Non-Isothermal Gas-Based Direct Reduction Behavior of High Chromium Vanadium-Titanium Magnetite Pellets and the Melting Separation of Metallized Pellets
Abstract
:1. Introduction
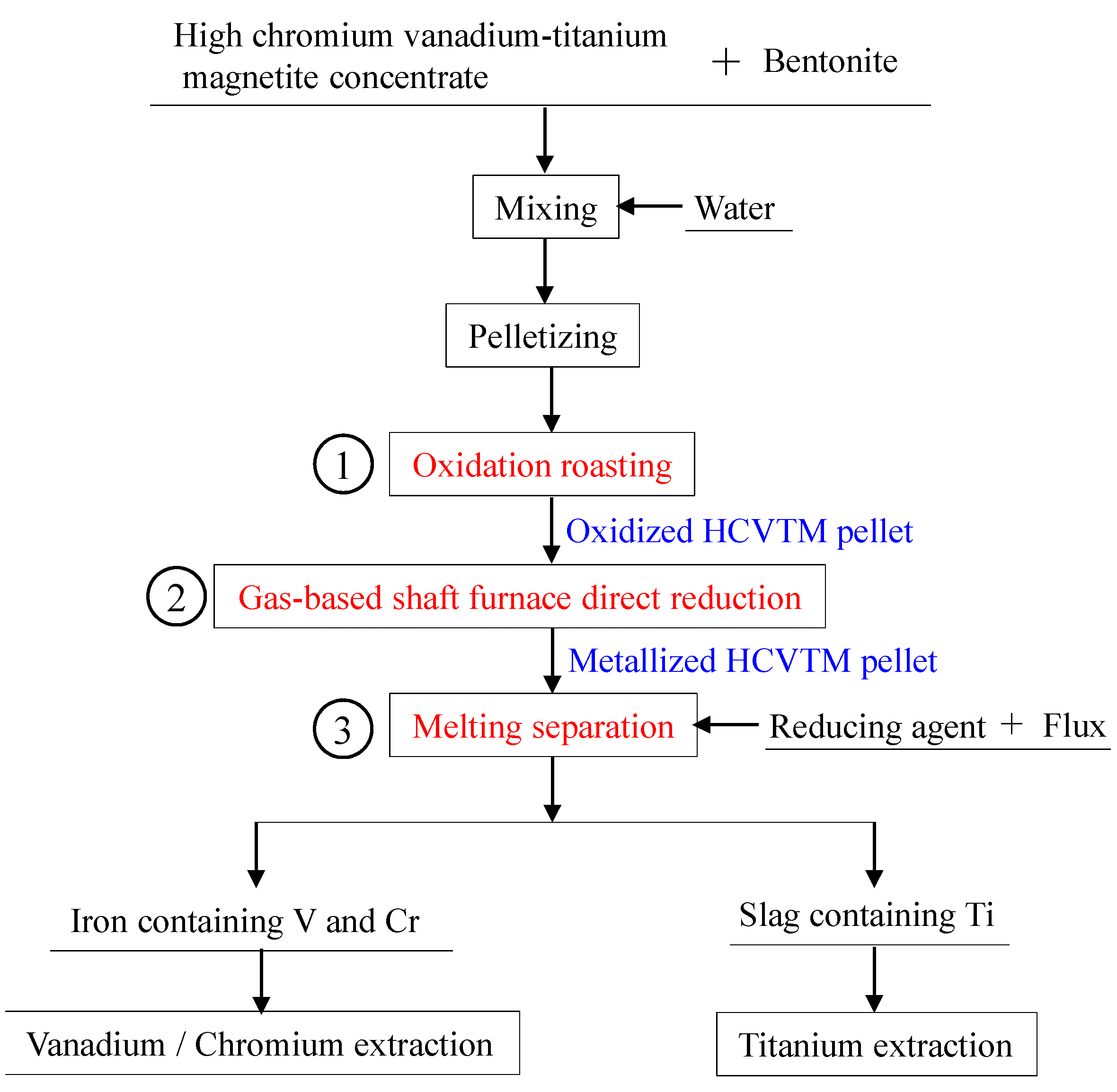
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedure
2.2.1. Pelletizing and Oxidizing of HCVTM
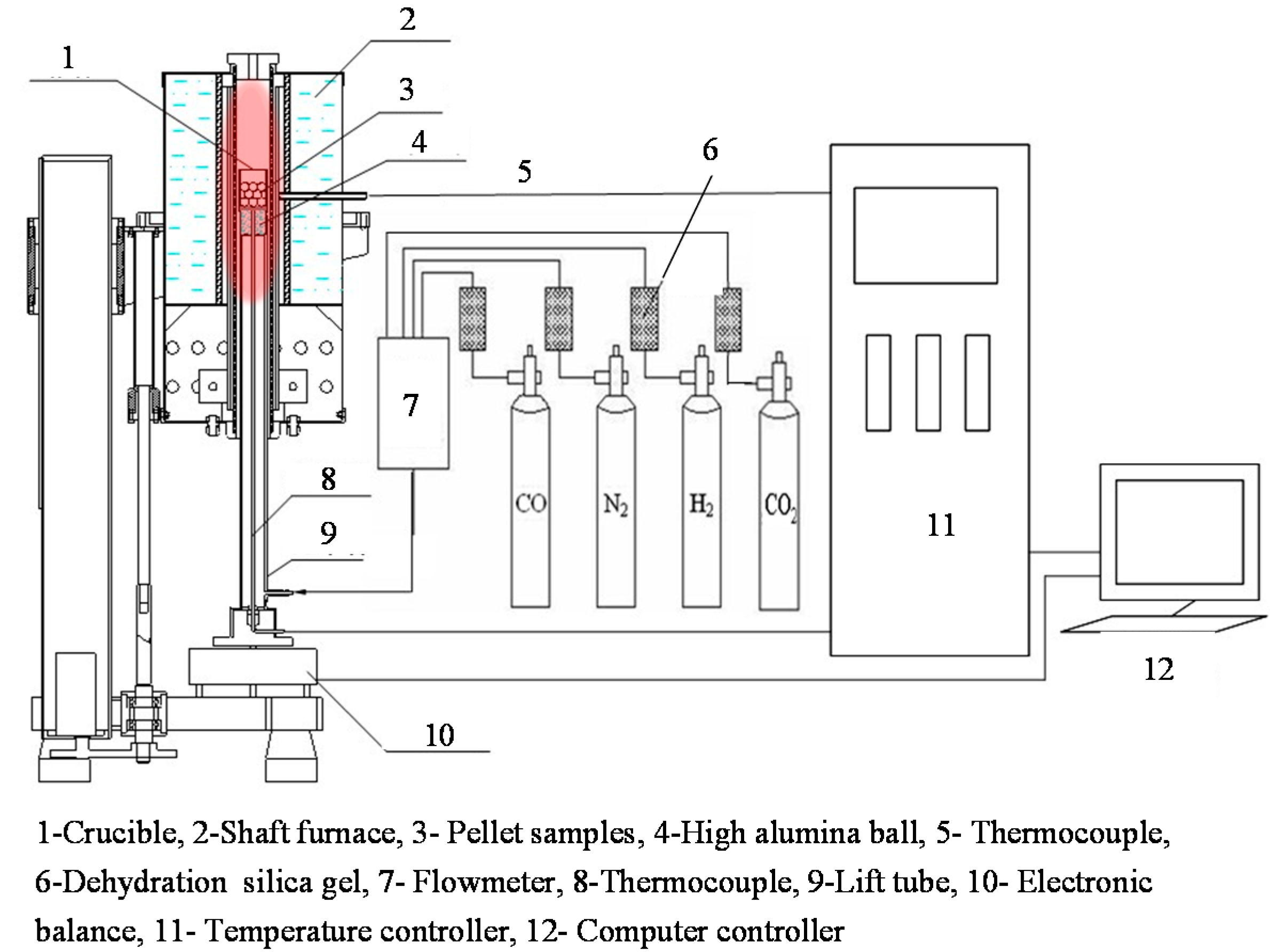
2.2.2. Gas-Based Shaft Furnace Direct Reduction of Oxidized HCVTM Pellets
