Microstructure and Corrosion Behavior of Simulated Welding HAZ of Q315NS Steel in Sulfuric Acid Solution †
Abstract
:1. Introduction
2. Experimental
2.1. HAZ Thermal Simulation
2.2. Microstructure Observation
2.3. Electrochemical Experiments
3. Results and Discussion
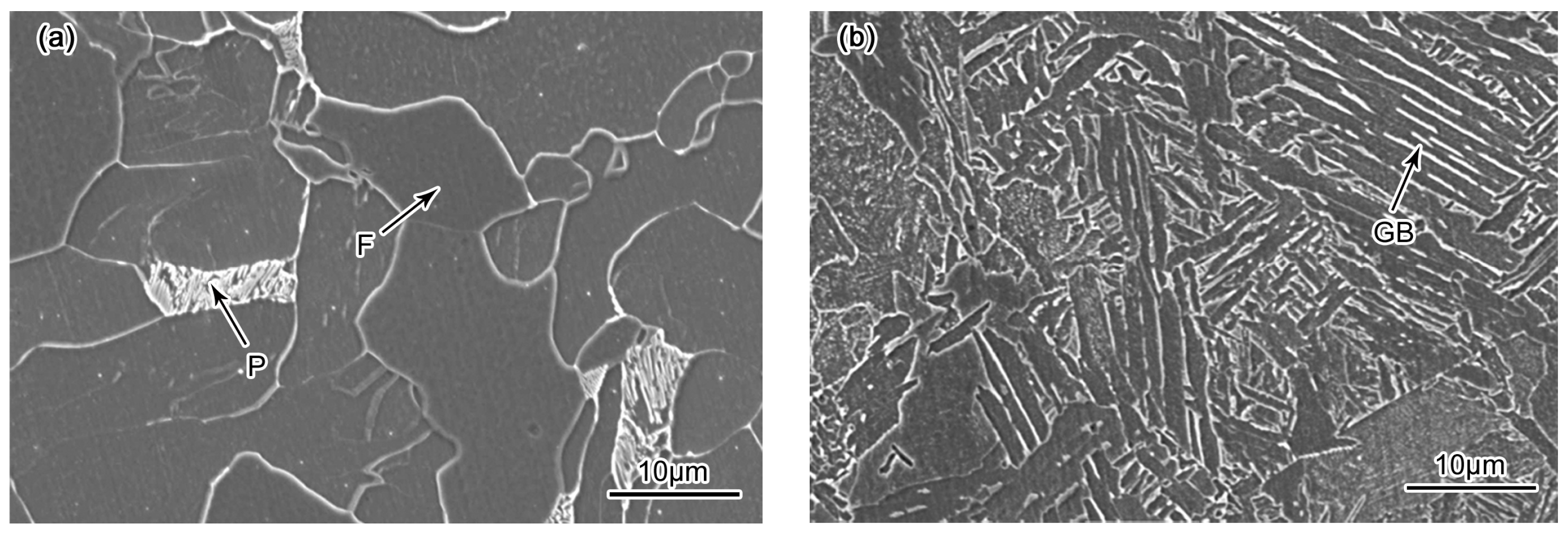
3.1. Microstructure Evolution
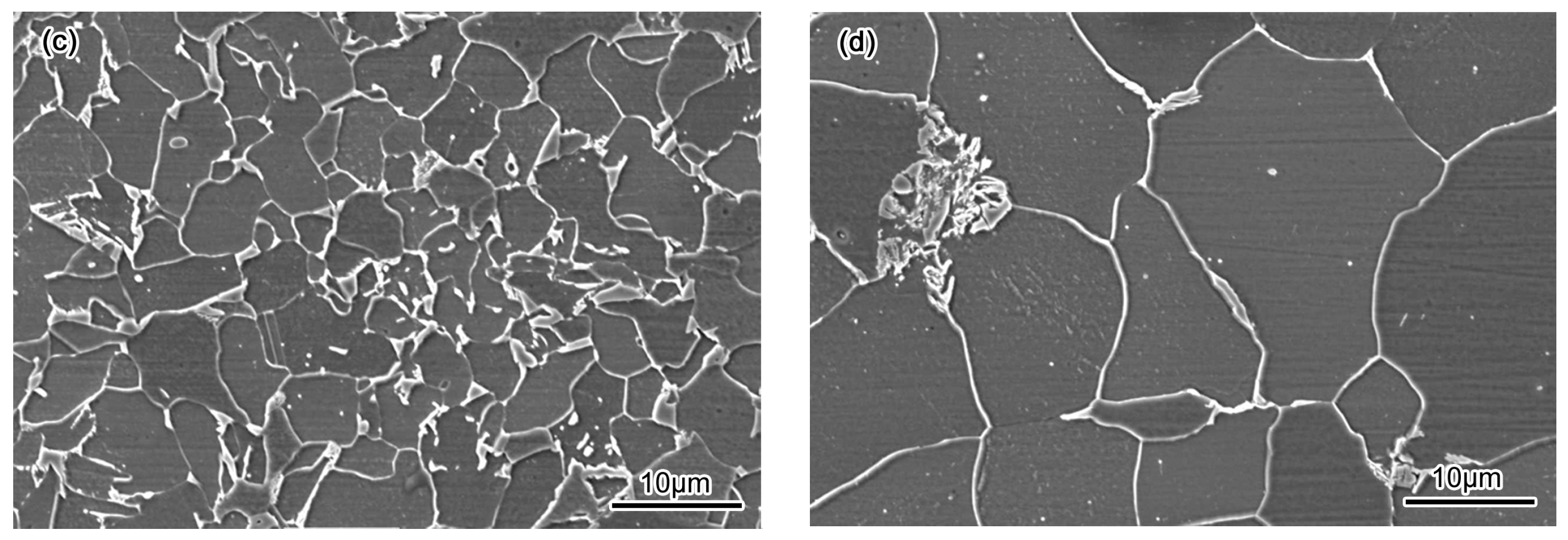
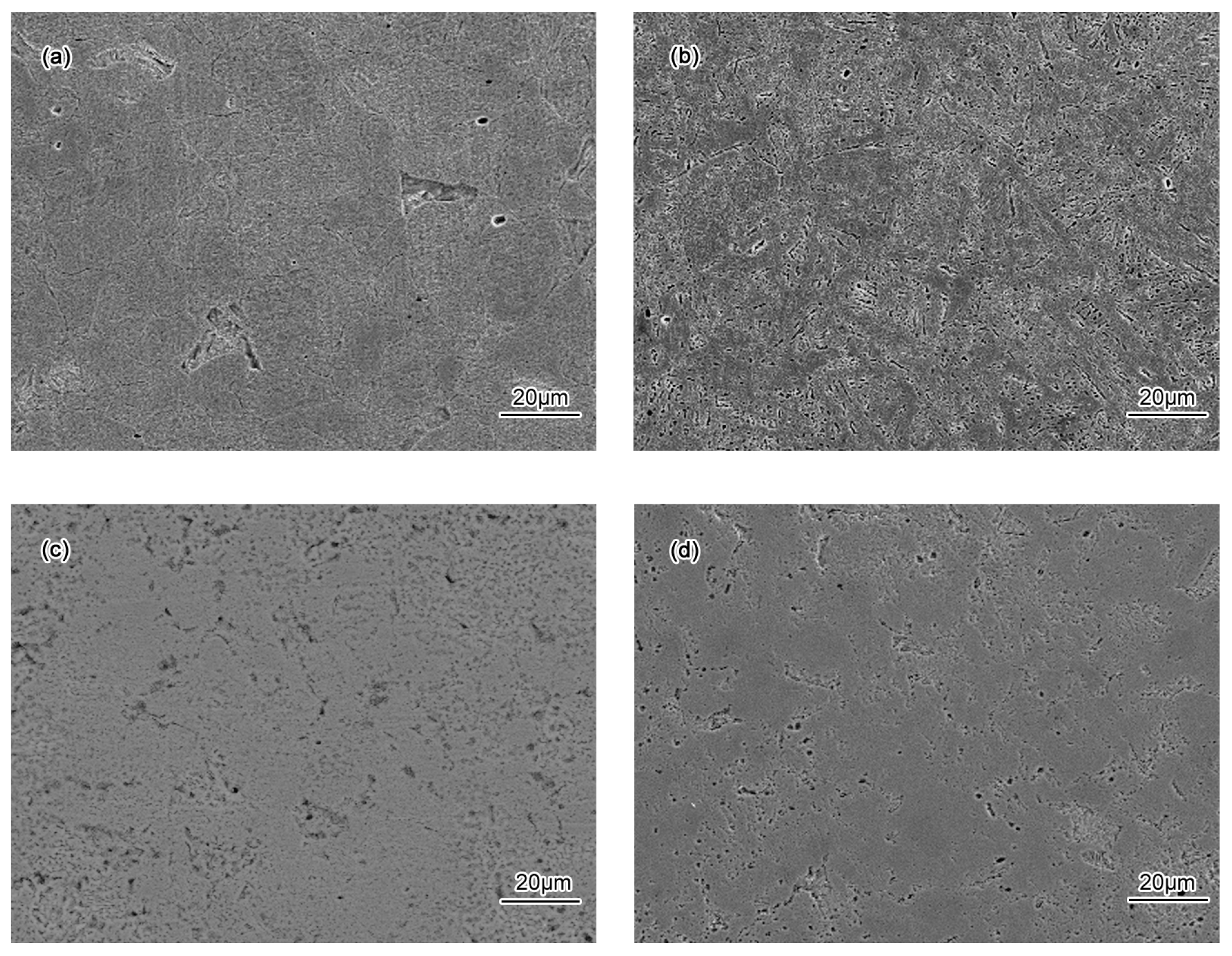
3.2. Corrosion Behavior
3.3. Electrochemical Characteristics in H2SO4 solution
4. Conclusions
- (1)
