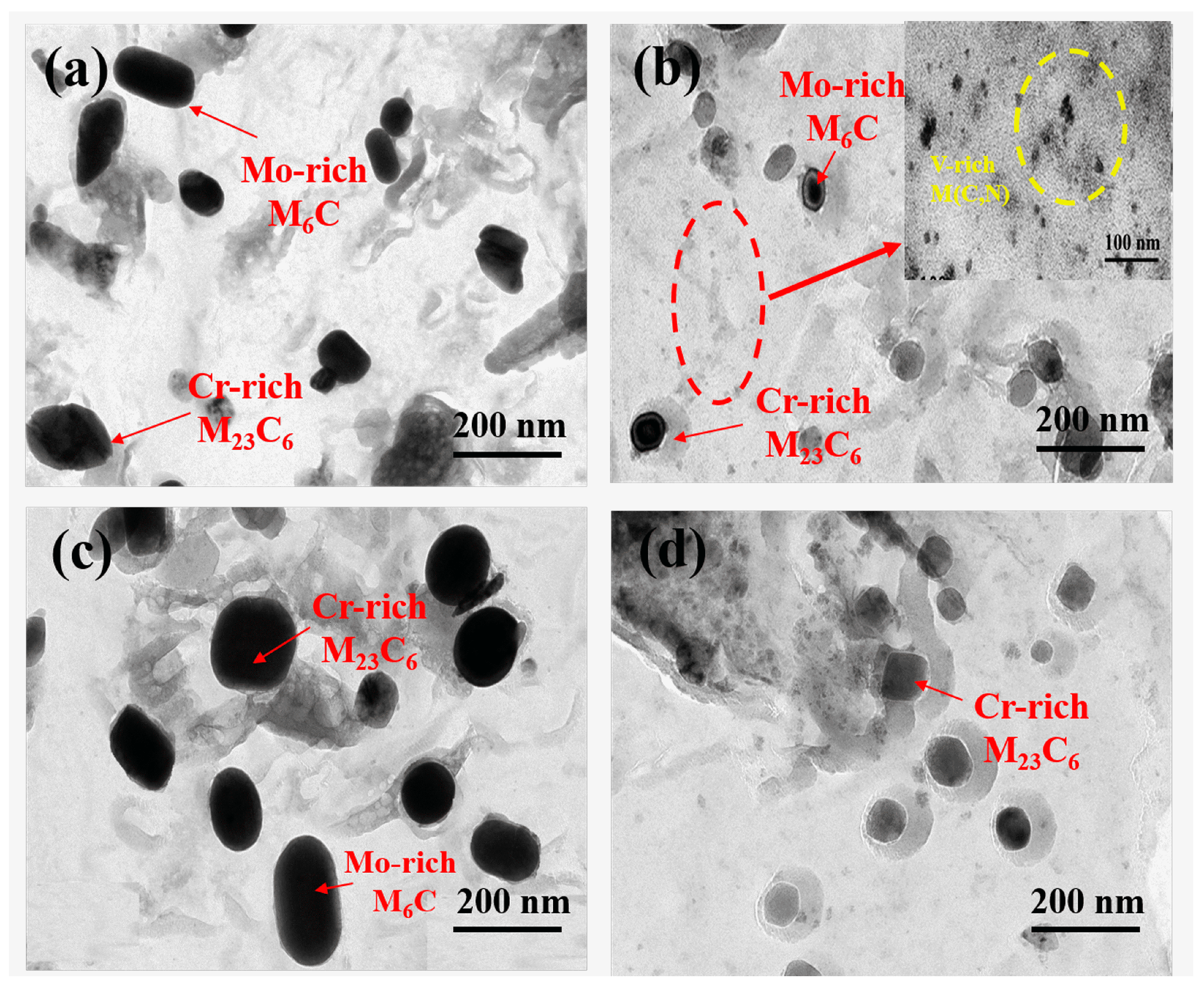
3.3. Precipitates
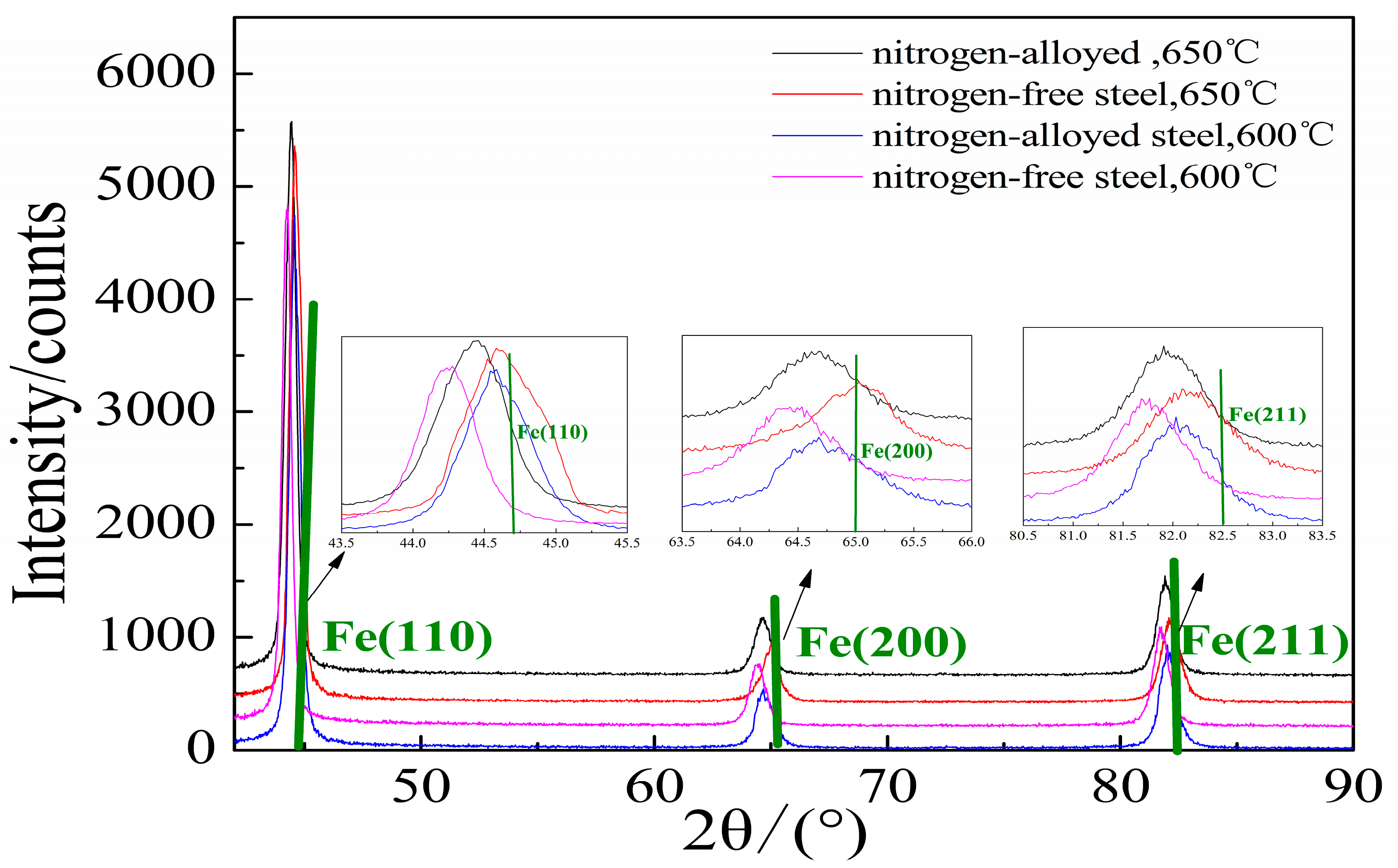
3.4. Lattice Distortion
4. Discussion
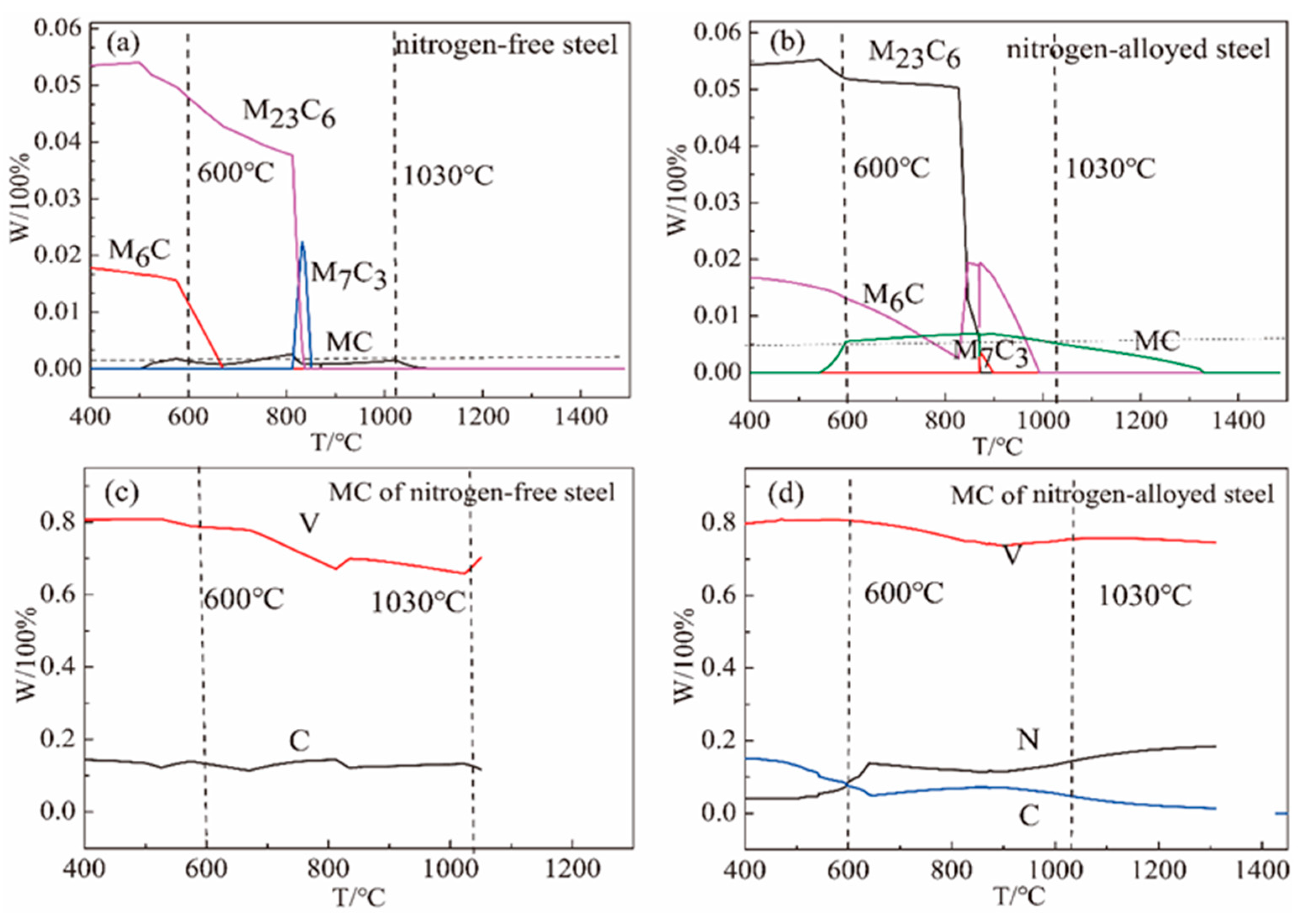
4.1. Effect of Nitrogen on Precipitates
4.2. Effect of V(C, N) on Refining Prior Austenitic Grains
4.3. Effect of Nitrogen on Martensitic Transformation
5. Conclusions
- (1)
- Nitrogen addition can increase hardness and temperability of 4Cr5Mo2V steel without toughness loss with a reasonable heat treatment procedure. The fair match of strength and high toughness of the nitrogen-alloyed 4Cr5Mo2V steel is associated with the refinement of the prior austenite grain size, the solution hardening of nitrogen atoms after tempering, and the increase of retained austenite.
- (2)
- During the heating stage of the quenching process, nitrogen promoted the precipitation of vanadium as finer V(C, N), imposing a stronger effect on restricting the growth of prior austenitic grains than VC and increasing grain refining efficiency of VC by 6.8 times. During the quenching process, the nitrogen decreases the MS of the martensitic transformation, increasing retained austenite which is a benefit for toughness.