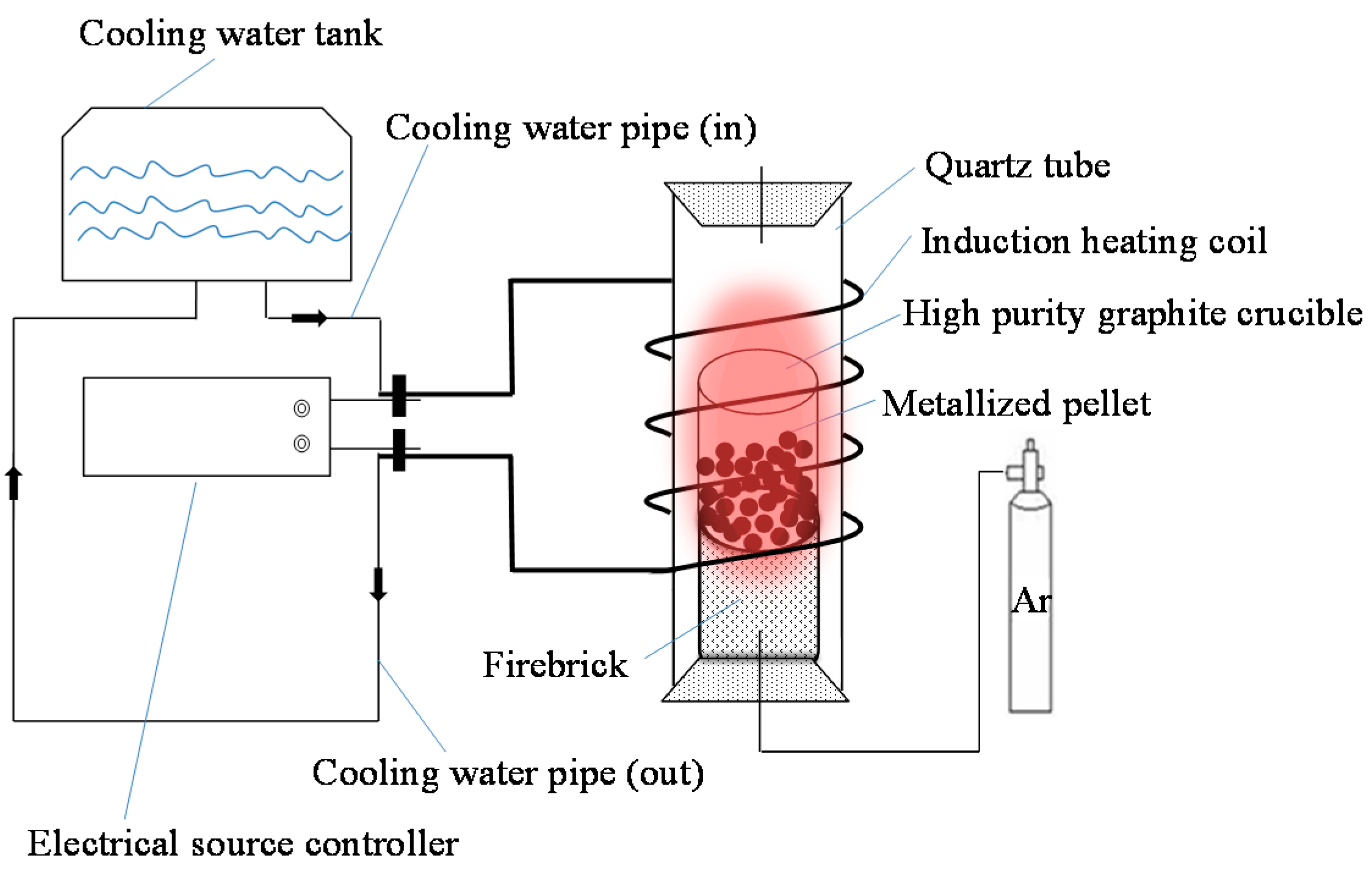
2.2.3. Melting Separation of the Metallized HCVTM Pellets
3. Results and Discussion
3.1. Properties of Oxidized HCVTM Pellets
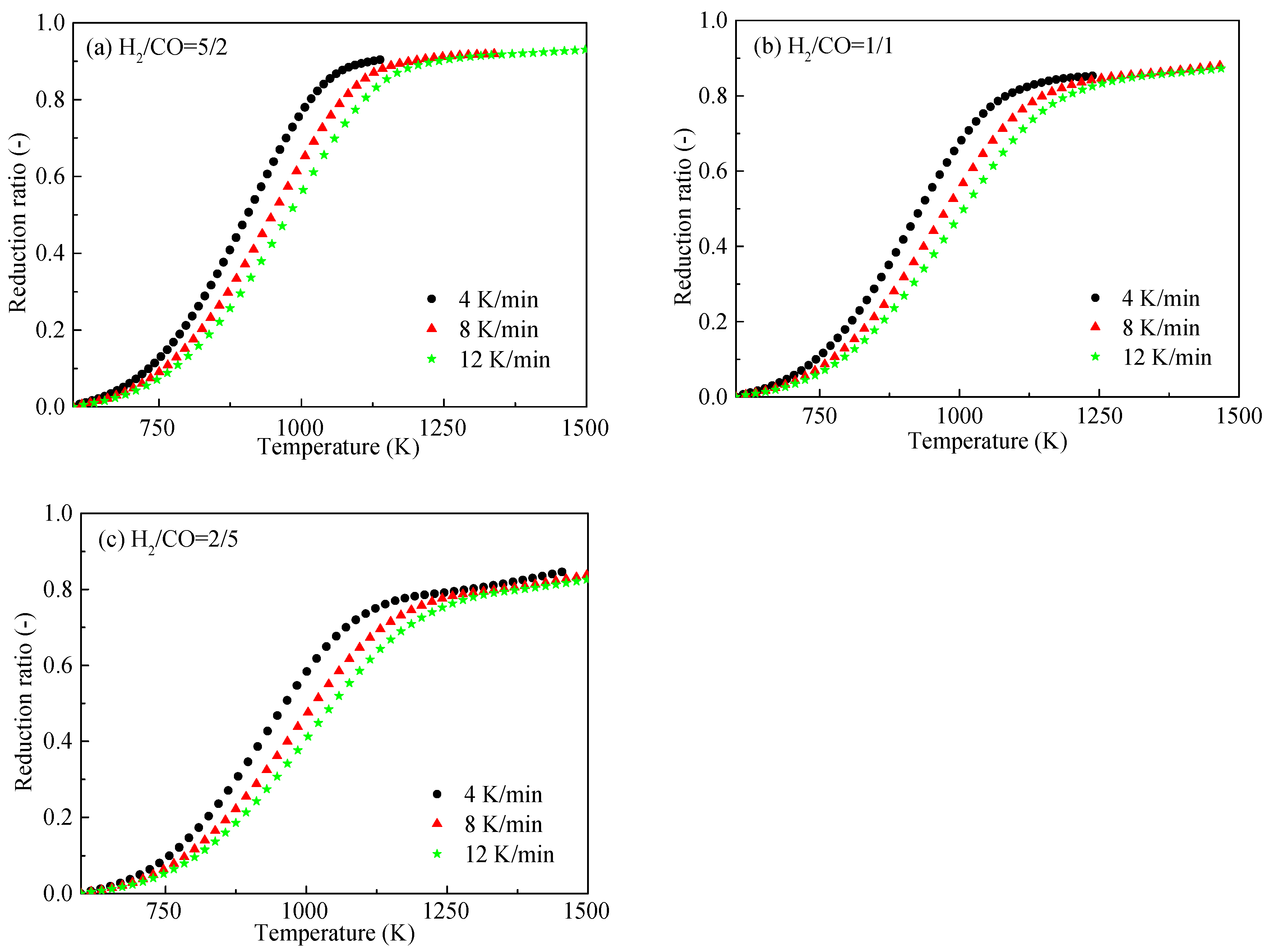
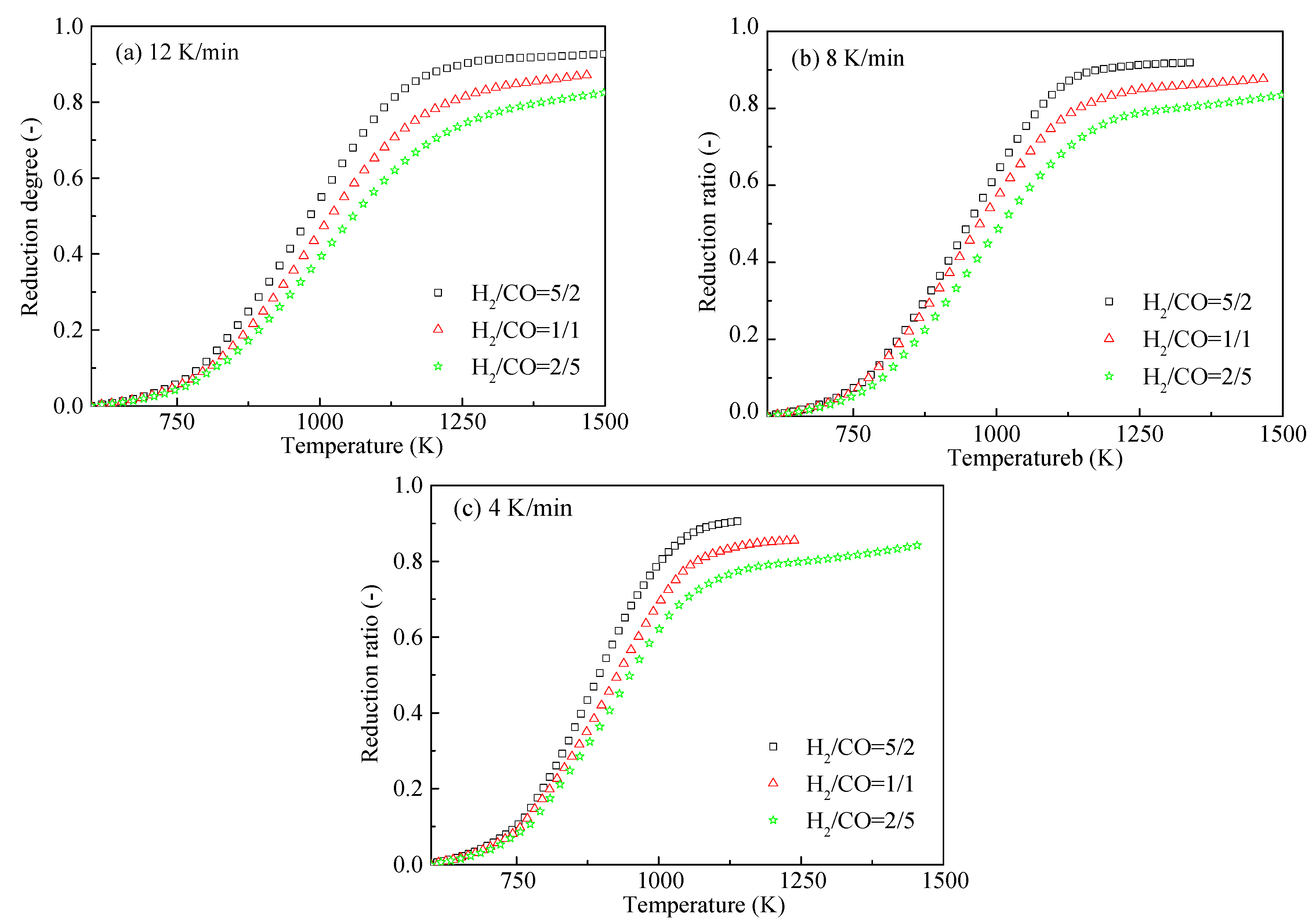
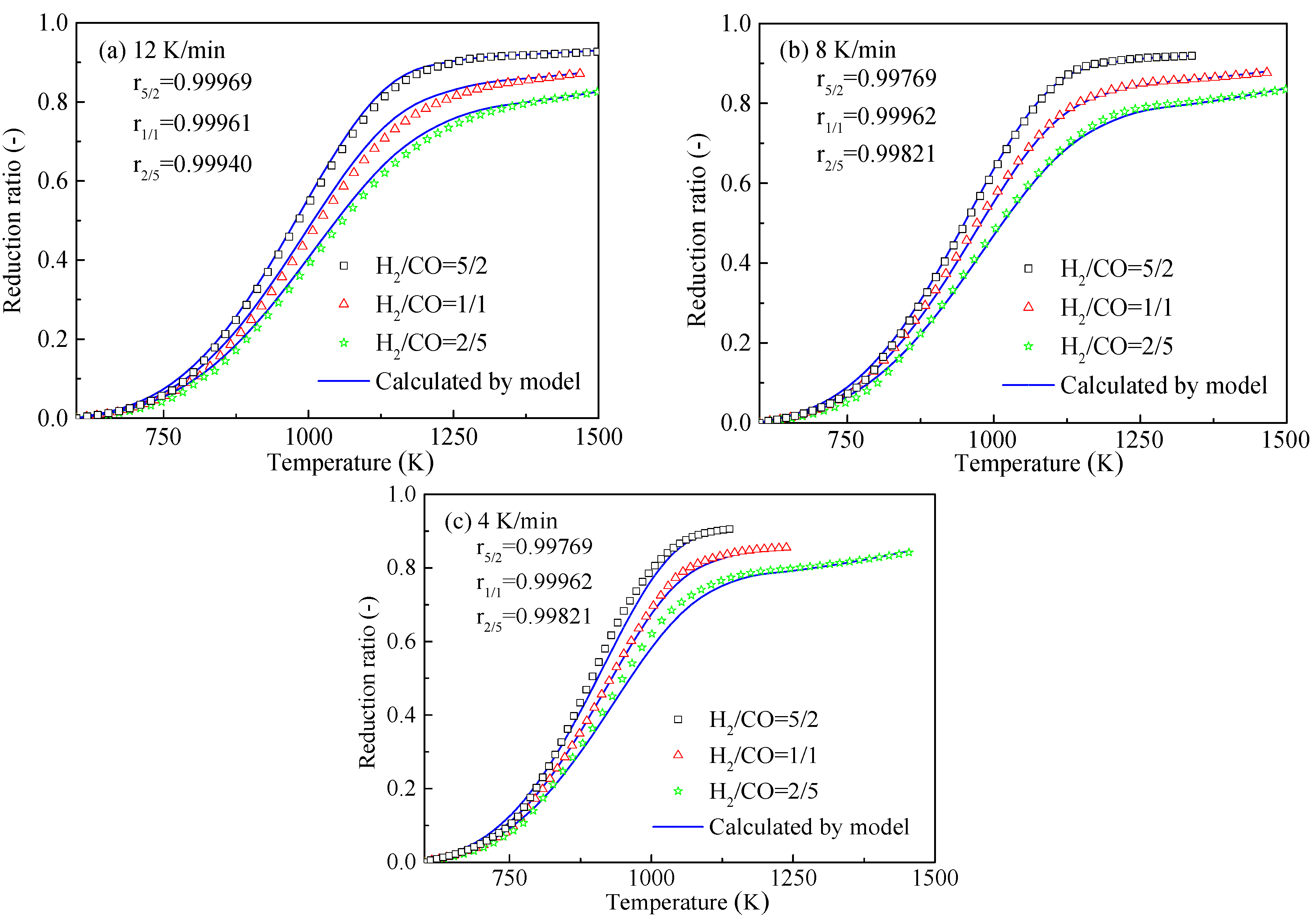
3.2. Effects of Heating Rate and Gas Composition on the HCVTM Pellet Reduction Ratio
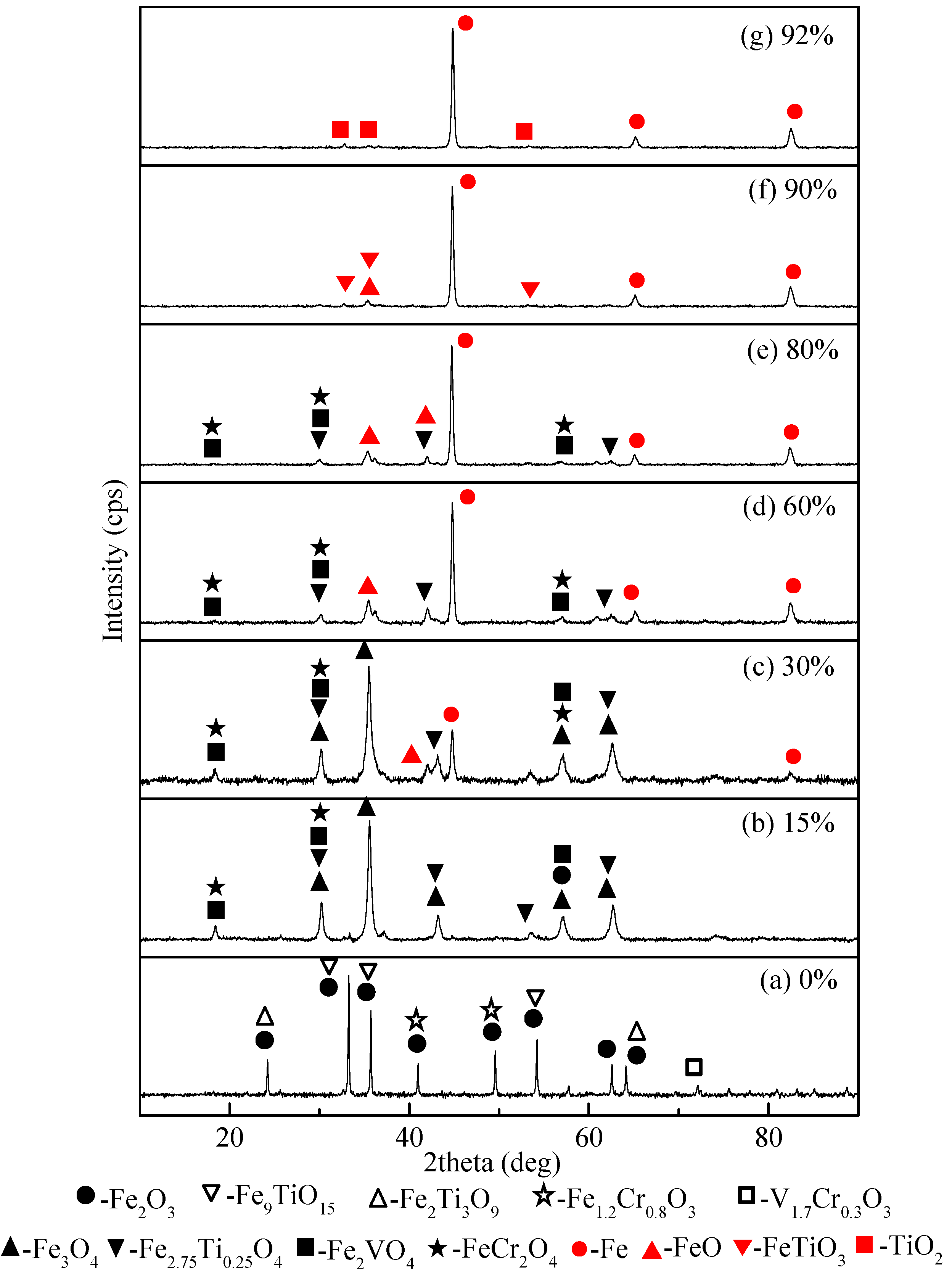
