Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles
Abstract
:1. Introduction
2. Materials and Methods
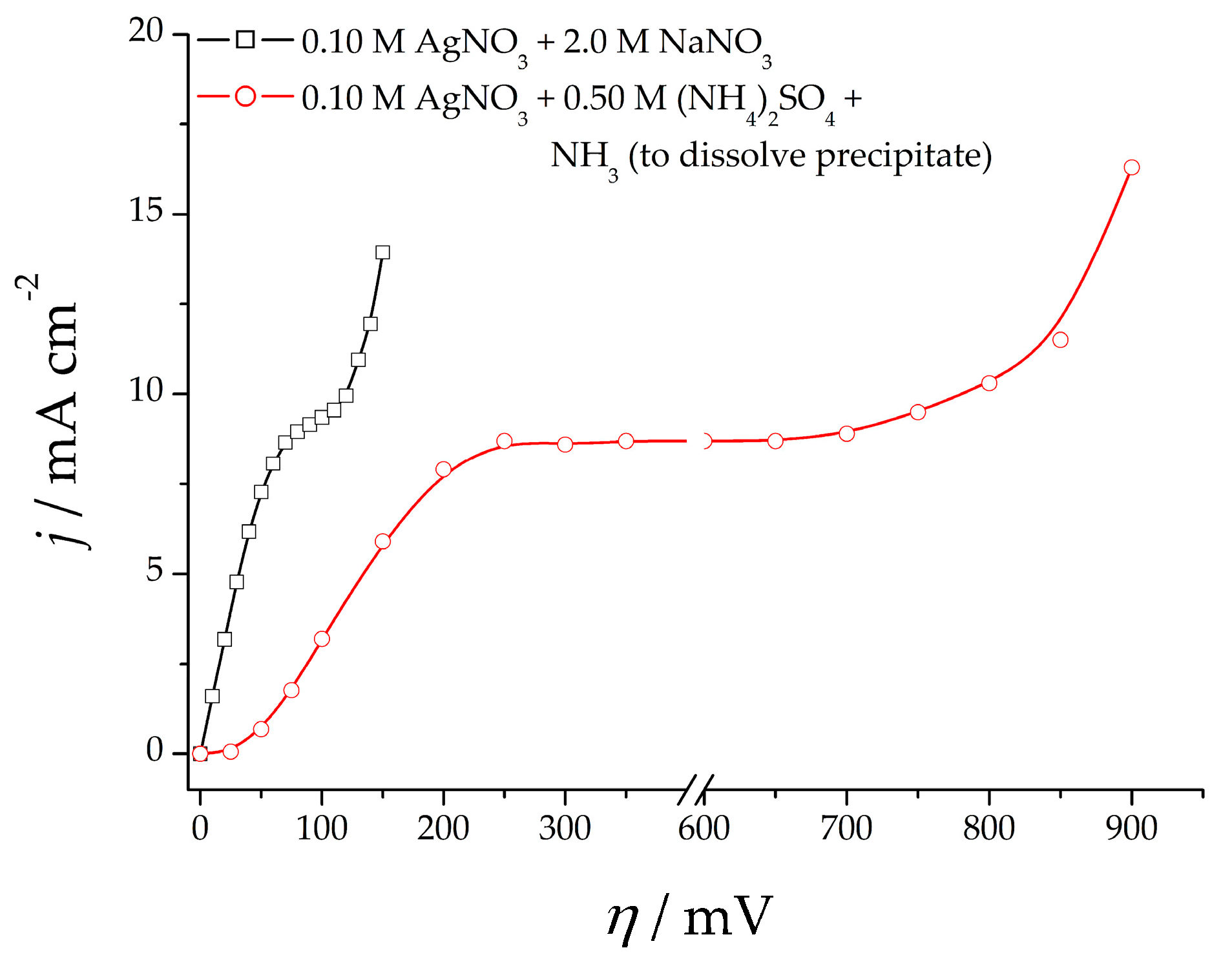
2.1. Electrochemical Experiments
- (a)
- 0.10 M AgNO3 in 2.0 M NaNO3 (the nitrate electrolyte), and
- (b)
- 0.10 M AgNO3 in 0.50 M (NH4)2SO4 with the addition of NH3 to dissolve the silver sulfate precipitate (the ammonium electrolyte).