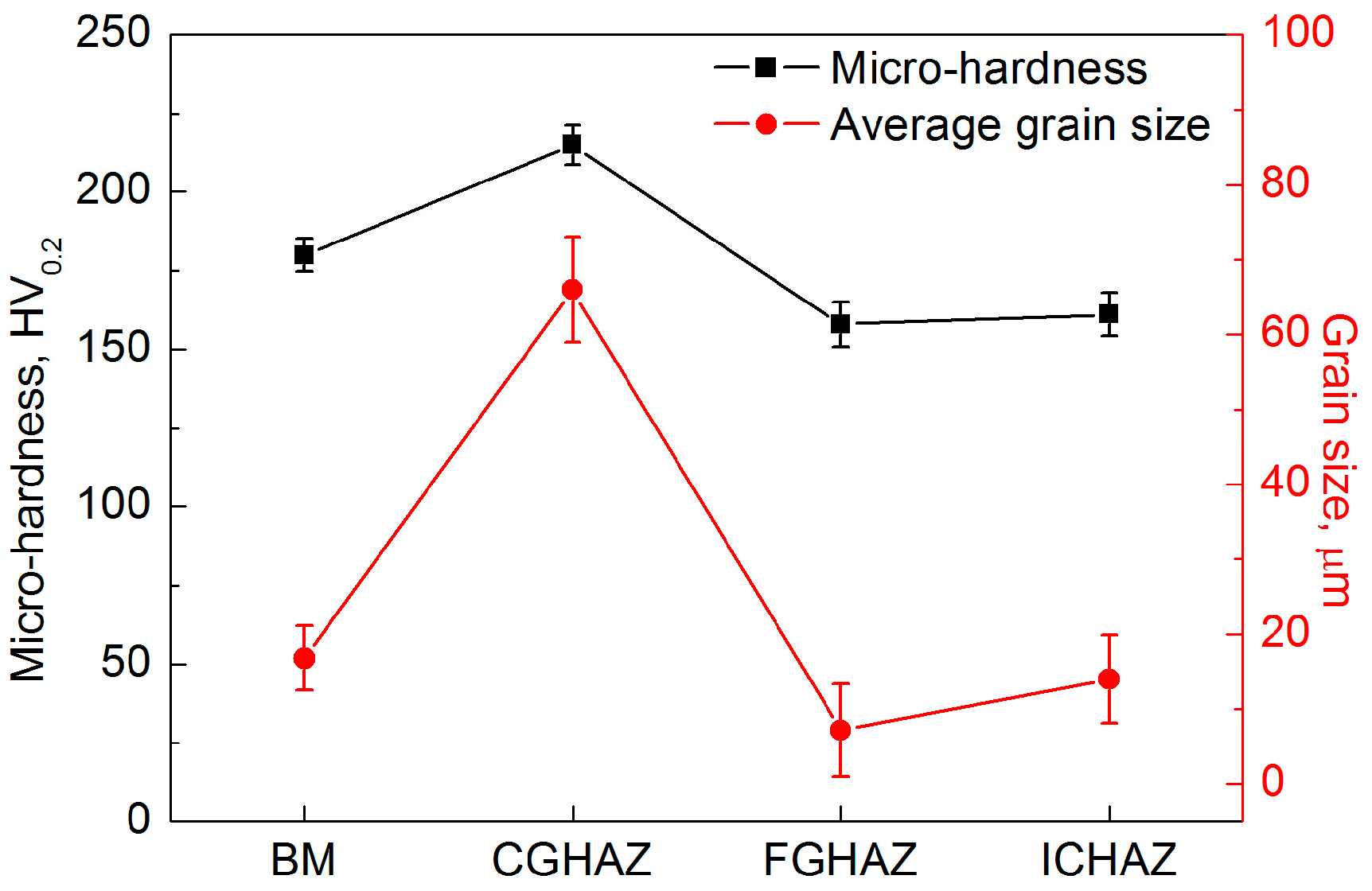
- The BM, FGHAZ and ICHAZ consisted of ferrite and pearlite, whereas the microstructure in the CGHAZ was mainly granular bainite. The CGHAZ has the highest microhardness and the largest average grain size due to the highest peak temperature.