3.3. Phase Transformation and Morphologic Changes of the HCVTM Pellets During Reduction
- (1)
- When the reduction begins:Meanwhile, the phases containing V or Cr also cause some changes, as shown in Equations (2)–(5).36Fe2O3 + 2Fe2Ti3O9 + 2Fe9TiO15 + 20H2 = 2Fe3O4 + 8 (3Fe3O4·Fe2TiO4) + 20H2OV1.7Cr0.3O3 = 0.85V2O3 + 0.15Cr2O3Fe1.2Cr0.8O3 = 0.4Cr2O3 + 0.6Fe2O3Fe3O4 + 3Cr2O3 + H2 = 3FeCr2O4 + H2O4Fe3O4 + 3V2O3 + H2 = 6Fe2VO4 + H2O
- (2)
- When the reduction ratio is 15–90%:Fe3O4 + 3Fe3O4·Fe2TiO4 + 14H2 = 11FeO + FeTiO3 + 14H2O
- (3)
- When the reduction ratio is more than 90%:FeO + FeTiO3 + 2H2 = 2Fe + TiO2 + 2H2O
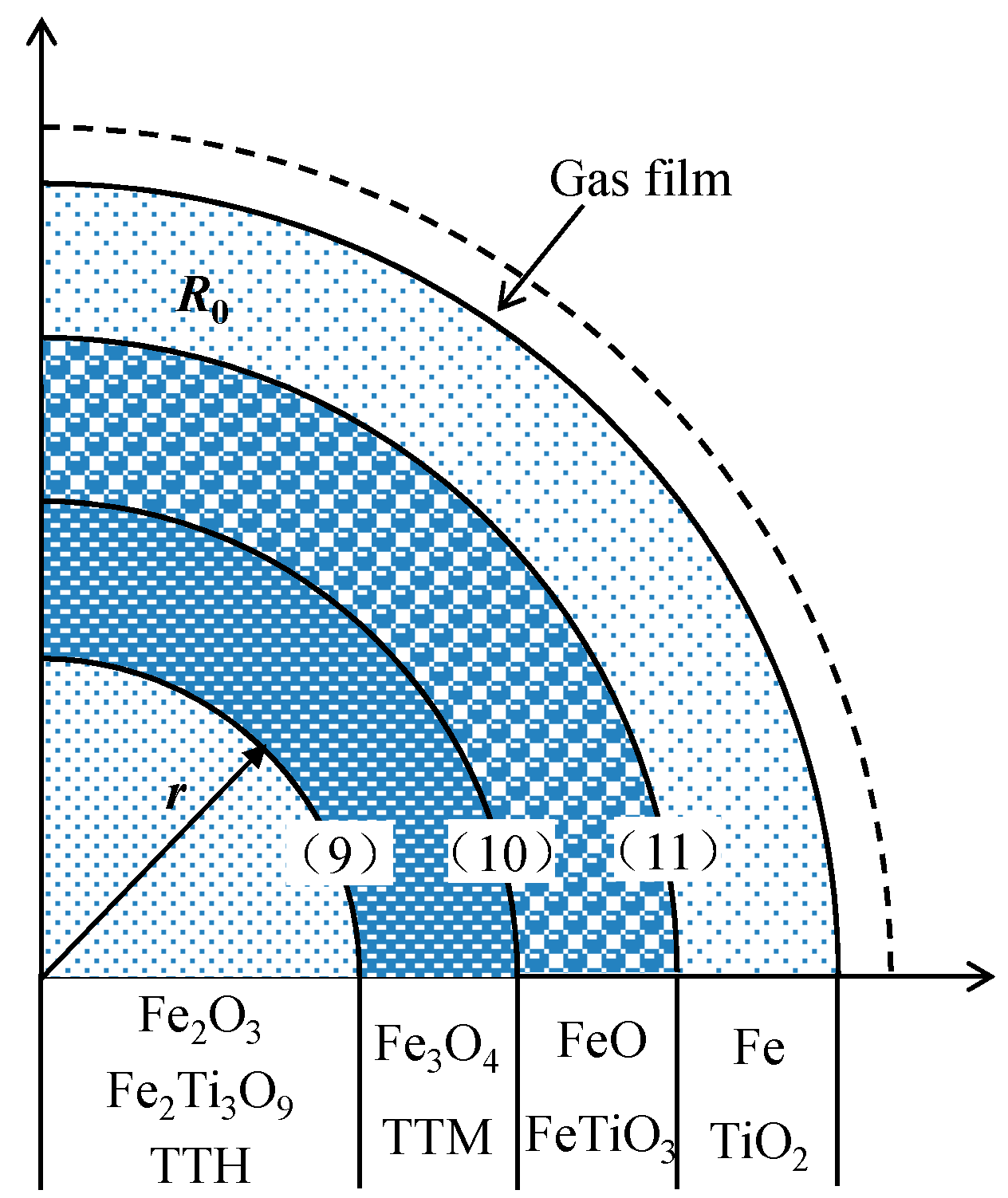
3.4. Non-Isothermal Gas-Based Direct Reduction Kinetics of HCVTM Pellets
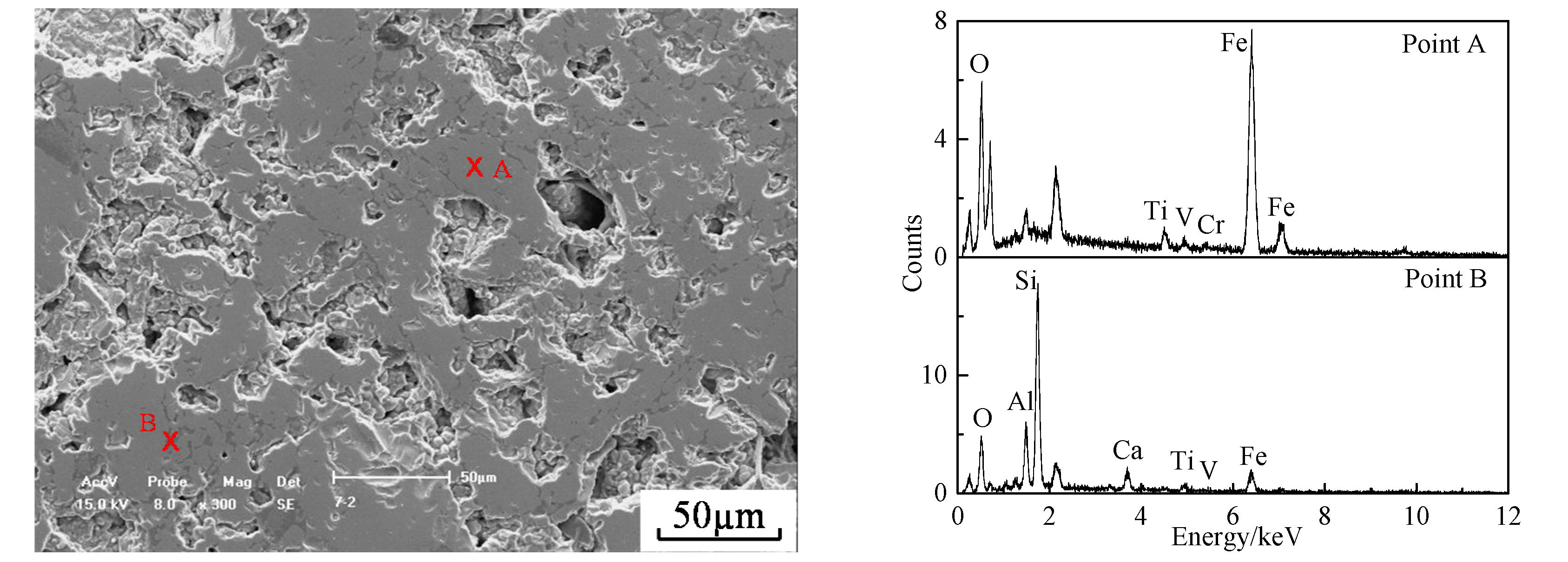
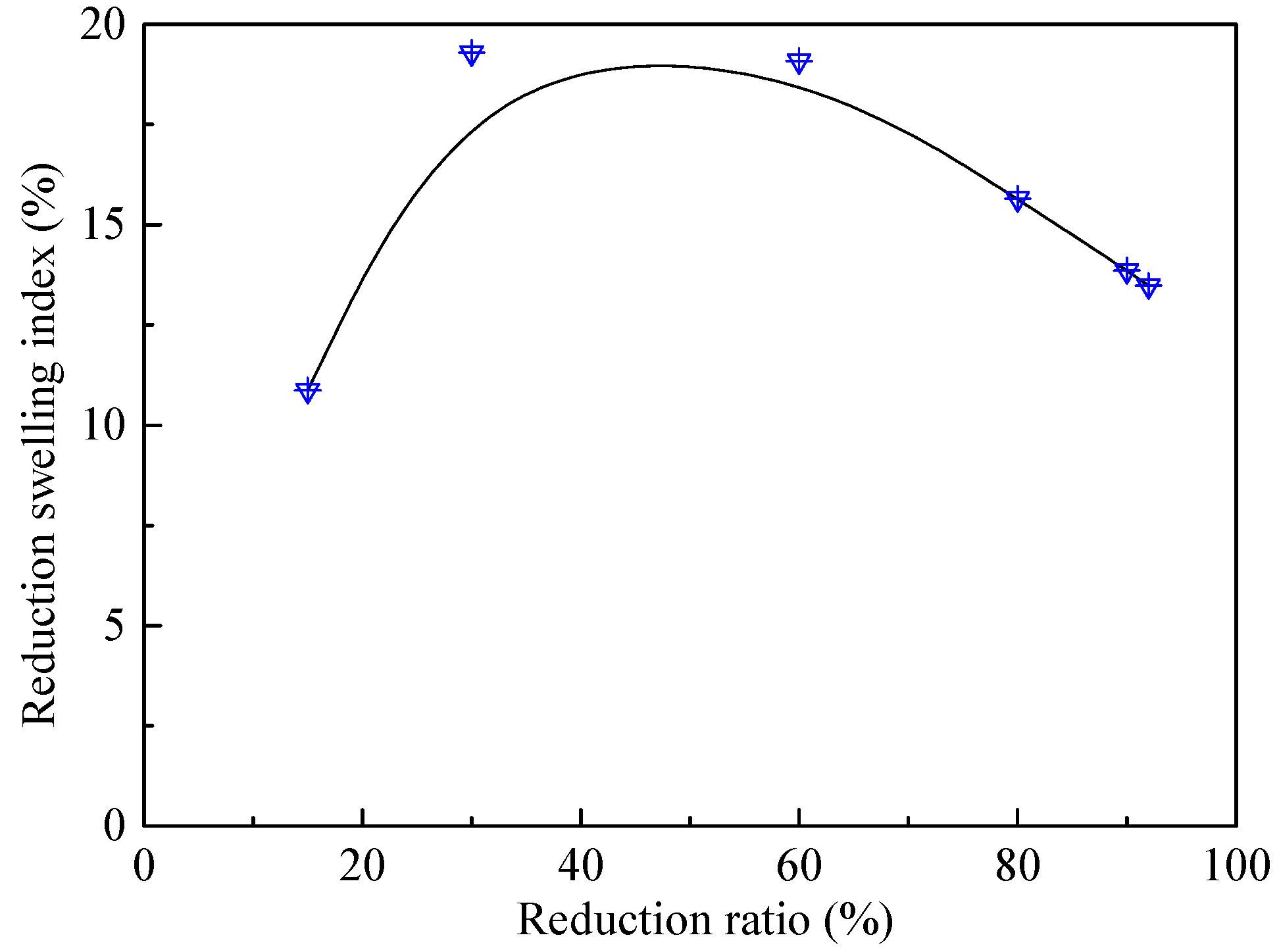
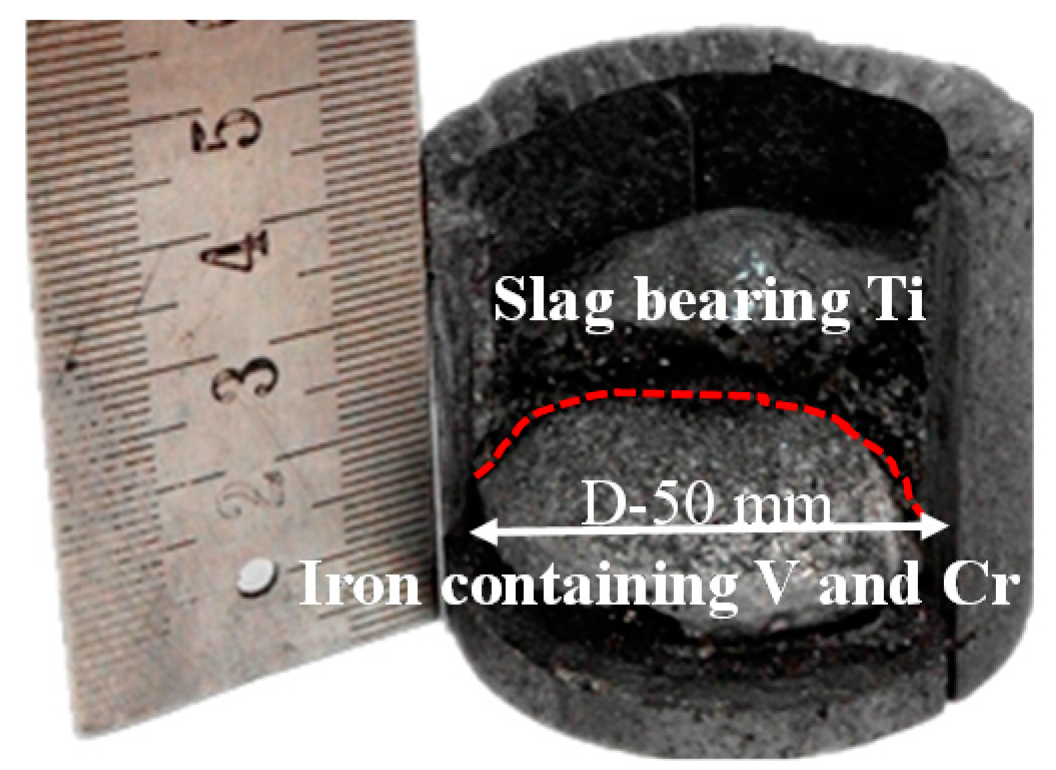
3.5. Melting Separation of the Metallized HCVTM Pellets
4. Conclusions
- (1)
- With the increase of the heating rate, the plot of the reduction ratio shifted to the right. The HCVTM pellets reached nearly the same reduction ratio under a given reducing gas composition, although the heating rates were different. Under the same heating rate, the gas mixture with more H2 was conducive for obtaining a higher reduction ratio.