2.2. Chemical Synthesis
2.3. Characterization
3. Results
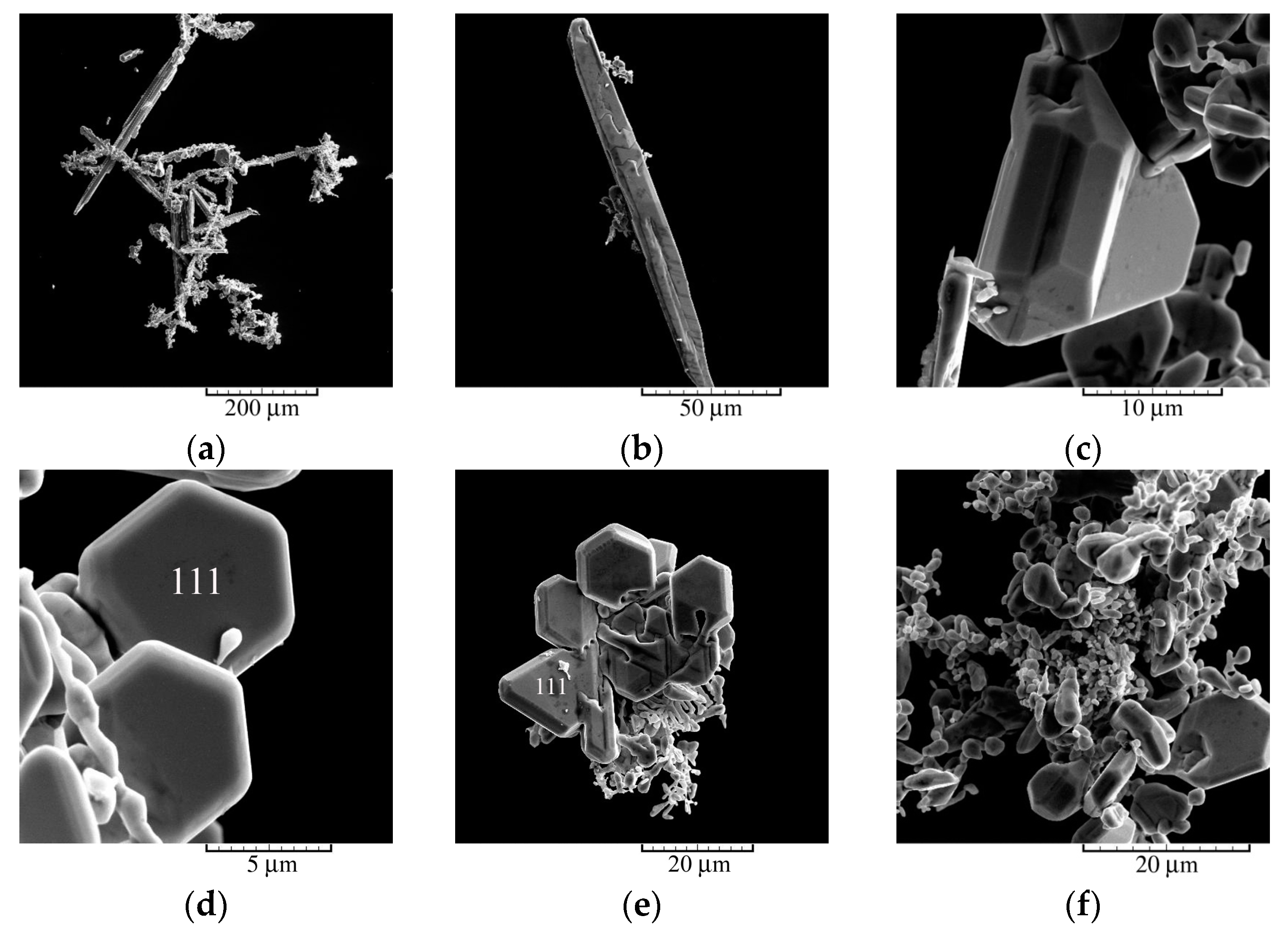
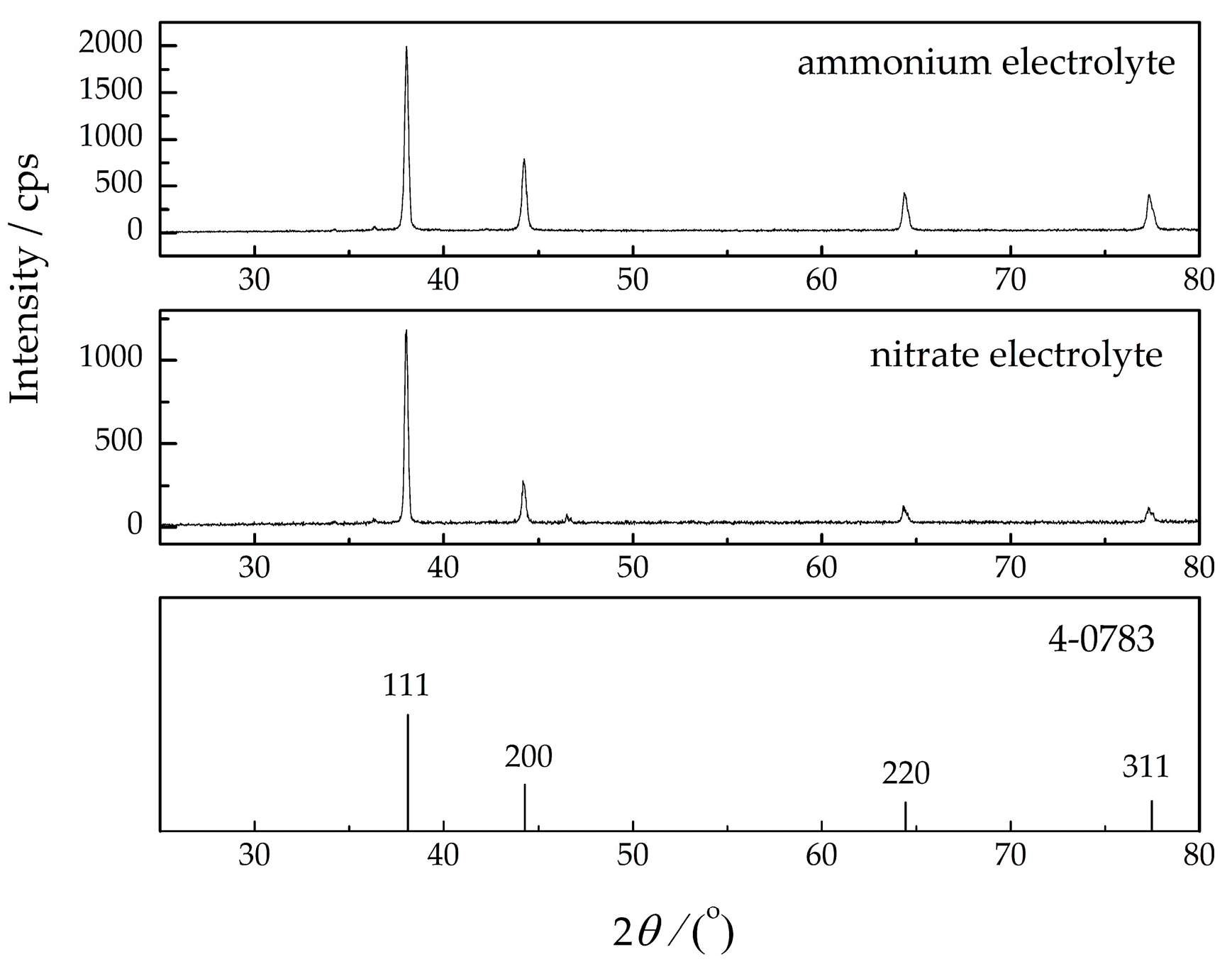
3.1. Electrochemical Production of Silver Particles: Polarization, Morphological, and Crystallographic Analysis
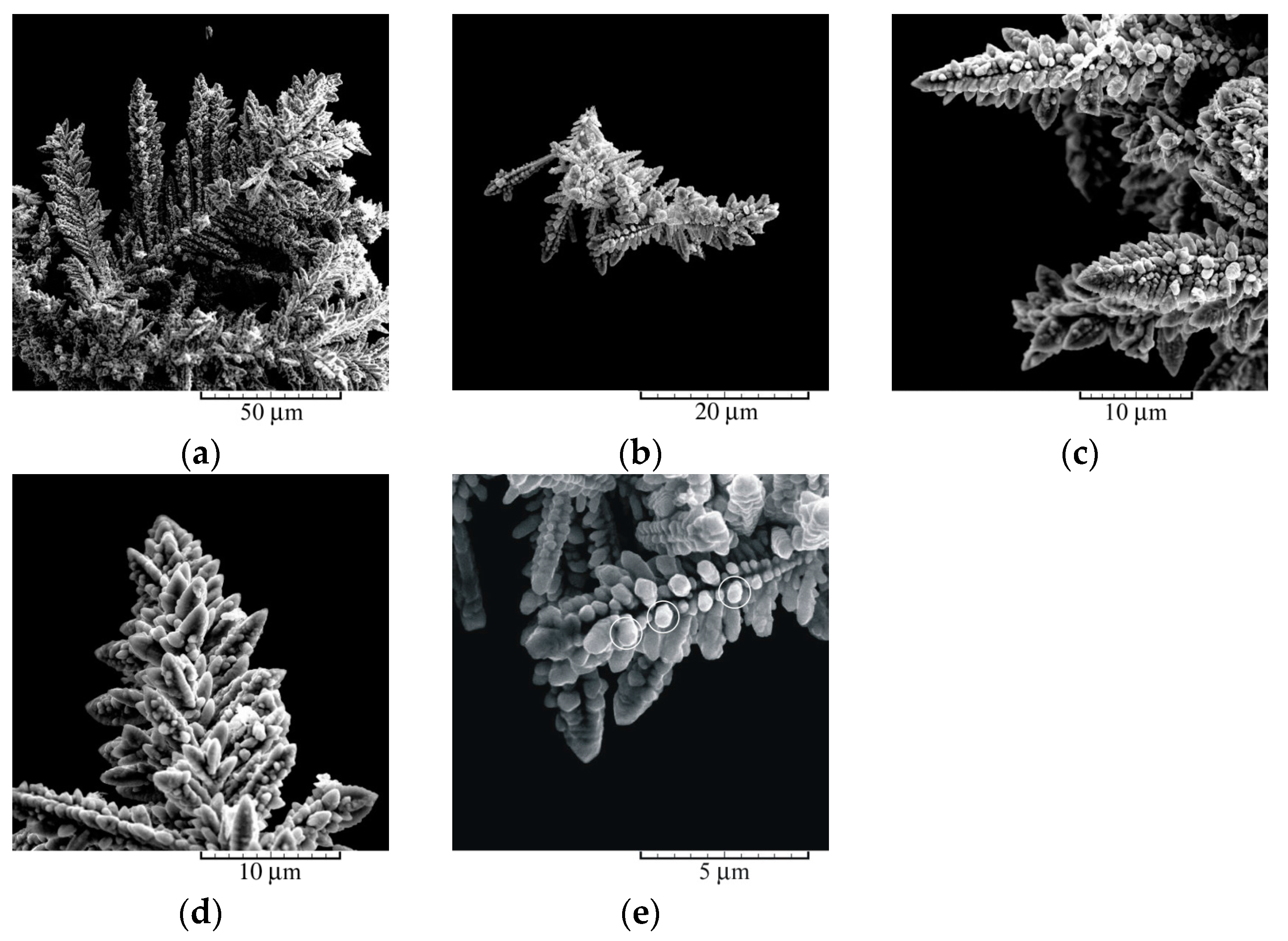
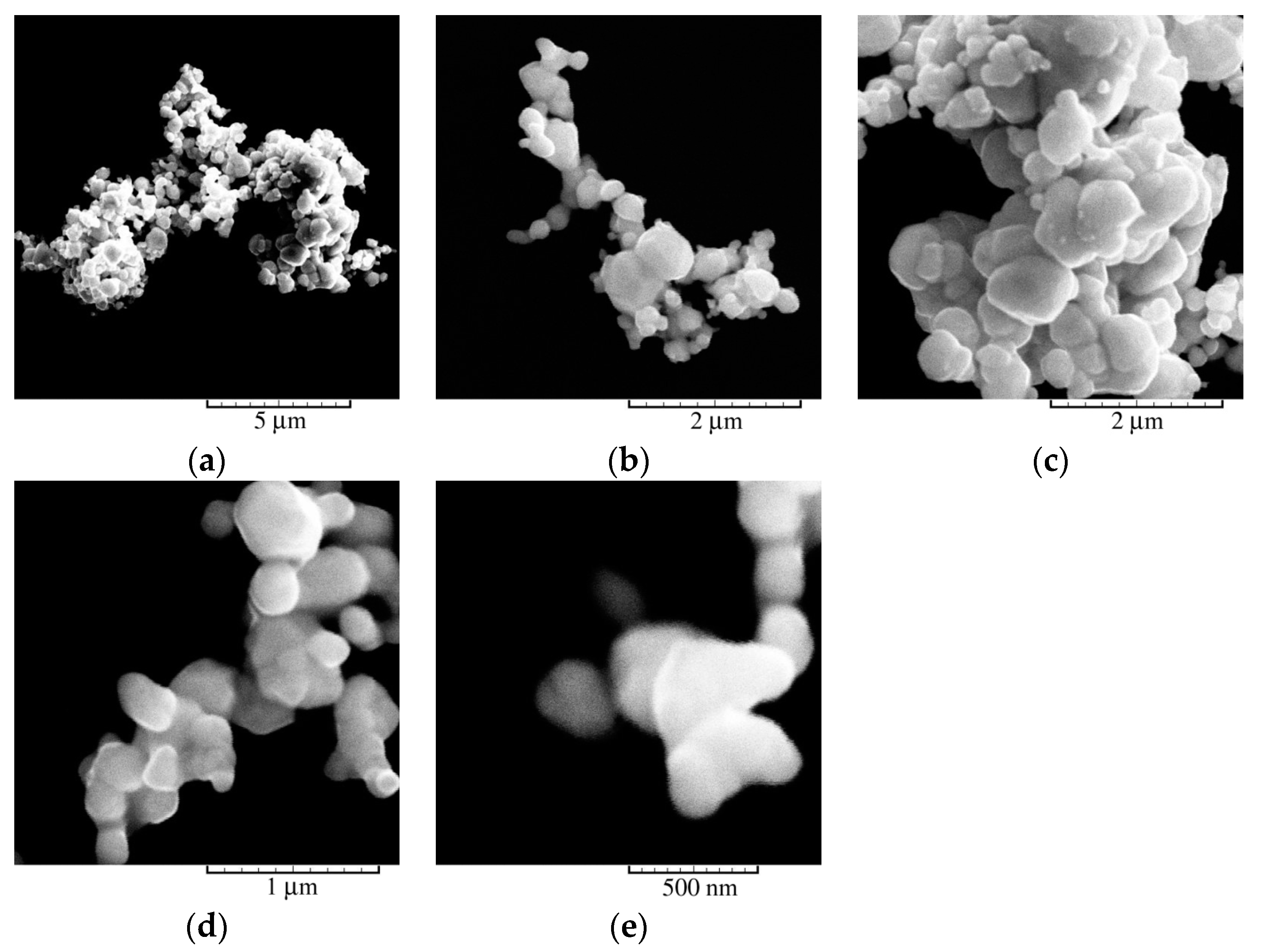
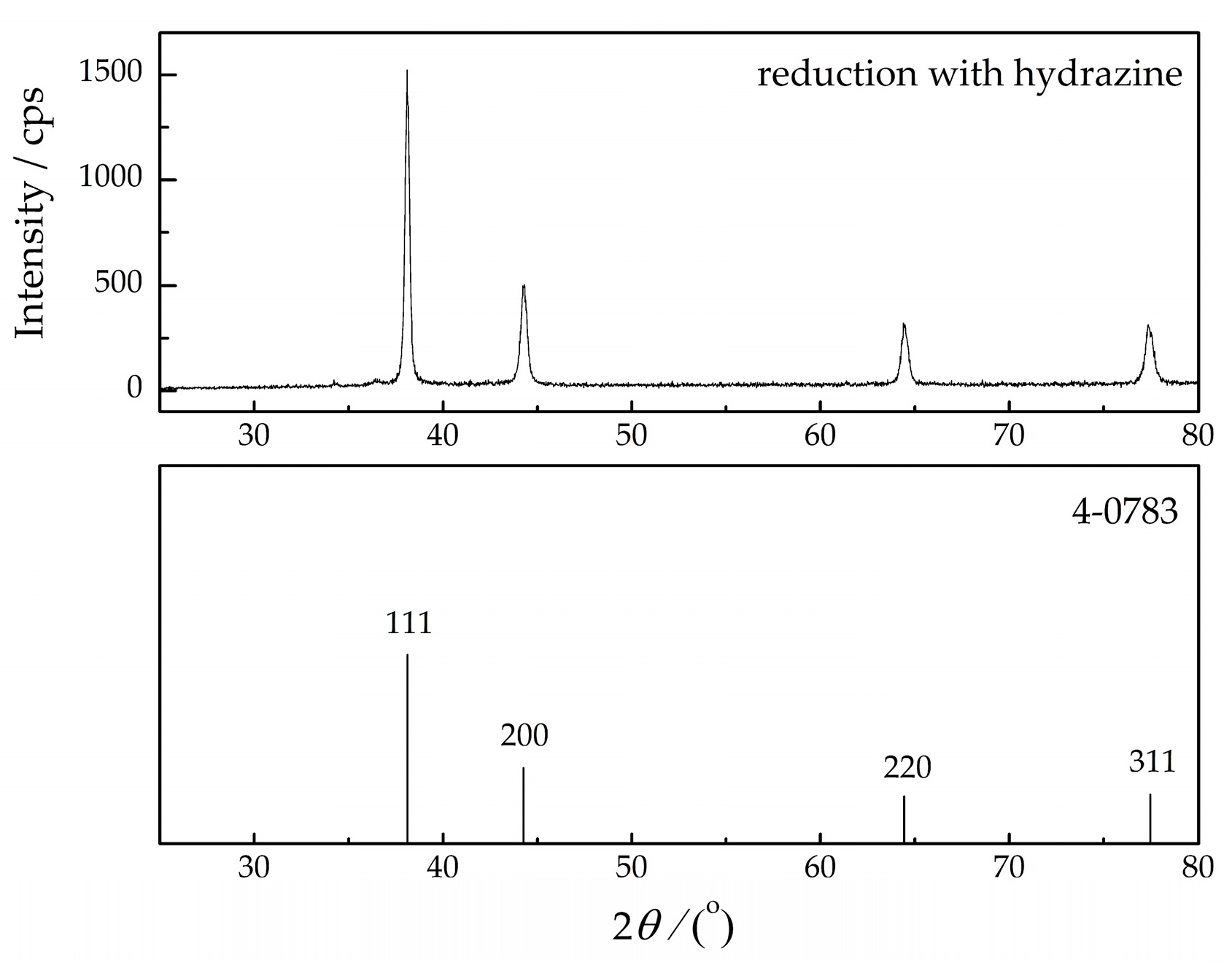
3.2. Chemical Synthesis of Ag Powder: Morphological and Crystallographic Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antony, L.V.M.; Reddy, R.G. Processes for Production of High-Purity Metal Powders. JOM 2003, 55, 14–18. [Google Scholar] [CrossRef]
- Chaubey, A.K.; Scudino, S.; Khoshkhoo, M.S.; Prashanth, K.G.; Mukhopadhyay, N.K.; Mishra, B.K.; Eckert, J. Synthesis and Characterization of Nanocrystalline Mg-7.4%Al Powders Produced by Mechanical Alloying. Metals 2013, 3, 58–68. [Google Scholar] [CrossRef]
- Calusaru, A. Electrodeposition of Metal Powders; Elsevier: New York, NY, USA, 1979; pp. 1–544. [Google Scholar]
- Orhan, G.; Hapci, G. Effect of electrolysis parameters on the morphologies of copper powder obtained in a rotating cylinder electrode cell. Powder Technol. 2010, 201, 57–63. [Google Scholar] [CrossRef]
- Amiri, M.; Nouhi, S.; Azizian-Kalandaragh, Y. Facile synthesis of silver nanostructures by using various deposition potential and time: A nonenzymatic sensors for hydrogen peroxide. Mater. Chem. Phys. 2015, 155, 129–135. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. Morphology of Electrochemically and Chemically Deposited Metals; Springer: New York, NY, USA, 2016; pp. 1–368. [Google Scholar]