- (3)
- In the process of tempering, some of the N atoms in M(C, N) were dissolved in the matrix, causing crystal lattice distortions, thus boosting the solution reinforcing effect. Meanwhile, the solid-dissolved nitrogen inhibits the diffusion of carbon, decreasing the growth rate of the carbides, and increasing tempering resistance.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gopalsamy, B.M.; Mondal, B.; Ghosh, S.; Arntz, K.; Klocke, F. Experimental investigations while hard machining of DIEVAR tool steel (50 HRC). Int. J. Adv. Manuf. Technol. 2010, 51, 853–869. [Google Scholar] [CrossRef]
- Cong, D.L.; Zhou, H.; Ren, Z.N.; Zhang, Z.H.; Zhou, H.F.; Meng, C.; Wang, C.W. The thermal fatigue resistance of H13 steel repaired by a biomimetic laser remelting process. Mater. Des. 2014, 55, 597–604. [Google Scholar] [CrossRef]
- Pérez, M.; Belzunce, F.J. The effect of deep cryogenic treatments on the mechanical properties of an AISI H13 steel. Mater. Sci. Eng. 2015, 624, 32–40. [Google Scholar] [CrossRef]
- Silva, L.L.G.D.; Ueda, M.; Mello, C.B.; Codaro, E.N.; Lepienski, C.M. Effects of the high temperature plasma immersion ion implantation (PIII) of nitrogen in AISI H13 steel. Mater. Sci. 2008, 43, 5989–5997. [Google Scholar] [CrossRef]
- Sola, R.; Giovanardi, R.; Parigi, G.; Veronesi, P. A novel method for fracture toughness evaluation of tool steels with post-tempering cryogenic treatment. Metals 2017, 7, 75. [Google Scholar] [CrossRef]
- Podgornik, B.; Paulin, I.; Zajec, B.; Jacobson, S.; Leskovšek, V. Deep cryogenic treatment of tool steels. J. Mater. Process. Technol. 2016, 229, 398–406. [Google Scholar] [CrossRef]
- Kheirandish, S.; Noorian, A. Effect of niobium on microstructure of cast AISIH13 hot work tool steel. J. Iron Steel Res. Int. 2008, 15, 61–66. [Google Scholar] [CrossRef]
- Wang, M.; Dangshen, M.A.; Liu, Z.; Zhou, J.; Chi, H.; Dai, J. Effect of Nb on segregation, primary carbides and toughness of H13 steel. Acta Metall. Sin. 2014, 50, 285–293. [Google Scholar]
- Gao, J.Z.; Fu, P.X.; Liu, H.W.; Li, D.Z. Effects of rare earth on the microstructure and impact toughness of H13 Steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef]
- Schino, A.D.; Barteri, M.; Kenny, J.M. Grain size dependence of mechanical, corrosion and tribological properties of high nitrogen stainless steels. J. Mater. Sci. 2003, 38, 3257–3262. [Google Scholar] [CrossRef]
- Hänninen, H.; Romu, J.; Ilola, R.; Laitinen, A. Effects of processing and manufacturing of high nitrogen-containing stainless steels on their mechanical, corrosion and wear properties. J. Mater. Process. Technol. 2001, 117, 424–430. [Google Scholar] [CrossRef]
- Jung, J.G.; Bae, J.H.; Lee, Y.K. Quantitative evaluation of dynamic precipitation kinetics in a complex Nb-Ti-V microalloyed steel using electrical resistivity measurements. Met. Mater. Int. 2013, 19, 1159–1162. [Google Scholar] [CrossRef]
- Simmons, J.W. Overview: High-Nitrogen Alloying of Stainless. Mater. Sci. Eng. 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Werner, E. Solid solution and grain size hardening of nitrogen-alloyed austenitic steels. Mater. Sci. Eng. 1988, 101, 93–98. [Google Scholar]
- Behjati, P.; Kermanpur, A.; Najafizadeh, A. Influence of nitrogen alloying on properties of Fe318Cr312Mn3XN austenitic stainless steels. Mater. Sci. Eng. 2013, 588, 43–48. [Google Scholar] [CrossRef]
- Gu, J.B.; Li, J.Y.; Huo, J.H. Effect of precipitation on hardening and toughening of nitrogen-alloyed H13 steel. Steel Res. Int. 2017. [Google Scholar] [CrossRef]
- SISC IAS, version 8.0; Scientific Instrument Software Co. Ltd.: Beijing, China, 2003.