- (2)
- The phase transformations of the HCVTM pellets during the non-isothermal reduction by the gas mixtures were ordered as follows: Fe2O3 → Fe3O4 → FeO → Fe; Fe9TiO15 + Fe2Ti3O9 → Fe2.75Ti0.25O4 → FeTiO3 → TiO2; V1.7Cr0.3O3 → V2O3 → Fe2VO4; Fe1.2Cr0.8O3 → Cr2O3 → FeCr2O4.
- (3)
- Based on the unreacted core model with multiple reaction interfaces, the kinetic model for the non-isothermal reduction of the HCVTM pellets by the gas mixtures was established as follows:where and are the mole fractions in the gas mixtures, respectively, %; T is the temperature at time t, K; T0 is the initial temperature, K; and β is the heating rate, K/min. The correlation coefficients were all greater than 0.99, revealing that the kinetic model could be properly used to describe the non-isothermal reduction of HCVTM pellets by gas mixtures.
- (4)
- Through the melting separation of the metallized HCVTM pellets, the iron containing V and Cr along with the Ti-rich slag was obtained. The mass fraction and recovery rates of Fe, V, and Cr in iron were 93.87% and 99.45%, 0.91% and 98.83%, and 0.72% and 95.02%, respectively. The mass fraction and recovery rate of the TiO2 in the slag were 38.12% and 95.08%, respectively.
- (5)
- The gas-based direct reduction process is a really promising technology for the disposal of HCVTM. It has low carbon emission and is environment-friendly. Furthermore, the recoveries of valuable elements including Fe, V, Cr, and Ti could be improved significantly through the novel process. The subsequent assessment of production cost and other things should be considered comprehensively in future research.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, M.; Liu, L.; Lv, X.; Bai, C.; Zhang, S. Crystallization Behavior of Perovskite in the Synthesized High-Titanium-Bearing Blast Furnace Slag Using Confocal Scanning Laser Microscope. Metall. Mater. Trans. B 2014, 45, 76–85. [Google Scholar] [CrossRef]
- Long, H.; Chun, T.; Wang, P.; Meng, Q.; Di, Z.; Li, J. Grinding Kinetics of Vanadium-Titanium Magnetite Concentrate in a Damp Mill and Its Properties. Metall. Mater. Trans. B 2016, 47, 1765–1772. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Zhang, J.; Li, G. Oxidation Kinetics and Oxygen Capacity of Ti-Bearing Blast Furnace Slag under Dynamic Oxidation Conditions. Metals 2016, 6, 105. [Google Scholar] [CrossRef]
- Zhou, W.; Xie, B.; Tan, W.F.; Diao, J.; Zhang, X.; Li, H.Y. Non-isothermal Crystallization Kinetics of Spinels in Vanadium Slag with High CaO Content. JOM 2016, 68, 2520–2524. [Google Scholar] [CrossRef]
- Tuo, X.; Xu, Z.; Teng, Y.; Mu, K. The Geochemical Characteristics of Heavy Metals in Soils in the Panzhihua V-Ti Magnetite Mine and the Pollution Evaluation. Bull. Mineral. Petrol. Geochem. 2007, 26, 127–131. [Google Scholar]
- Zhou, Y.; Yang, D.; Xie, Y.; Wang, P. Distribution Characteristics of Heavy Metals in Soils in Panzhihua V-Ti Magnetite Mine and Pollution Evaluation. Southwest China J. Agric. Sci. 2010, 23, 777–781. [Google Scholar]