- Nikolić, N.D.; Branković, G.; Pavlović, M.G. Correlate between morphology of powder particles obtained by the different regimes of electrolysis and the quantity of evolved hydrogen. Powder Technol. 2012, 221, 271–277. [Google Scholar]
- Yao, C.-Z.; Liu, M.; Zhang, P.; He, X.-H.; Li, G.-R.; Zhao, W.-X.; Liu, P.; Tong, Y.-X. Tuning the architectures of lead deposits on metal substrates by electrodeposition. Electrochim. Acta 2008, 54, 247–253. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Hong, J. Hierarchical Pb microstructures: A facile electrochemical synthesis, shape evolution and influencing factors. CrystEngComm 2011, 13, 934–940. [Google Scholar] [CrossRef]
- Cherevko, S.; Xing, X.; Chung, C.-H. Hydrogen template assisted electrodeposition of sub-micrometer wires composing honeycomb-like porous Pb films. Appl. Surf. Sci. 2011, 257, 8054–8061. [Google Scholar] [CrossRef]
- Yang, M. Fern-shaped bismuth dendrites electrodeposited at hydrogen evolution potentials. J. Mater. Chem. 2011, 21, 3119–3124. [Google Scholar] [CrossRef]
- Bengoa, L.N.; Bruno, S.; Lazzarino, H.A.; Seré, P.R.; Egli, W.A. Study of dendritic growth of zinc crystals on the edges of steel sheet. J. Appl. Electrochem. 2014, 44, 1261–1269. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Zhang, L.; Zhang, Y.; Jiang, C. Electrolytic approach towards the controllable synthesis of symmetric, hierarchical, and highly ordered nickel dendritic crystals. CrystEngComm 2012, 14, 1629–1636. [Google Scholar] [CrossRef]