- (2)
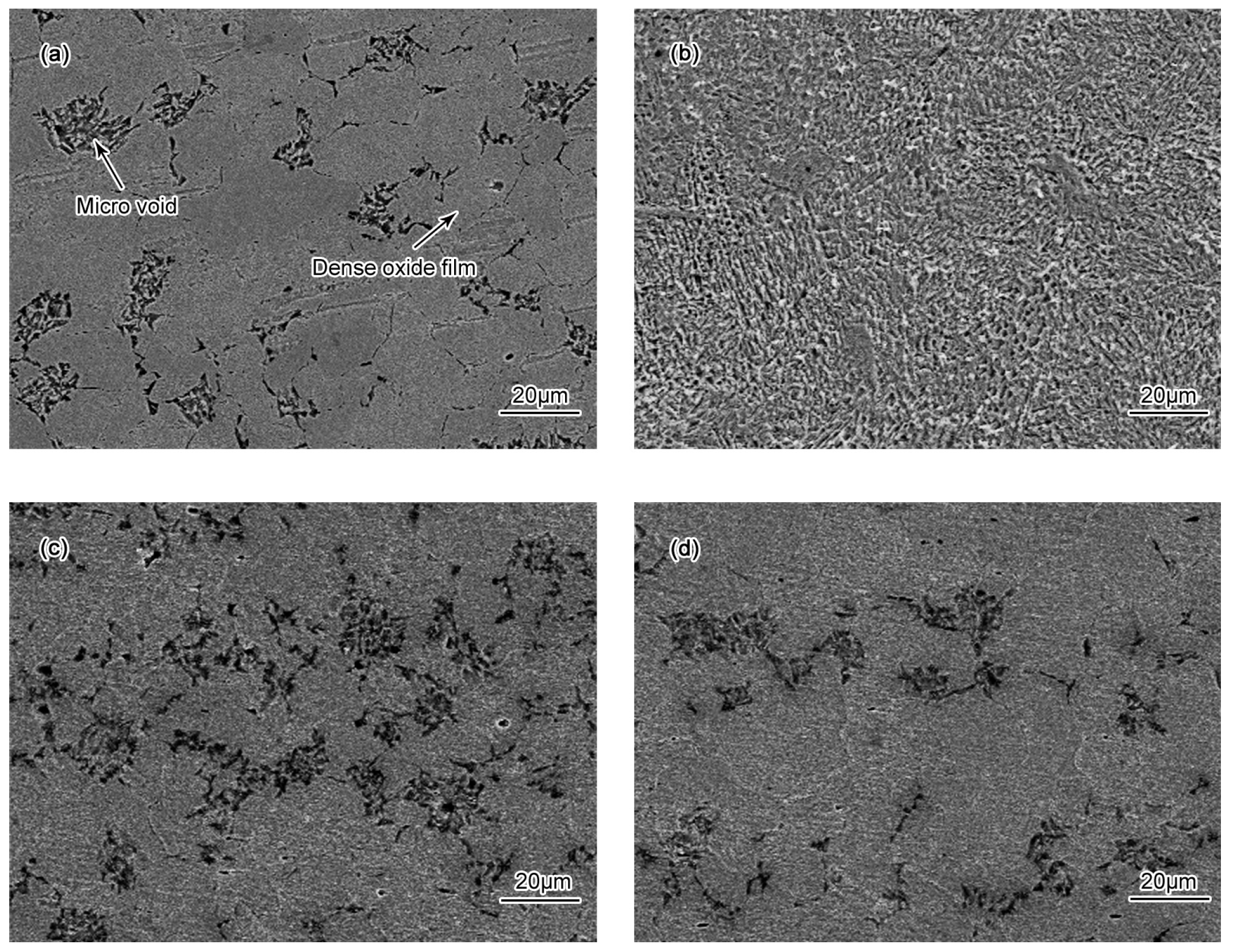
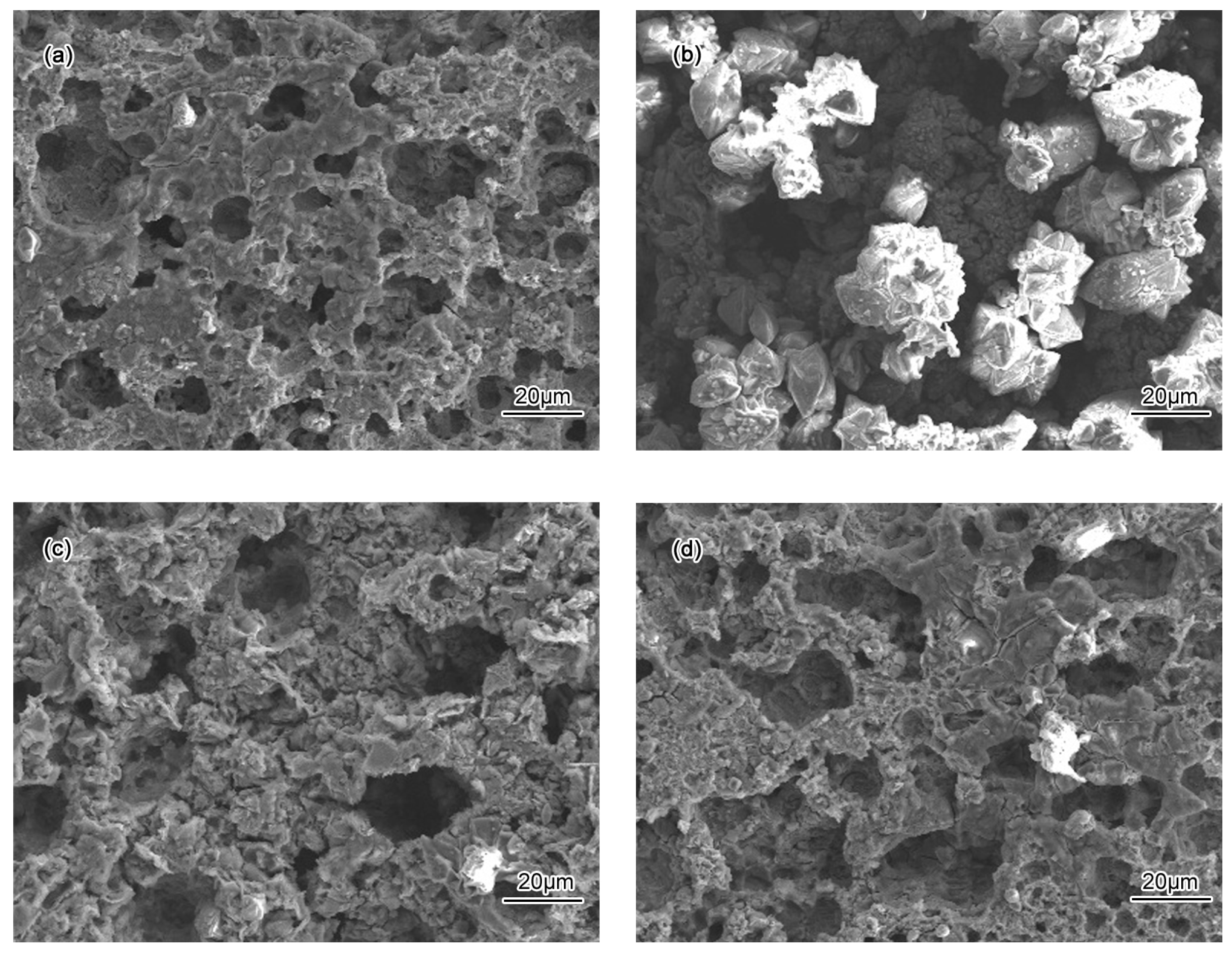
- Passivation process occured in all specimens in 50 wt % H2SO4 solution. A porous structure product was formed on the surface of the BM while a rod-shaped structure corrosion product was formed on CGHAZ after immersion for 72 h in 50 wt % H2SO4 solution.