- ASTM. Standard Test Methods for Determining Average Grain Size; ASTME 112-10; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Ning, A.G.; Guo, H.J.; Chen, X.C. Precipitation behaviors and strengthening of carbides in H13 steel during annealing. Mater. Trans. 2015, 56, 581–586. [Google Scholar]
- Chen, C.; Zhang, F.C.; Yang, Z.N.; Zheng, C.L. Superhardenability behavior of vanadium in 40CrNiMoV steel. Mater. Des. 2015, 83, 422–430. [Google Scholar] [CrossRef]
- Wang, T.S.; Zhang, M.; Wang, Y.H.; Yang, J.; Zhang, F.C. Martensitic transformation behaviour of deformed supercooled austenite. Scr. Mater. 2013, 68, 162–165. [Google Scholar] [CrossRef]
- Xu, L.Q.; Zhang, D.T.; Liu, Y.C.; Ning, B.Q. Precipitation behavior and martensite lath coarsening during tempering of T/P92 ferritic heat-resistant steel. Int. J. Min. Met. Mater. 2014, 21, 438–447. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, H.; Guo, C.H.; Jiang, F.C. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Zagonel, L.F.; Bettini, J.; Basso, R.L.O.; Paredez, P. Nanosized precipitates in H13 tool steel low temperature plasma nitriding. Surf. Coat. Technol. 2012, 207, 72–78. [Google Scholar] [CrossRef]
- Chen, Y.L.; Liu, B.; Li, J.Y. Study on heat treatment of nitrogen H13 steel. Adv. Mater. Res. 2010, 146, 1885–1888. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, Y.L.; Huo, J.H. Mechanism of improvement on strength and toughness of H13 die steel by nitrogen. Mater. Sci. Eng. 2015, 640, 16–23. [Google Scholar] [CrossRef]
- Gavriljuk, V.G. Nitrogen in Iron and Steel. ISIJ Int. 1996, 36, 738–745. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, G.G.; Chen, L.; Zhang, Y.D.; Yan, Q.Z. Characteristics and Generating Mechanism of Large Precipitates in Nb–Ti-microalloyed H13 Tool Steel. ISIJ Int. 2016, 995–1002. [Google Scholar] [CrossRef]
- Michaud, P.; Delagnes, D.; Lamesle, P.; Mathon, M.H.; Levaillant, C. The effect of the addition of alloying elements on carbide precipitation and mechanical properties in 5% chromium martensitic steels. Acta Mater. 2007, 55, 4877–4889. [Google Scholar] [CrossRef]
- Huang, B.X.; Wang, C.Z.; Wang, X.Y.; Rong, Y.H. Effect of nitrogen on martensitic transformation and mechanical properties of TWIP steel. Acta Metall. Sin. 2012, 48, 769–774. [Google Scholar] [CrossRef]
- Song, W.W.; Radulescu, A.; Liu, L.L.; Bleck, W. Study on a high entropy alloy by high energy synchrotron X-Ray diffraction and small angle neutron scattering. Steel Res. Int. 2017. [Google Scholar] [CrossRef]
- Moon, J.; Lee, T.H.; Shin, J.H.; Lee, J.W. Hot working behavior of a nitrogen-alloyed Fe–18Mn–18Cr–N austenitic stainless steel. Mater. Sci. Eng. 2014, 594, 302–308. [Google Scholar] [CrossRef]
- Saklakoğlu, N. Characterization of surface mechanical properties of H13 steel implanted by plasma immersion ion implantation. J. Mater. Process. Technol. 2007, 189, 367–373. [Google Scholar] [CrossRef]