- Lv, Z.-Y.; Li, A.-Q.; Fei, Y.; Li, Z.; Chen, J.-R.; Wang, A.-J.; Feng, J.-J. Facile and controlled electrochemical route to three-dimensional hierarchical dendritic gold nanostructures. Electrochim. Acta 2013, 109, 136–144. [Google Scholar] [CrossRef]
- Ostanina, T.N.; Rudoi, V.M.; Patrushev, A.V.; Darintseva, A.B.; Farlenkov, A.S. Modelling the Dynamic Growth of Copper and Zinc Dendritic Deposits under the Galvanostatic Electrolysis Conditions. J. Electroanal. Chem. 2015, 750, 9–18. [Google Scholar] [CrossRef]
- Jović, V.D.; Jović, B.M.; Maksimović, V.M.; Pavlović, M.G. Electrodeposition and morphology of Ni, Co and Ni–Co alloy powders: Part II. Ammonium chloride supporting electrolyte. Electrochim. Acta 2007, 52, 4254–4263. [Google Scholar]
- Nikolić, N.D.; Pavlović, Lj.J.; Pavlović, M.G.; Popov, K.I. Morphologies of electrochemically formed copper powder particles and their dependence on the quantity of evolved hydrogen. Powder Technol. 2008, 185, 195–201. [Google Scholar]
- Djokić, S.S. Production of Metallic Powders from Aqueous Solutions without an External Current Source. In Electrochemical Production of Metal Powders, Series: Modern Aspects of Electrochemistry; Djokić, S.S., Ed.; Springer: New York, NY, USA, 2012; Volume 54, pp. 369–398. [Google Scholar]
- Djokić, S.S.; Djokić, N.S. Electroless deposition of metallic powders. J. Electrochem. Soc. 2011, 158, D204–D209. [Google Scholar]