- (3)
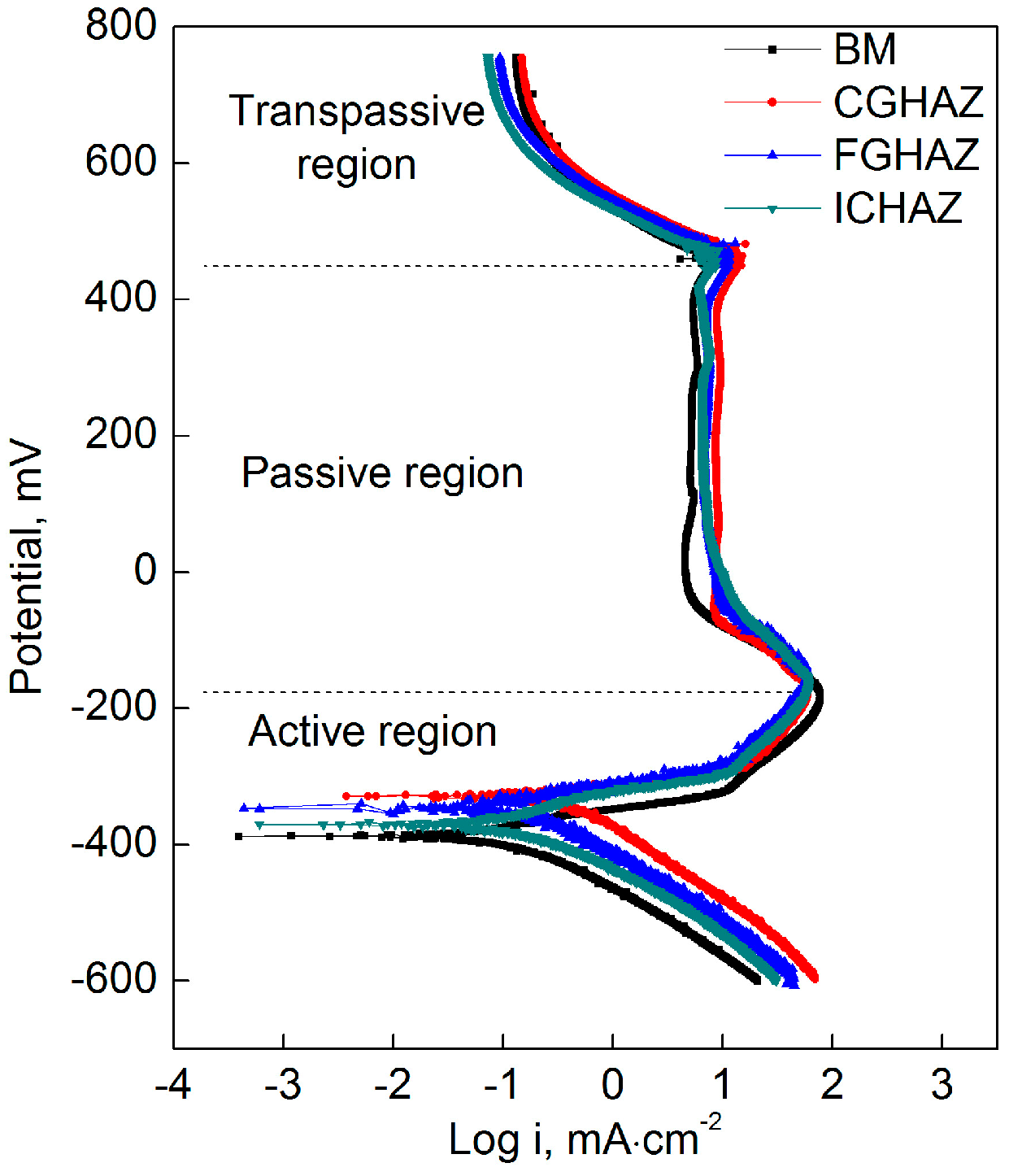
- All specimens underwent the similar polarization process. The electrochemical tests showed that the corrosion current density of CGHAZ was the largest while the corrosion current density of BM was the lowest.
