Effect of Fe-Content on the Mechanical Properties of Recycled Al Alloys during Hot Compression
Abstract
:1. Introduction
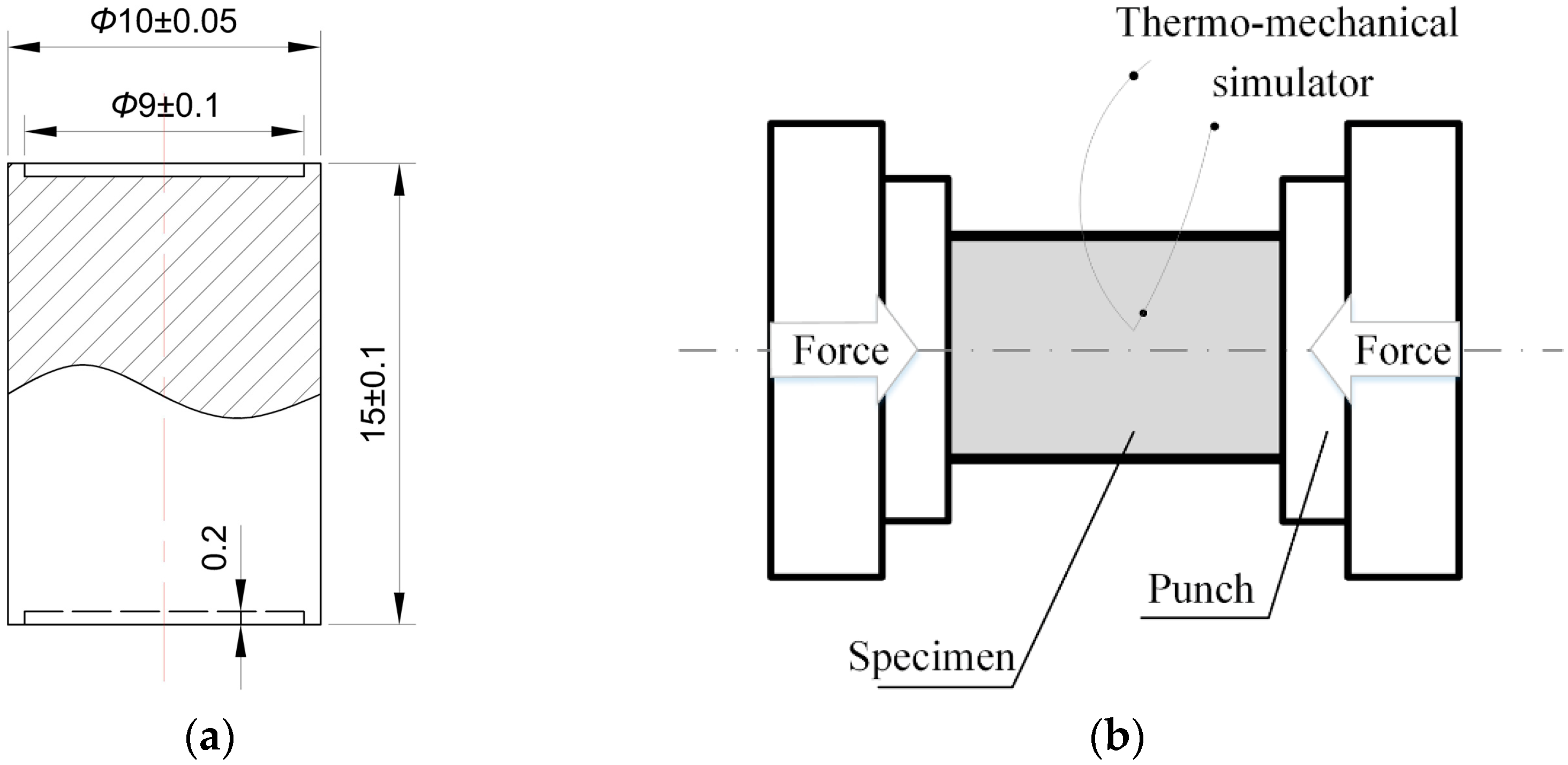
2. Materials and Methods
3. Results
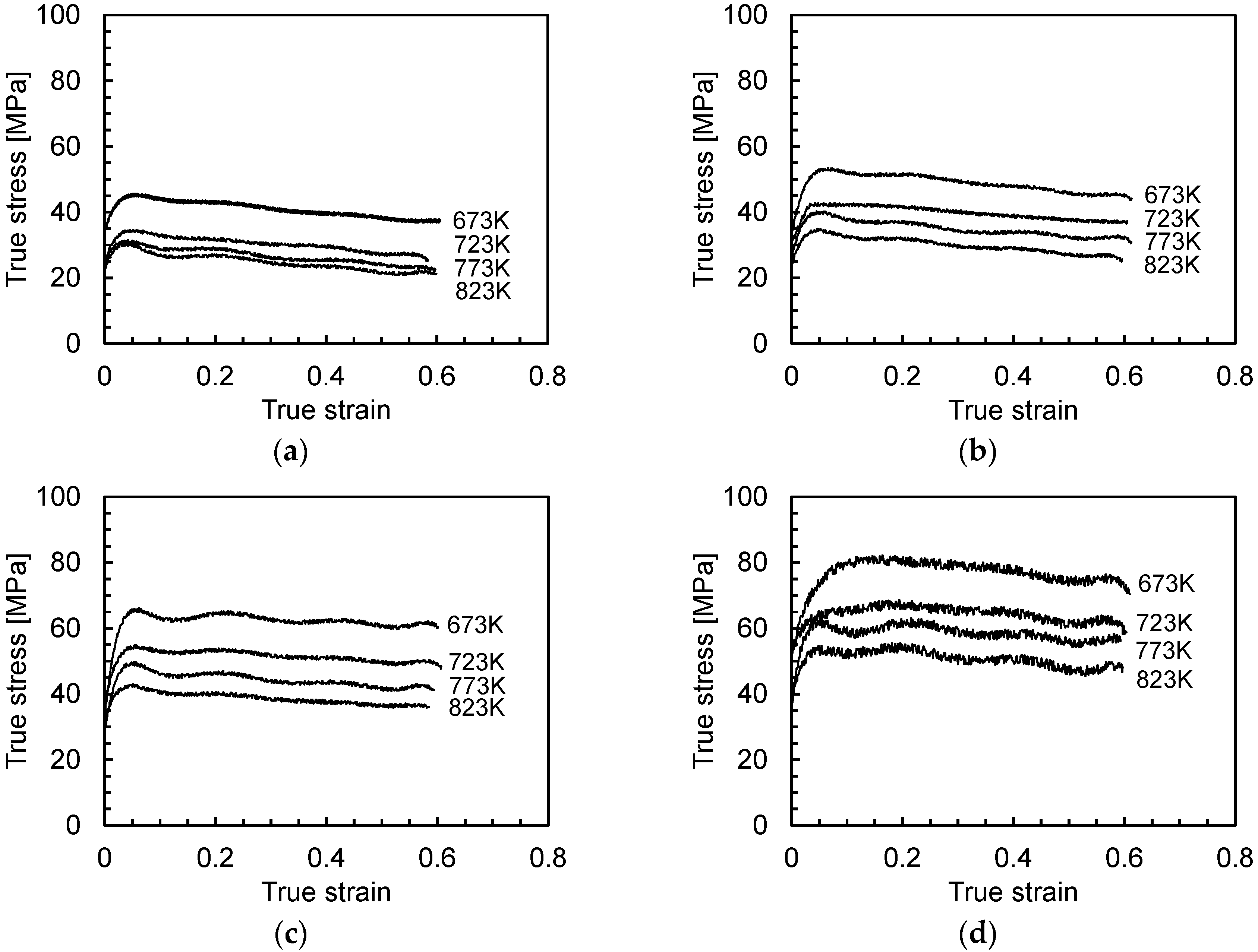
3.1. Flow Curves
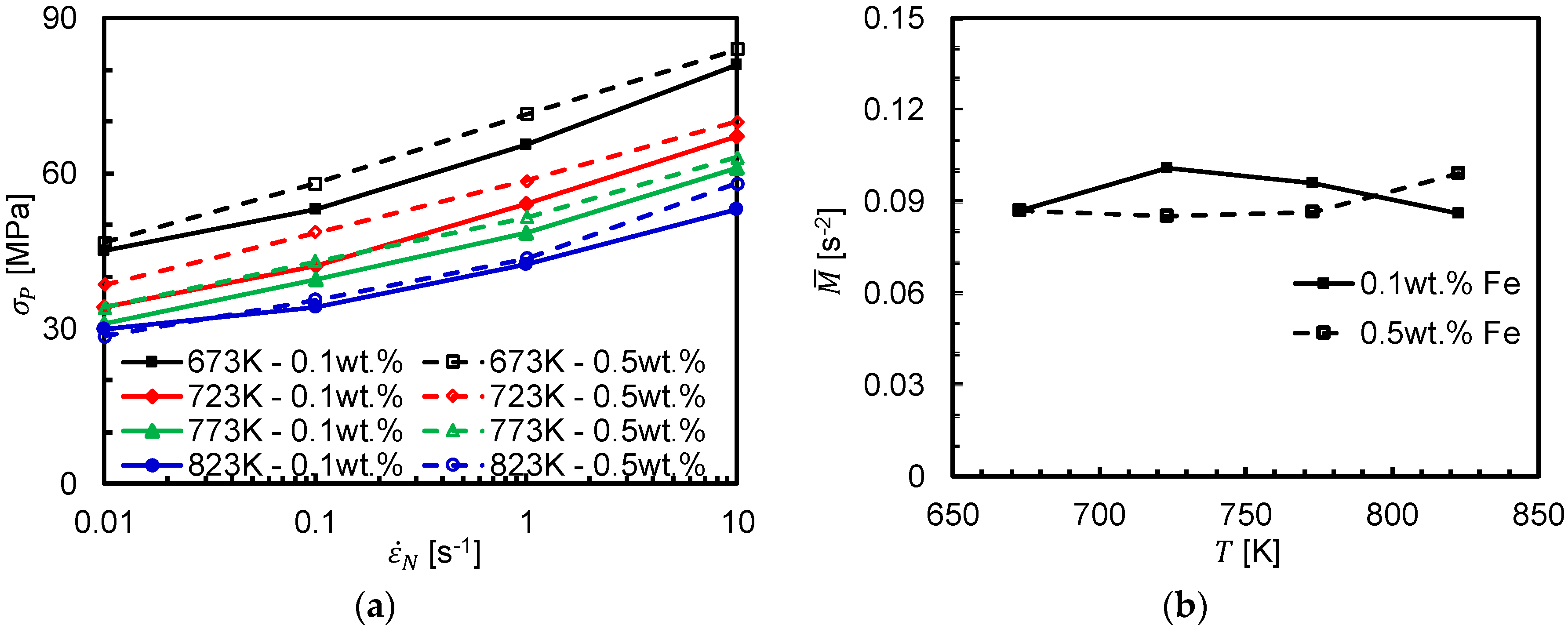
3.2. Strain Rate Sensitivity
3.3. Activation Energy for Dynamic Recrystallization and Peak Stress Model
3.3.1. Governing Equations
3.3.2. Identification of Parameters
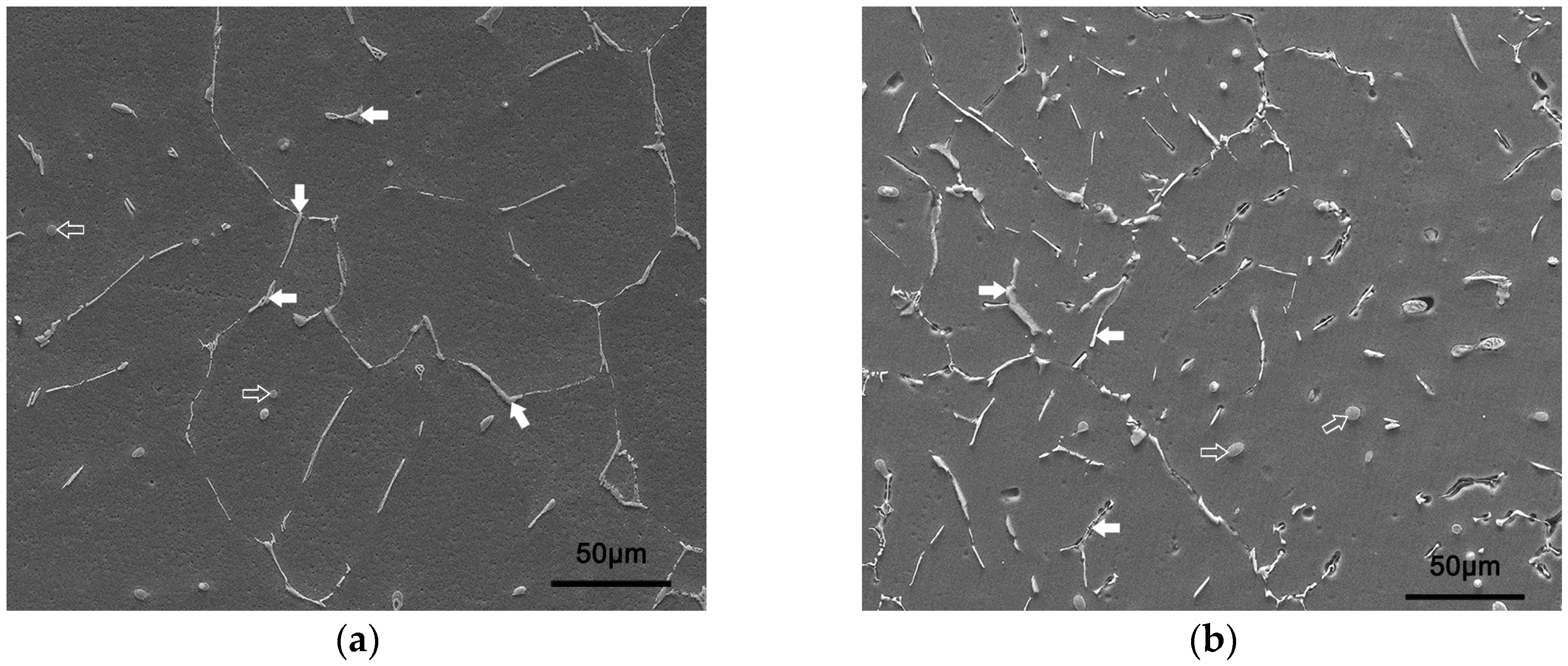
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choate, W.T.; Green, J.A.S. Modeling the impact of secondary recovery (recycling) on the U.S. Aluminum supply and nominal energy requirements. In Light Metals, 2004th ed.; Tabereaux, A.T., Ed.; John Wiley and Sons Ltd.: Warrendale, PA, USA, 2004; pp. 913–918. [Google Scholar]
- Das, S.K. Designing aluminium alloys for a recycling friendly world. Mater. Sci. Forum 2006, 519–521, 1239–1244. [Google Scholar] [CrossRef]
- Sarkar, J.; Kutty, T.R.G.; Conlon, K.T.; Wilkinson, D.S.; Embury, J.D.; Lloyd, D.J. Tensile and bending properties of AA5754 aluminum alloys. Mater. Sci. Eng. A 2001, 316, 52–59. [Google Scholar] [CrossRef]