- Chen, S.; Chu, M. Metalizing Reduction and Magnetic Separation of Vanadium Titano-magnetite Based on Hot Briquetting. J. Miner. Metall. Mater. 2014, 21, 225–233. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Han, Y.; She, X.; Xue, Q. Reduction Mechanism of Titanomagnetite Concentrate by Hydrogen. Int. J. Miner. Process. 2013, 125, 122–128. [Google Scholar] [CrossRef]
- Park, E.; Ostrovski, O. Reduction of Titania-Ferrous Ore by Carbon Monoxide. ISIJ Int. 2003, 43, 1316–1325. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.; Li, F.; Tang, Y.; Liu, Z.; Xue, X. Reduction Mechanism of High-chromium Vanadium–titanium Magnetite pellets by H2-CO-CO2 Gas Mixtures. Int. J. Miner. Metall. Mater. 2015, 22, 562–572. [Google Scholar] [CrossRef]
- Park, E.; Lee, S.B.; Ostrovski, O.; Min, D.J.; Rhee, C.H. Reduction of the Mixture of Titanomagnetite Ironsand and Hematite Iron Ore Fines by Carbon Monoxide. ISIJ Int. 2004, 44, 214–216. [Google Scholar] [CrossRef]
- Zhou, L.; Tao, D.; Fang, M.; Zeng, F.; Pu, X. Carbothermic Reduction of V-Ti Magnetite Ore. Chin. J. Rare Met. 2009, 33, 406–410. [Google Scholar]
- Jiang, T.; Wang, S.; Guo, Y.; Chen, F.; Zheng, F. Effects of Basicity and MgO in Slag on the Behaviors of Smelting Vanadium Titanomagnetite in the Direct Reduction-Electric Furnace Process. Metals 2016, 6, 107. [Google Scholar] [CrossRef]
- Hu, T.; Lv, X.; Bai, C.; Lun, Z.; Qiu, G. Reduction Behavior of Panzhihua Titanomagnetite Concentrates with Coal. Metall. Mater. Trans. B. 2013, 44, 252–260. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, J.; Liu, Z.; Chu, M.; Xue, X. Non-isothermal Reduction Mechanism and Kinetics of High Chromium Vanadium–titanium Magnetite pellets. Ironmak. Steelmak. 2015, 42, 17–26. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, S.; Wang, X.; Chen, B.; Yang, H.; Wang, S.; Luo, P. Recovery of Iron from Chromium Vanadium-Bearing Titanomagnetite Concentrate by Direct Reduction. JOM 2016, 68, 2698–2703. [Google Scholar] [CrossRef]
- Zhang, P. Basic Technology Study on Gas-based Direct Reduction for Indonesia Vanadium-Titanium Magnetite. Master’s Thesis, Northeastern University, Shenyang, China, 2014. [Google Scholar]
- Li, Y. Basic Research on Gas-based Reduction of Indonesia Ironsand Oxidized Pellets. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Zhang, J.; Zheng, C.; Tang, Y.; Chai, F. Reduction of Panzhihua titanium-bearing oxidized pellets by CO-N2-H2 gas. J. Cent. South Univ. 2016, 23, 1015–1022. [Google Scholar] [CrossRef]
- Park, E.; Ostrovski, O. Reduction of Titania-ferrous Ore by Hydrogen. ISIJ Int. 2004, 44, 999–1005. [Google Scholar] [CrossRef]
- Ono, N.H.; Yonezawa, T.; Usui, T. Effect of Water-Gas Shift Reaction on Reduction of Iron Oxide Powder Packed Bed with H2-CO Mixtures. ISIJ Int. 2003, 43, 1502–1511. [Google Scholar]
- He, G.; Du, X.; Zhang, K.; Tang, Z.; Lou, T.; Tu, G. Research on the Process of Deep Reduction-smelting Separation of Hongge Ore and the Trend of V, Cr. J. Mater. Metall. 2014, 13, 15–19. [Google Scholar]