- (4)
- The corrosion process of all specimens was mainly controlled by charge transportation process. The charge transfer resistance of BM and CGHAZ were the greatest and the weakest respectively. The corrosion product of CGHAZ had the biggest capacitance due to the loosest distribution character.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lins, V.F.C.; Guimarães, E.M. Failure of a heat exchanger generated by an excess of SO2 and H2 in the sulfur recovery unit of a petroleum refinery. J. Loss Prev. Process Ind. 2007, 20, 91–97. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Sun, F.L.; Lv, S.J.; Li, X.G. A new steel with good low-temperature sulfuric acid dew point corrosion resistance. Mater. Corros. 2012, 63, 598–606. [Google Scholar] [CrossRef]
- Yang, Y.G.; Zhang, T.; Shao, Y.W.; Meng, G.Z.; Wang, F.H. In situ study of dew point corrosion by electrochemical measurement. Corros. Sci. 2013, 71, 62–71. [Google Scholar] [CrossRef]
- Wang, Y.G.; Ma, H.D.; Liang, Z.Y.; Chen, H.; Zhao, Q.X.; Jin, X. Experimental study on dew point corrosion characteristics of the heating surface in a 65 t/h biomass-fired circulating fluidized bed boiler. Appl. Therm. Eng. 2016, 96, 76–82. [Google Scholar] [CrossRef]
- Luk-Cyr, J.; El-Bawab, R.; Champliaud, H.; Lanteigne, J.; Vadean, A. Mechanical properties of 75% Ar/25% CO2 flux-cored arc welded E309L austenitic stainless steel. Mat. Sci. Eng. A 2016, 678, 197–203. [Google Scholar] [CrossRef]
- Haugen, V.G.; Rogne, B.R.S.; Akselsen, O.M.; Thaulow, C.; Østby, E. Local mechanical properties of intercritically reheated coarse grained heat affected zone in low alloy steel. Mater. Des. 2014, 59, 135–140. [Google Scholar] [CrossRef]
- You, Y.; Shang, C.J.; Chen, L.; Subramanian, S. Investigation on the crystallography of the transformation products of reverted austenite in intercritically reheated coarse grained heat affected zone. Mater. Des. 2013, 43, 485–491. [Google Scholar] [CrossRef]
- Li, X.D.; Fan, Y.R.; Ma, X.P.; Subramanian, S.V.; Shang, C.J. Influence of Martensite–Austenite constituents formed at different intercritical temperatures on toughness. Mater. Des. 2015, 67, 457–463. [Google Scholar] [CrossRef]
- Li, X.D.; Ma, X.P.; Subramanian, S.V.; Shang, C.J.; Misra, R.D.K. Influence of prior austenite grain size on martensite–austenite constituent and toughness in the heat affected zone of 700 MPa high strength linepipe steel. Mat. Sci. Eng. A 2014, 616, 141–147. [Google Scholar] [CrossRef]
- Kim, S.; Kang, D.; Kim, T.W.; Lee, J.; Lee, C. Fatigue crack growth behavior of the simulated HAZ of 800 MPa grade high-performance steel. Mater. Sci. Eng. A 2011, 528, 2331–2338. [Google Scholar] [CrossRef]
- Moeinifar, S.; Kokabi, A.H.; Hosseini, H.R.M. Influence of peak temperature during simulation and real thermal cycles on microstructure and fracture properties of the reheated zones. Mater. Des. 2010, 31, 2948–2955. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, X.M.; Shang, C.J.; Misra, R.D. K Characterization of the multi-pass weld metal and the impact of retained austenite obtained through intercritical heat treatment on low temperature toughness. Mater. Sci. Eng. A 2016, 649, 282–292. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Kuzmikova, L.; Li, H.J.; Barbaro, F. Effect of inter-critically reheating temperature on microstructure and properties of simulated inter-critically reheated coarse grained heat affected zone in X70 steel. Mater. Sci. Eng. A 2014, 605, 8–13. [Google Scholar] [CrossRef]
- Dastgiri, M.S.; Mohammadi, J.; Behnamian, Y.; Eghlimi, A.; Mostafaei, A. Metallurgical investigations and corrosion behavior of failed weld joint in AISI 1518 low carbon steel pipeline. Eng. Fail. Anal. 2015, 53, 78–96. [Google Scholar] [CrossRef]