- Das, S.K.; Green, J.A.S.; Kaufman, J.G. The development of recycle-friendly automotive aluminum alloys. JOM 2007, 59, 47–51. [Google Scholar] [CrossRef]
- Gesing, A.; Berry, L.; Dalton, R.; Wolanski, R. Assuring continued recyclability of automotive aluminum alloys: Grouping of wrought alloys by color, X-ray absorption and chemical composition-based sorting. In Proceedings of the TMS 2002 Annual Meeting: Automotive Alloys and Aluminum Sheet and Plate Rolling and Finishing Technology Symposia, Warrendale, PA, USA, 18–21 Feburary 2002; pp. 3–15. [Google Scholar]
- Pan, F.; Zhang, D. Aluminium Alloy and Application; Chemical Industry Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Staley, J.T.; Lege, D.J. Advances in aluminium alloy products for structural applications in transportation. J. Phys. IV 1993, 3, 179–190. [Google Scholar] [CrossRef]
- Shabestari, S.G. The effect of iron and manganese on the formation of intermetallic compounds in aluminum-silicon alloys. Mater. Sci. Eng. A 2004, 383, 289–298. [Google Scholar] [CrossRef]
- Matsubara, H.; Izawa, N.; Nakanishi, M. Macroscopic segregation in Al-11 mass % Si slloy containing 2 mass % Fe solidified under centrifugal force. J. Jpn. Inst. Light Met. 1998, 48, 93–97. [Google Scholar] [CrossRef]