- Delagnes, D.; Lamesle, P.; Mathon, M.H.; Mebarki, N.; Levaillant, C. Influence of silicon content on the precipitation of secondary carbides and fatigue properties of a 5% Cr tempered martensitic steel. Mater. Sci. Eng. 2005, 394, 435–444. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, J.; Zhang, Y.W.; Geng, P. Analysis of carbides in spheroidized H13 steel. Trans. Mater. Heat Treat. 2012, 33, 100–105. [Google Scholar]
- Kang, M.; Park, G.; Jung, J.G.; Kim, B.H.; Lee, Y.K. The effects of annealing temperature and cooling rate on carbide precipitation behavior in H13 hot-work tool steel. J. Alloys Compd. 2015, 627, 359–366. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steels, 1st ed.; Metallurgical Industry Press: Beijing, China, 2006; pp. 228–247. (In Chinese) [Google Scholar]
- Prokoshkina, V.; Kaputkina, L. Peculiarities of martensitic transformations and martensite structure in high nitrogen steels. Mater. Sci. Eng. 2007, 481, 762–765. [Google Scholar] [CrossRef]
- Yang, H.S.; Bhadeshia, H.K.D.H. Uncertainties in dilatometric determination of martensite start temperature. Mater. Sci. Technol. 2007, 23, 556–560. [Google Scholar] [CrossRef]
- Lacroix, G.; Pardoen, T.; Jacques, P.J. The fracture toughness of TRIP-assisted multiphase steels. Acta Mater. 2008, 56, 3900–3913. [Google Scholar] [CrossRef]
| Element | C | Si | Mn | Cr | Mo | V | N 1 |
|---|---|---|---|---|---|---|---|
| Nitrogen steel | 0.33 | 0.5 | 0.42 | 5.3 | 2.4 | 0.68 | 0.08 |
| Nitrogen-free steel | 0.33 | 0.5 | 0.40 | 5.4 | 2.4 | 0.63 | - |
| Quenching | 1000 °C/0.5 h | 1030 °C/0.5 h | |||
| Tempering | 580 °C/6 h | 600 °C/2 h | 600 °C/2 h | 600 °C/4 h | 600 °C/6 h |
| Nitrogen-alloyed steel/Nitrogen-free steel | |||||
| Average hardness 1/HRC | 50.2/48.2 | 49.7/48.1 | 51.0/48.6 | 49.6/47.3 | 48.0/46.0 |
| Average impact toughness 1 (ak/J·cm−2) | 12.3/12.6 | 12.5/12.5 | 12.0/12.3 | 14.3/14.4 | 14.6/14.8 |
| Samples | Tempering Temperature (°C) | Standard Dm (Å) | Mean Dm (Å) | Change (Δd/d%) |
|---|---|---|---|---|
| Nitrogen-free | 600 | 2.027 | 2.043 | 0.79 |
| Nitrogen-alloyed | 600 | 2.027 | 2.064 | 1.82 |
| Nitrogen-free | 650 | 2.027 | 2.037 | 0.50 |
| Nitrogen-alloyed | 650 | 2.027 | 2.056 | 1.43 |
| Precipitates | Lattice Constant (nm) | Average Diameter 1 (nm) | Volume Fraction (f) |
|---|---|---|---|
| VC | 0.4182 | 50 | 0.21% |
| V(C, N) | 0.4154 | 20 | 0.68% |
| Precipitates | Lattice Mismatch (100%) | Interface Energy (J/m2) | Fm (N/m2) | Fm(V(C, N))/Fm(VC) |
|---|---|---|---|---|
| VC | 0.1182 | 0.4449 | 5.6 × 104 | 7.8 |
| V(C, N) | 0.1122 | 0.4338 | 4.4 × 105 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Li, J.; Chen, Y. Microstructure and Strengthening-Toughening Mechanism of Nitrogen-Alloyed 4Cr5Mo2V Hot-Working Die Steel. Metals 2017, 7, 310. https://doi.org/10.3390/met7080310
Gu J, Li J, Chen Y. Microstructure and Strengthening-Toughening Mechanism of Nitrogen-Alloyed 4Cr5Mo2V Hot-Working Die Steel. Metals. 2017; 7(8):310. https://doi.org/10.3390/met7080310
Chicago/Turabian StyleGu, Jinbo, Jingyuan Li, and Yulai Chen. 2017. "Microstructure and Strengthening-Toughening Mechanism of Nitrogen-Alloyed 4Cr5Mo2V Hot-Working Die Steel" Metals 7, no. 8: 310. https://doi.org/10.3390/met7080310