- Zhao, L.; Wang, L.; Chen, D.; Zhao, H.; Liu, Y.; Qi, T. Behaviors of Vanadium and Chromium in Coal-based Direct Reduction of High-chromium Vanadium-bearing Titanomagnetite Concentrates Followed by Magnetic Separation. Trans. Nonferrous Met. Soc. China 2015, 25, 1325–1331. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, S.; Jiang, T.; Xue, X. Influence of MgO in Form of Magnesite on Properties and Mineralogy of High Chromium, Vanadium, Titanium Magnetite Sinters. Ironmak. Steelmak. 2015, 42, 217–225. [Google Scholar] [CrossRef]
- Dang, J.; Chou, K.; Hu, X.; Zhang, G. Reduction Kinetics of Metal Oxides by Hydrogen. Steel Res. Int. 2013, 84, 526–533. [Google Scholar] [CrossRef]
- Chou, K. A Kinetic Model for Oxidation of Si-Al-O-N Materials. J. Am. Ceram. Soc. 2006, 89, 1568–1576. [Google Scholar] [CrossRef]
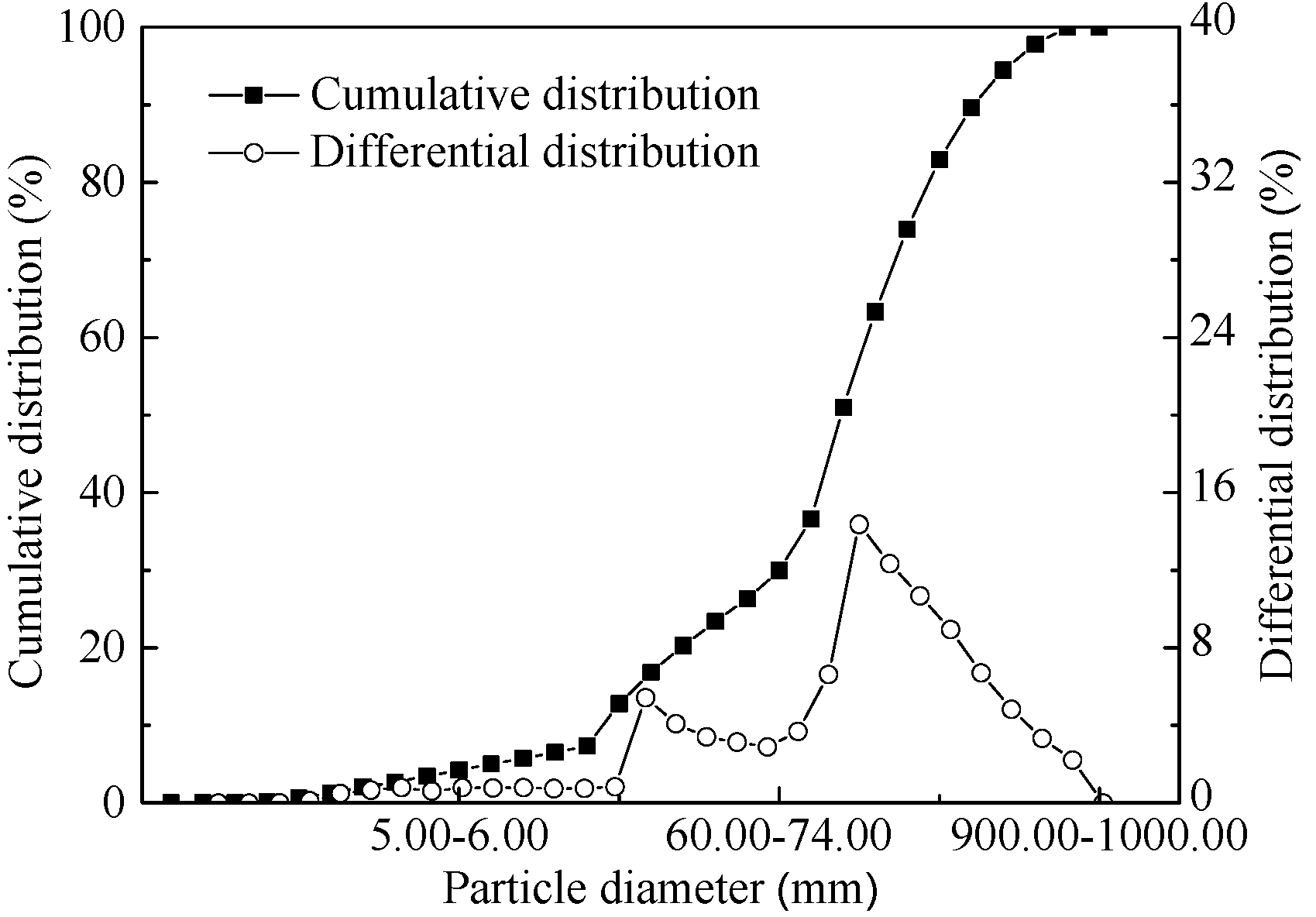
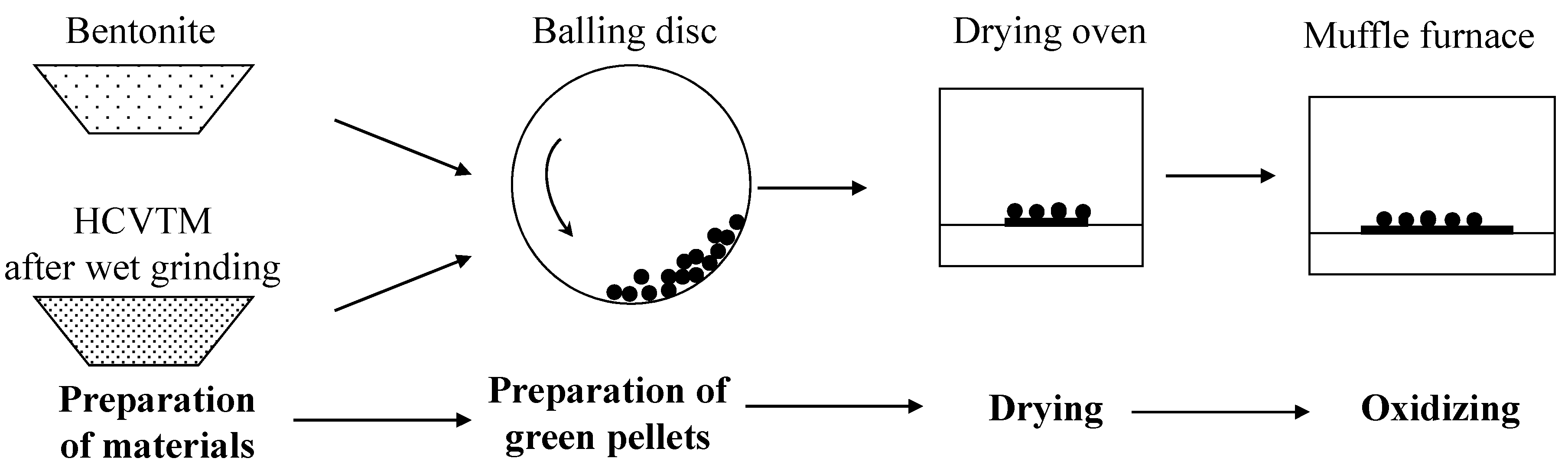
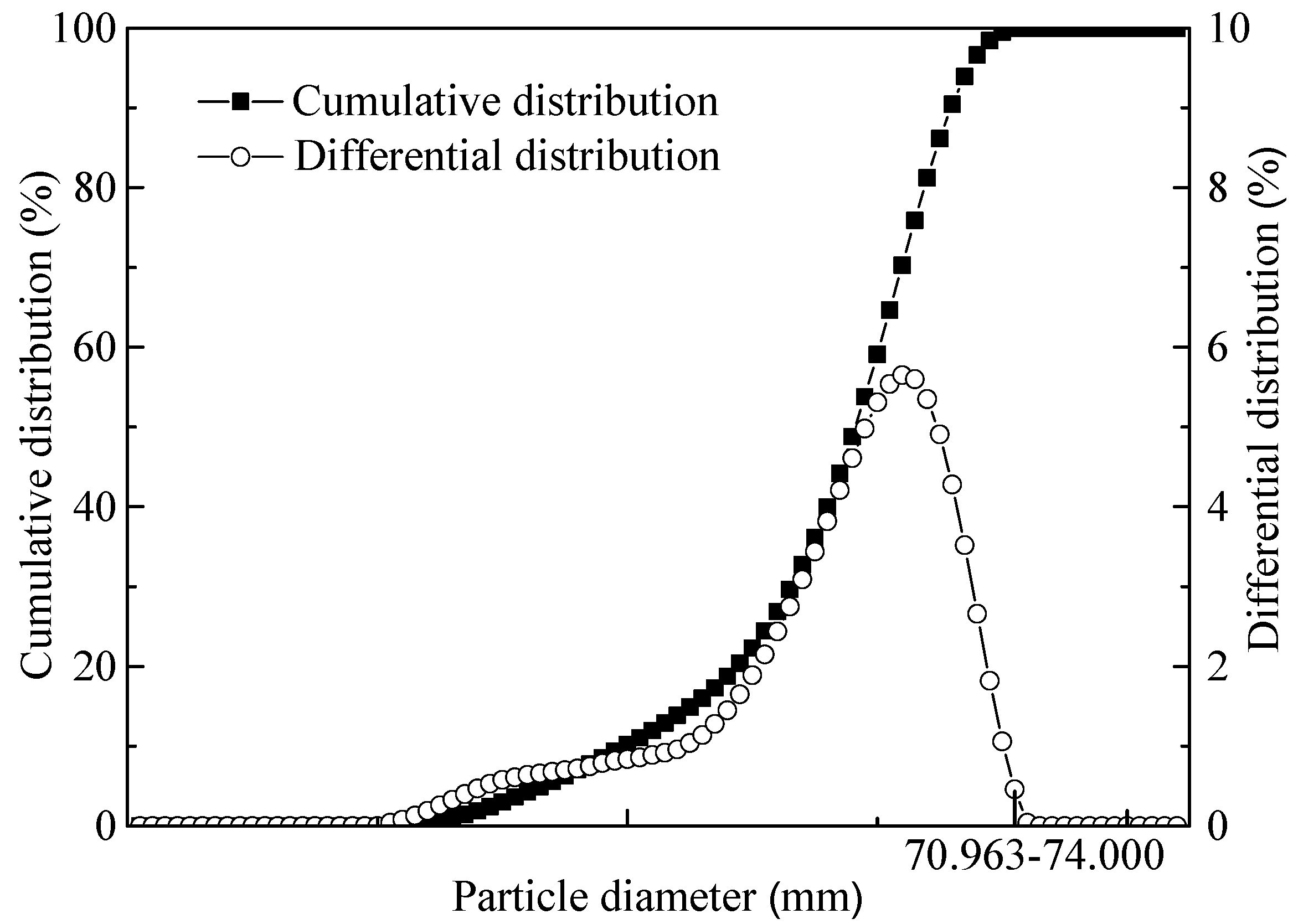





| Item | TFe | V2O5 | Cr2O3 | TiO2 | Al2O3 | SiO2 | MgO | CaO | S | P | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCVTM | 62.12 | 0.95 | 0.61 | 5.05 | 3.18 | 2.12 | 0.92 | 0.22 | 0.04 | 0.01 | - | - |
| Bentonite | - | - | - | - | 14..47 | 67.45 | 4.61 | 2.47 | - | - | 1.68 | 1.19 |
| TFe | FeO | TiO2 | V2O5 | Cr2O3 | Al2O3 | SiO2 | MgO | CaO |
|---|---|---|---|---|---|---|---|---|
| 59.46 | 0.50 | 4.49 | 0.91 | 0.59 | 3.07 | 2.03 | 1.03 | 0.21 |
| Reduction Ratio (%) | Phase Composition |
|---|---|
| 0 | Fe2O3, Fe9TiO15, V1.7Cr0.3O3, Fe1.2Cr0.8O3, Fe2Ti3O9 |
| 15 | Fe3O4, Fe2.75Ti0.25O4, Fe2VO4, FeCr2O4 |
| 30 | Fe, Fe3O4, FeO, Fe2.75Ti0.25O4, Fe2VO4, FeCr2O4 |
| 60 | Fe, Fe3O4, FeO, Fe2.75Ti0.25O4, Fe2VO4, FeCr2O4 |
| 80 | Fe, Fe3O4, FeO, Fe2.75Ti0.25O4, Fe2VO4, FeCr2O4 |
| 90 | Fe, FeO, FeTiO3 |
| 92 | Fe, TiO2 |
| Parameter | (J/mol) | |||||
| Value | 90.64 | 83.98 | 171.80 | 145.94 | 158.51 | 83.79 |
| Parameter | (-) | |||||
| Value | 0.68 | 8.86 | 4.60 | 1.56 | 7.10 | 4.20 |
| TFe | MFe | TiO2 | V2O5 | Cr2O3 | Al2O3 | SiO2 | MgO | CaO |
|---|---|---|---|---|---|---|---|---|
| 76.64 | 70.01 | 9.241 | 1.334 | 0.899 | 4.26 | 3.16 | 1.51 | 0.34 |
| Composition of Iron/% | |||||||
| TFe | V | Cr | Ti | Si | S | P | - |
| 93.87 | 0.91 | 0.72 | 0.56 | 0.034 | 0.027 | 0.041 | - |
| Composition of Slag/% | |||||||
| TFe | V | Cr | TiO2 | CaO | SiO2 | MgO | Al2O3 |
| 1.82 | 0.038 | 0.133 | 38.21 | 21.30 | 13.45 | 6.55 | 18.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Chu, M.-S.; Ying, Z.-W.; Li, F.; Feng, C.; Liu, Z.-G. Non-Isothermal Gas-Based Direct Reduction Behavior of High Chromium Vanadium-Titanium Magnetite Pellets and the Melting Separation of Metallized Pellets. Metals 2017, 7, 153. https://doi.org/10.3390/met7050153
Tang J, Chu M-S, Ying Z-W, Li F, Feng C, Liu Z-G. Non-Isothermal Gas-Based Direct Reduction Behavior of High Chromium Vanadium-Titanium Magnetite Pellets and the Melting Separation of Metallized Pellets. Metals. 2017; 7(5):153. https://doi.org/10.3390/met7050153
Chicago/Turabian StyleTang, Jue, Man-Sheng Chu, Zi-Wei Ying, Feng Li, Cong Feng, and Zheng-Gen Liu. 2017. "Non-Isothermal Gas-Based Direct Reduction Behavior of High Chromium Vanadium-Titanium Magnetite Pellets and the Melting Separation of Metallized Pellets" Metals 7, no. 5: 153. https://doi.org/10.3390/met7050153