- Joon-Pyou, P.; Sassa, K.; Asai, S. Elimination of iron in molten Al-Si alloys by electromagnetic force. J. Jpn. Inst. Light Met. 1995, 59, 312–318. [Google Scholar]
- Haghdadi, N.; Cizek, P.; Beladi, H.; Hodgson, P.D. A novel high-strain-rate ferrite dynamic softening mechanism facilitated by the interphase in the austenite/ferrite microstructure. Acta Mater. 2017, 126, 44–57. [Google Scholar] [CrossRef]
- Min, J.; Hector, L.G., Jr.; Zhang, L.; Sun, L.; Carsley, J.E.; Lin, J. Plastic instability at elevated temperatures in a trip-assisted steel. Mater. Des. 2016, 95, 370–386. [Google Scholar] [CrossRef]
- Ji, H.S.; Ji, H.K.; Wagoner, R.H. A plastic constitutive equation incorporating strain, strain-rate, and temperature. Int. J. Plast. 2010, 26, 1746–1771. [Google Scholar]
- Jonas, J.J.; Sellars, C.M.; Tegart, W.J.M. Strength and structure under hot-working conditions. Int. Mater. Rev. 1969, 14, 1–24. [Google Scholar] [CrossRef]
- Huang, C.-Q.; Diao, J.-P.; Deng, H.; Li, B.-J.; Hu, X.-H. Microstructure evolution of 6016 aluminum alloy during compression at elevated temperatures by hot rolling emulation. Trans. Nonferr. Met. Soc. China 2013, 23, 1576–1582. [Google Scholar] [CrossRef]
- Shi, H.; Mclaren, A.J.; Sellars, C.M.; Shahani, R.; Bolingbroke, R. Constitutive equations for high temperature flow stress of aluminium alloys. Mater. Sci. Technol. 1997, 13, 210–216. [Google Scholar] [CrossRef]