- Tan, W.; Xu, B.S.; Han, W.A.; Feng, S.W.; Feng, J.L.; Zhong, Q.P. HAZ corrosion of 22SiMn2TiB ultra-strength steel weldment in 3.5% NaCl solution. Acta. Metall. Sin. 2004, 40, 197–201. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization of corrosion of welded X70 pipeline steel in near-neutral pH solution. Corros. Sci. 2009, 51, 1714–1724. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization and Mott–Schottky analysis of corrosion of welded X70 pipeline steel in carbonate/bicarbonate solution. Electrochim. Acta 2009, 55, 316–324. [Google Scholar] [CrossRef]
- Bordbar, S.; Alizadeh, M.; Hashemi, S.H. Effects of microstructure alteration on corrosion behavior of welded joint in API X70 pipeline steel. Mater. Des. 2013, 45, 597–604. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Corrosion of the Heat-Affected Zones (HAZs) of API-X100 pipeline steel in dilute bicarbonate solutions at 90 °C—An electrochemical evaluation. Corros. Sci. 2013, 74, 297–307. [Google Scholar] [CrossRef]
- Yu, X.H.; Caron, J.L.; Babu, S.S.; Lippold, J.C.; Isheim, D.; Seidman, D.N. Characterization of microstructural strengthening in the heat-affected zone of a blast-resistant naval steel. Acta Mater. 2010, 58, 5596–5609. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Kannan, R.; Li, L.J. Characterization of as-welded microstructure of heat-affected zone in modified 9Cr–1Mo–V–Nb steel weldment. Mater. Charact. 2016, 118, 225–234. [Google Scholar] [CrossRef]
- Hu, J.; Du, L.X.; Xie, H.; Dong, F.T.; Misra, R.D.K. Effect of weld peak temperature on the microstructure, hardness, and transformation kinetics of simulated heat affected zone of hot rolled ultra-low carbon high strength Ti–Mo ferritic steel. Mater. Des. 2014, 60, 302–309. [Google Scholar] [CrossRef]
- Hayat, F.; Uzun, H. Effect of heat treatment on microstructure mechanical properties and fracture behaviour of ship and dual phase steels, J. Iron Steel Res. Int. 2011, 18, 65–72. [Google Scholar] [CrossRef]
- Zhang, W.Y. Welding Metallurgy (Basic Principle); China Machine Press: Beijing, China, 1996; pp. 167–174. [Google Scholar]
- Cui, Z.Q.; Liu, B.X. Metallurgy and Treatment Theory, 3rd ed.; Harbin Institute of Technology Press: Harbin, China, 2007; pp. 206–209. [Google Scholar]
- Wang, L.W.; Liu, Z.Y.; Cui, Z.Y.; Du, C.W.; Wang, X.H.; Li, X.G. In situ corrosion characterization of simulated weld heat affected zone on API X80 pipeline steel. Corros. Sci. 2014, 85, 401–410. [Google Scholar] [CrossRef]
- Olasolo, M.; Uranga, P.; Rodriguez-Ibabe, J.M.; López, B. Effect of austenite microstructure and cooling rate on transformation characteristics in a low carbon Nb–V microalloyed steel. Mater. Sci. Eng. A 2011, 528, 2559–2569. [Google Scholar] [CrossRef]
- Moon, J; Lee, J.; Lee, C. Prediction for the austenite grain size in the presence of growing particles in the weld HAZ of Ti-microalloyed steel. Mater. Sci. Eng. A 2007, 459, 40–46. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Fundamentals of Materials Science: The Microstructure-Property Relationship Using Metals as Model Systems, 1st ed.; Springer: Heidelberg, Germany, 2010; pp. 349–354. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 352–355. [Google Scholar]
- Li, X.G.; Zhang, D.W.; Liu, Z.Y.; Li, Z.; Du, C.W.; Dong, C.F. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zou, Y.; Matsuda, K.; Zou, Z.D. Corrosion behaviour of reheated CGHAZ of X80 pipeline steel in H2S-containing environments. Mater. Des. 2016, 99, 44–56. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, C.P.; Du, C.W.; Li, X.G. Effect of applied potentials on stress corrosion cracting of X80 pipeline steel in simulated Yingtan soil solution. Acta. Metall. Sin. 2011, 47, 1434–1439. [Google Scholar] [CrossRef]
- Wang, Z.H; Huang, Y.H.; Li, J.; Yang, L.; Xie, D.H. Effect of Nb on corrosion behavior of simulated weld HAZs of X80 pipeline steel in simulated seawater environments corresponding to shallow sea and deep sea. J. Chin. Soc. Corros. Prot. 2016, 36, 604–610. [Google Scholar] [CrossRef]
- Cao, C.N. Principles of Electrochemisty of Corrosion, 3rd ed.; Chemical Industry Press: Beijing, China, 2008; pp. 74–79. [Google Scholar]