- Djokić, S.S.; Djokić, N.S.; Guthy, C.; Thundat, T. Deposition of copper, silver and gold from aqueous solutions onto germanium substrates via galvanic displacement. Electrochim. Acta 2013, 109, 475–481. [Google Scholar]
- Yun, H.D.; Seo, D.M.; Lee, M.Y.; Kwon, S.Y.; Park, L.S. Effective Synthesis and Recovery of Silver Nanowires Prepared by Tapered Continuous Flow Reactor for Flexible and Transparent Conducting Electrode. Metals 2016, 6, 14. [Google Scholar] [CrossRef]
- Maksimović, V.M.; Pavlović, M.G.; Pavlović, Lj.J.; Tomić, M.V.; Jović, V.D. Morphology and growth of electrodeposited silver powder particles. Hydrometallurgy 2007, 86, 22–26. [Google Scholar]
- Popov, K.I.; Živković, P.M.; Nikolić, N.D. Formation of Disperse Silver Deposits by the Electrodeposition Processes at High Overpotentials. Int. J. Electrochem. Sci. 2012, 7, 686–696. [Google Scholar]
- Fu, L.; Tamanna, T.; Hu, W.-J.; Yu, A. Chemical preparation and applications of silver dendrites. Chem. Pap. 2014, 68, 1283–1297. [Google Scholar] [CrossRef]
- Jiang, Z.; Lin, Y.; Xie, Z. Structural investigations and growth mechanism of well-defined Ag dendrites prepared by conventional redox displacement. Mater. Chem. Phys. 2012, 134, 762–767. [Google Scholar] [CrossRef]
- Giannini, C.; Ladisa, M.; Altamura, D.; Siliqi, D.; Sibillano, T.; De Caro, L. X-ray Diffraction: A Powerful Technique for the Multiple-Length-Scale Structural Analysis of Nanomaterials. Crystals 2016, 6, 87. [Google Scholar] [CrossRef]