- Zener, C.; Hollomon, J.H. Effect of strain rate upon plastic flow of steel. J. Appl. Phys. 1944, 15, 22–32. [Google Scholar] [CrossRef]
- Jeniski, R.A.; Thanaboonsombut, B.; Sanders, T.H. The effect of iron and manganese on the recrystallization behavior of hotrolled and solution-heat-treated aluminum alloy 6013. Metall. Mater. Trans. A 1996, 27, 19–27. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Han, Y.; Wu, W.; Chen, J. Hot deformation behavior of 2026 aluminum alloy during compression at elevated temperature. Mater. Sci. Eng. A 2010, 527, 485–490. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, H.; Han, Y.; Wu, W.; Chen, J. Hot deformation behavior of 7150 aluminum alloy during compression at elevated temperature. Mater. Charact. 2009, 60, 530–536. [Google Scholar] [CrossRef]
- Haghdadi, N.; Zarei-Hanzaki, A.; Abedi, H.R. The flow behavior modeling of cast A356 aluminum alloy at elevated temperatures considering the effect of strain. Mater. Sci. Eng. A 2012, 535, 252–257. [Google Scholar] [CrossRef]
- Lee, K.J.; Woo, K.D. Effect of the hot-rolling microstructure on texture and surface roughening of Al-Mg-Si series aluminum alloy sheets. Met. Mater. Int. 2011, 17, 689–695. [Google Scholar] [CrossRef]