- Naderi, E.; Ehteshamzadeh, M.; Jafari, A.H.; Hosseini, M.G. Effect of carbon steel microstructure and molecular structure of two new Schiff base compounds on inhibition performance in 1 M HCl solution by DC, SEM and XRD studies. Mater. Chem. Phys. 2010, 120, 134–141. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X. Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim. Acta 2009, 54, 1881–1887. [Google Scholar] [CrossRef]
- Achouri, M.E.; Kertit, S.; Gouttaya, H.M.; Nciri, B.; Bensouda, Y.; Perez, L.; Infante, M.R.; Elkacemi, K. Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α,ω-bis-(dimethyl tetradecyl ammonium bromide). Prog. Org. Coat. 2001, 43, 267–273. [Google Scholar] [CrossRef]
- Wang, X.X.; Gao, Y.M.; Li, K.; Yan, J.B.; Li, Y.F.; Feng, J.B. Effect of yttrium on the corrosion behaviour of 09CrCuSb alloy in concentrated sulphuric acid. Corros. Sci. 2013, 69, 369–375. [Google Scholar] [CrossRef]
- Wang, X.X.; Gao, Y.M.; Li, Y.F.; Yang, T. Effect of yttrium on the corrosion behavior of 09CrCuSb alloy in NaCl Solution. Corros. Sci. 2014, 87, 211–217. [Google Scholar] [CrossRef]


| Simulated Welding HAZ | Peak Temperature, °C | Heating Rate, °C/s | Holding Time at Peak Temperature, s | Cooling Rate, °C/s |
|---|---|---|---|---|
| CGHAZ | 1320 | 150 | 1 | 20 |
| FGHAZ | 930 | 150 | 1 | 20 |
| ICHAZ | 830 | 150 | 1 | 20 |
| Electrode | BM | CGHAZ | FGHAZ | ICHAZ |
|---|---|---|---|---|
| OCP value, mV | −431.24 | −339.15 | −379.22 | −353.73 |
| Specimens | Ecorr, mV | icorr, µA/cm2 | βc, mV/dec | βa, mV/dec | Epp, mV | Eb, mV | ip, µA/cm2 | Corrosion Rate, mm/a |
|---|---|---|---|---|---|---|---|---|
| BM | −371.9 | 121.2 | 91.6 | 22.2 | −184 | 425 | 5.19 | 3.313 |
| CGHAZ | −321.3 | 316.9 | 98.7 | 23.6 | −159 | 403 | 8.71 | 7.39 |
| FGHAZ | −333.9 | 148.4 | 91.7 | 25.3 | −165 | 409 | 6.87 | 3.927 |
| ICHAZ | −358.8 | 124.3 | 89.1 | 22.4 | −162 | 420 | 6.60 | 3.419 |
| Specimens | Immersion Time, h | Rsol, Ω∙cm2 | Cc, μF | Rc, Ω∙cm2 | Rct, Ω∙cm2 | CPE | |
|---|---|---|---|---|---|---|---|
| Y, S·sn/cm2 | n | ||||||
| BM | 0 | 1.004 × 10−13 | 1.177 | 0.947 | 81.86 | 6.966 × 10−5 | 0.9628 |
| 4 | 1.225 × 10−9 | 0.9849 | 0.9551 | 131.6 | 3.728 × 10−4 | 0.9381 | |
| 12 | 1.003 × 10−7 | 1.142 | 1.189 | 147.2 | 5.024 × 10−4 | 0.9647 | |
| 72 | 9.999 × 10−8 | 1.282 | 1.031 | 116.6 | 5.362 × 10−4 | 0.9626 | |
| CGHAZ | 0 | 9.924 × 10−8 | 1.929 | 0.669 | 31.38 | 1.381 × 10−4 | 0.9719 |
| 4 | 2.467 × 10−11 | 3.422 | 0.4024 | 48.74 | 8.371 × 10−4 | 0.9552 | |
| 12 | 8.983 × 10−12 | 2.967 | 0.4973 | 77.23 | 7.9 × 10−4 | 0.9681 | |
| 72 | 2.45 × 10−6 | 1.296 | 0.8698 | 65.55 | 9.823 × 10−4 | 0.9422 | |
| FGHAZ | 0 | 7.662 × 10−7 | 0.4751 | 1.576 | 41.24 | 5.826 × 10−5 | 0.9391 |
| 4 | 1 × 10−7 | 0.8941 | 1.171 | 78.75 | 7.8 × 10−4 | 0.9410 | |
| 12 | 1.269 × 10−9 | 0.9385 | 1.214 | 94.12 | 7.193 × 10−4 | 0.9564 | |
| 72 | 1.001 × 10−7 | 0.7065 | 1.699 | 99.41 | 6.427 × 10−4 | 0.9565 | |
| ICHAZ | 0 | 9.571 × 10−8 | 0.2447 | 2.681 | 62.06 | 4.027 × 10−5 | 0.9349 |
| 4 | 5.769 × 10−8 | 0.9239 | 1.321 | 91.47 | 4.287 × 10−4 | 0.9528 | |
| 12 | 7.769 × 10−12 | 0.6154 | 1.865 | 99.58 | 5.969 × 10−4 | 0.9573 | |
| 72 | 1.495 × 10−7 | 1.19 | 1.048 | 91.55 | 7.242 × 10−4 | 0.9582 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhao, H.; Shu, F.; He, W.; Wang, G. Microstructure and Corrosion Behavior of Simulated Welding HAZ of Q315NS Steel in Sulfuric Acid Solution. Metals 2017, 7, 194. https://doi.org/10.3390/met7060194
Zhang S, Zhao H, Shu F, He W, Wang G. Microstructure and Corrosion Behavior of Simulated Welding HAZ of Q315NS Steel in Sulfuric Acid Solution. Metals. 2017; 7(6):194. https://doi.org/10.3390/met7060194
Chicago/Turabian StyleZhang, Suqiang, Hongyun Zhao, Fengyuan Shu, Wenxiong He, and Guodong Wang. 2017. "Microstructure and Corrosion Behavior of Simulated Welding HAZ of Q315NS Steel in Sulfuric Acid Solution" Metals 7, no. 6: 194. https://doi.org/10.3390/met7060194