- Berube, L.P.; Esperance, G.L. A Quantitative Method of Determining of the Degree of Texture of Zinc Electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Mandke, M.V.; Han, S.-H.; Pathan, H.M. Growth of silver dendritic nanostructures via electrochemical route. CrystEngComm 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Ivanović, E.R.; Branković, G.; Lačnjevac, U.Č.; Stevanović, S.I.; Stevanović, J.S.; Pavlović, M.G. Electrochemical and crystallographic aspects of lead granular growth. Metall. Mater. Trans. B 2015, 46, 1760–1774. [Google Scholar]
- Winand, R. Electrodeposition of metals and alloys—New results and perspectives. Electrochim. Acta 1994, 39, 1091–1105. [Google Scholar] [CrossRef]
- Kozlov, V.M.; Peraldo Bicelli, L. Influence of the nature of metals on the formation of the deposit’s polycrystalline structure during electrocrystallization. J. Cryst. Growth 1999, 203, 255–260. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Popov, K.I.; Pavlović, L.J.; Pavlović, M.G. The effect of hydrogen codeposition on the morphology of copper electrodeposits. I. The concept of effective overpotential. J. Electroanal. Chem. 2006, 588, 88–98. [Google Scholar]
- Han, J.; Liu, J. Electrodeposition of Crystalline Dendritic Silver in 12-Tungstosilicate Acid System. J. Nanoeng. Nanomanuf. 2012, 2, 171–174. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Vaštag, D.D.; Živković, P.M.; Jokić, B.; Branković, G. Influence of the complex formation on the morphology of lead powder particles produced by the electrodeposition processes. Adv. Powder Technol. 2013, 24, 674–682. [Google Scholar]