| Si | Mg | Fe | Mn | Al |
|---|---|---|---|---|
| 1.05 | 0.54 | 0.10 | 0.069 | Balance |
| 0.97 | 0.56 | 0.50 | 0.071 | Balance |
| Al Alloys | m | β | α | n | Q [kJ mol−1] | A |
|---|---|---|---|---|---|---|
| 0.1 Fe 1 | 11.02 | 0.1963 | 0.01781 | 8.760 | 140.5 | 3.550 × 109 |
| 0.5 Fe 1 | 9.452 | 0.2013 | 0.02130 | 8.030 | 154.5 | 2.569 × 109 |
| Alloys | [s−1] | T [K] | [MPa] | [MPa] | δ [%] |
|---|---|---|---|---|---|
| 0.1 Fe | 0.01 | 673 | 45.0 | 43.0 | −4.38% |
| 723 | 34.0 | 36.3 | 6.69% | ||
| 773 | 31.0 | 31.1 | 0.26% | ||
| 823 | 30.0 | 27.0 | −9.87% | ||
| 0.1 | 673 | 53.0 | 53.3 | 0.48% | |
| 723 | 42.0 | 45.4 | 8.13% | ||
| 773 | 39.5 | 39.2 | −0.64% | ||
| 823 | 34.0 | 34.4 | 1.05% | ||
| 1 | 673 | 65.5 | 64.8 | −1.11% | |
| 723 | 54.0 | 56.0 | 3.66% | ||
| 773 | 48.5 | 48.9 | 0.80% | ||
| 823 | 42.5 | 43.2 | 1.53% | ||
| 10 | 673 | 81.0 | 77.3 | −4.53% | |
| 723 | 67.0 | 67.8 | 1.16% | ||
| 773 | 61.0 | 59.9 | −1.80% | ||
| 823 | 53.0 | 53.4 | 0.75% | ||
| 0.5 Fe | 0.01 | 673 | 46.5 | 47.8 | 2.90% |
| 723 | 38.5 | 39.8 | 3.31% | ||
| 773 | 34.0 | 33.6 | −1.29% | ||
| 823 | 28.5 | 28.7 | 0.86% | ||
| 0.1 | 673 | 58.0 | 58.8 | 1.37% | |
| 723 | 48.5 | 49.6 | 2.33% | ||
| 773 | 43.0 | 42.4 | −1.46% | ||
| 823 | 35.5 | 36.6 | 3.13% | ||
| 1 | 673 | 71.5 | 70.8 | −0.94% | |
| 723 | 58.5 | 60.8 | 3.90% | ||
| 773 | 51.5 | 52.6 | 2.16% | ||
| 823 | 43.5 | 46.0 | 5.65% | ||
| 10 | 673 | 84.0 | 83.7 | −0.39% | |
| 723 | 70.0 | 73.0 | 4.25% | ||
| 773 | 63.0 | 64.1 | 1.71% | ||
| 823 | 58.0 | 56.7 | −2.28% |
| Fe Content (wt %) | 0.1 | 0.5 |
|---|---|---|
| Numbers of lager intermetallic particles (1–200 μm) in Figure 6 [mm−2] | 2068 | 4826 |
| Area [%] | 1.839 | 3.706 |
| Max diameter [μm] | 18.20 | 18.04 |
| Average diameter [μm] | 3.425 | 3.339 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Hou, Z.; Ma, M.; Lu, G. Effect of Fe-Content on the Mechanical Properties of Recycled Al Alloys during Hot Compression. Metals 2017, 7, 262. https://doi.org/10.3390/met7070262
Lu H, Hou Z, Ma M, Lu G. Effect of Fe-Content on the Mechanical Properties of Recycled Al Alloys during Hot Compression. Metals. 2017; 7(7):262. https://doi.org/10.3390/met7070262
Chicago/Turabian StyleLu, Hongzhou, Zeran Hou, Mingtu Ma, and Guimin Lu. 2017. "Effect of Fe-Content on the Mechanical Properties of Recycled Al Alloys during Hot Compression" Metals 7, no. 7: 262. https://doi.org/10.3390/met7070262