- Wranglen, G. Dendrites and growth layers in the electrocrystallization of metals. Electrochim. Acta 1960, 2, 130–146. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Sangaranarayanan, M.V. Electrodeposition of silver nanostructures: From polygons to dendrites. CrystEngComm 2013, 15, 2052–2056. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y. Lim, B; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.; Reddy, A.K.N.; Gamboa-Aldeco, M. Modern Electrochemistry 2A, Fundamentals of Electrodics; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; p. 1333. [Google Scholar]
- Nikolić, N.D.; Maksimović, V.M.; Branković, G.; Živković, P.M.; Pavlović, M.G. Influence of the type of electrolyte on morphological and crystallographic characteristics of lead powder particles. J. Serbian Chem. Soc. 2013, 78, 1387–1395. [Google Scholar]
- Nikolić, N.D.; Maksimović, V.M.; Branković, G. Morphological and crystallographic characteristics of electrodeposited lead from the concentrated electrolyte. RSC Adv. 2013, 3, 7466–7471. [Google Scholar]
- Moudir, N.; Boukennous, Y.; Moulaï-Mostefa, N.; Bozetine, I.; Maoudj, M.; Kamel, N.; Kamel, Z.; Moudir, D. Preparation of silver powder used for solar cell paste by reduction process. Energy Procedia 2013, 36, 1184–1191. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Sangaranarayanan, M.V. A facile formation of silver dendrites on indium tin oxide surfaces using electrodeposition and amperometric sensing of hydrazine. Sens. Actuators B 2015, 213, 92–101. [Google Scholar] [CrossRef]
| The Type of Powder Particles | Ratio of Intensities | ||
|---|---|---|---|
| (111)/(200) | (111)/(220) | (111)/(311) | |
| The nitrate electrolyte | 4.2 | 9.3 | 9.9 |
| The ammonium electrolyte | 2.5 | 4.8 | 4.9 |
| Reduction with hydrazine | 3.0 | 4.7 | 4.8 |
| Ag standard (4-0783) | 2.5 | 4 | 3.8 |
| Plane (hkl) | R (in %) | Rs (in %) | TC | RTC (in %) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RNIT | RAM | RHYD | TCNIT | TCAM | TCHYD | RTCNIT | RTCAM | RTCHYD | ||
| (111) | 69.4 | 54.9 | 57.2 | 52.4 | 1.32 | 1.05 | 1.09 | 41.6 | 27.2 | 28.8 |
| (200) | 16.3 | 21.9 | 18.9 | 20.9 | 0.78 | 1.05 | 0.90 | 24.6 | 27.2 | 23.8 |
| (220) | 7.4 | 11.9 | 12.2 | 13.1 | 0.56 | 0.93 | 0.93 | 17.7 | 24.1 | 24.6 |
| (311) | 6.9 | 11.3 | 11.7 | 13.6 | 0.51 | 0.83 | 0.86 | 16.1 | 21.5 | 22.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramović, L.; Pavlović, M.M.; Maksimović, V.M.; Vuković, M.; Stevanović, J.S.; Bugarin, M.; Nikolić, N.D. Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles. Metals 2017, 7, 160. https://doi.org/10.3390/met7050160
Avramović L, Pavlović MM, Maksimović VM, Vuković M, Stevanović JS, Bugarin M, Nikolić ND. Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles. Metals. 2017; 7(5):160. https://doi.org/10.3390/met7050160
Chicago/Turabian StyleAvramović, Ljiljana, Miroslav M. Pavlović, Vesna M. Maksimović, Marina Vuković, Jasmina S. Stevanović, Mile Bugarin, and Nebojša D. Nikolić. 2017. "Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles" Metals 7, no. 5: 160. https://doi.org/10.3390/met7050160






