Preparation of Highly Pure Vanadyl Sulfate from Sulfate Solutions Containing Impurities of Iron and Aluminum by Solvent Extraction Using EHEHPA
Abstract
:1. Introduction
2. Materials and Methods
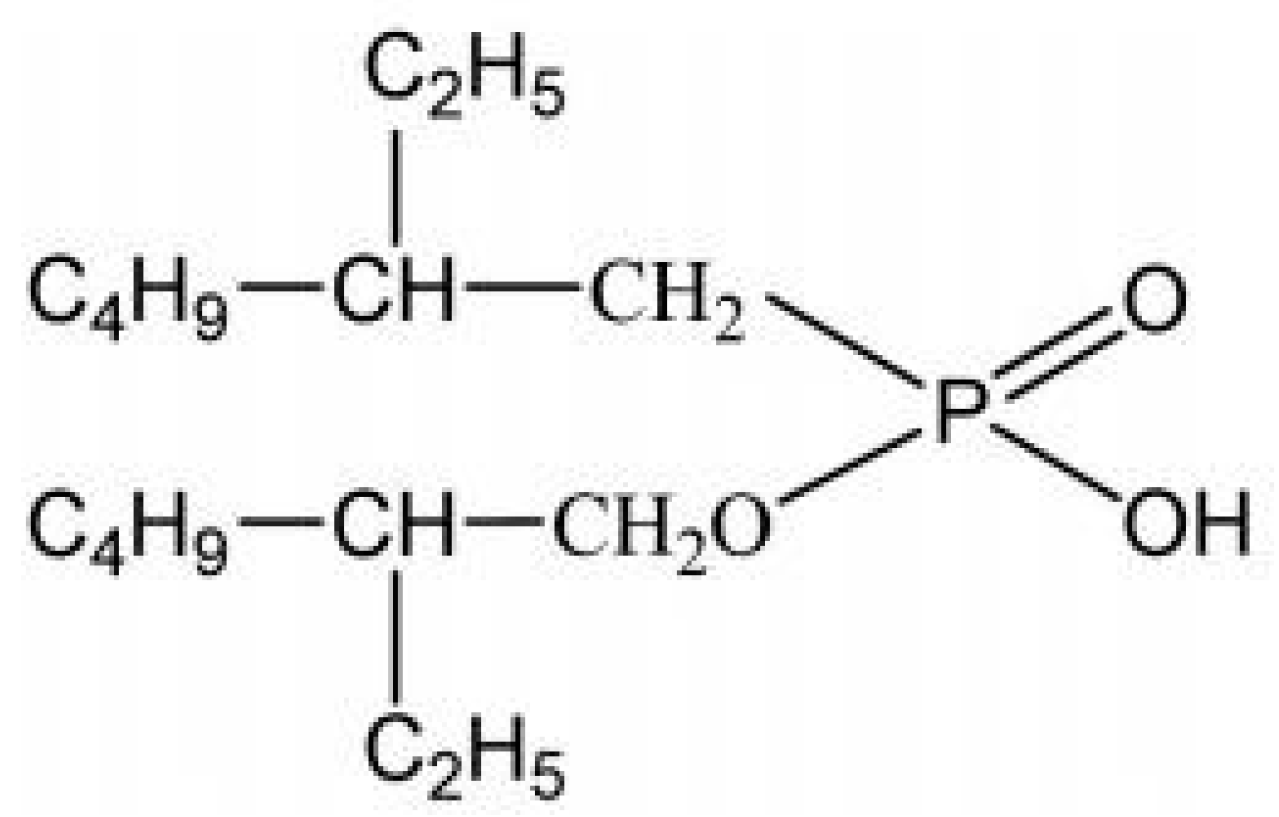
2.1. Materials and Reagents
2.2. Procedures and Apparatus
2.3. Data Treatment
3. Results and Discussion
3.1. Extraction Process
3.1.1. Effect of Initial pH
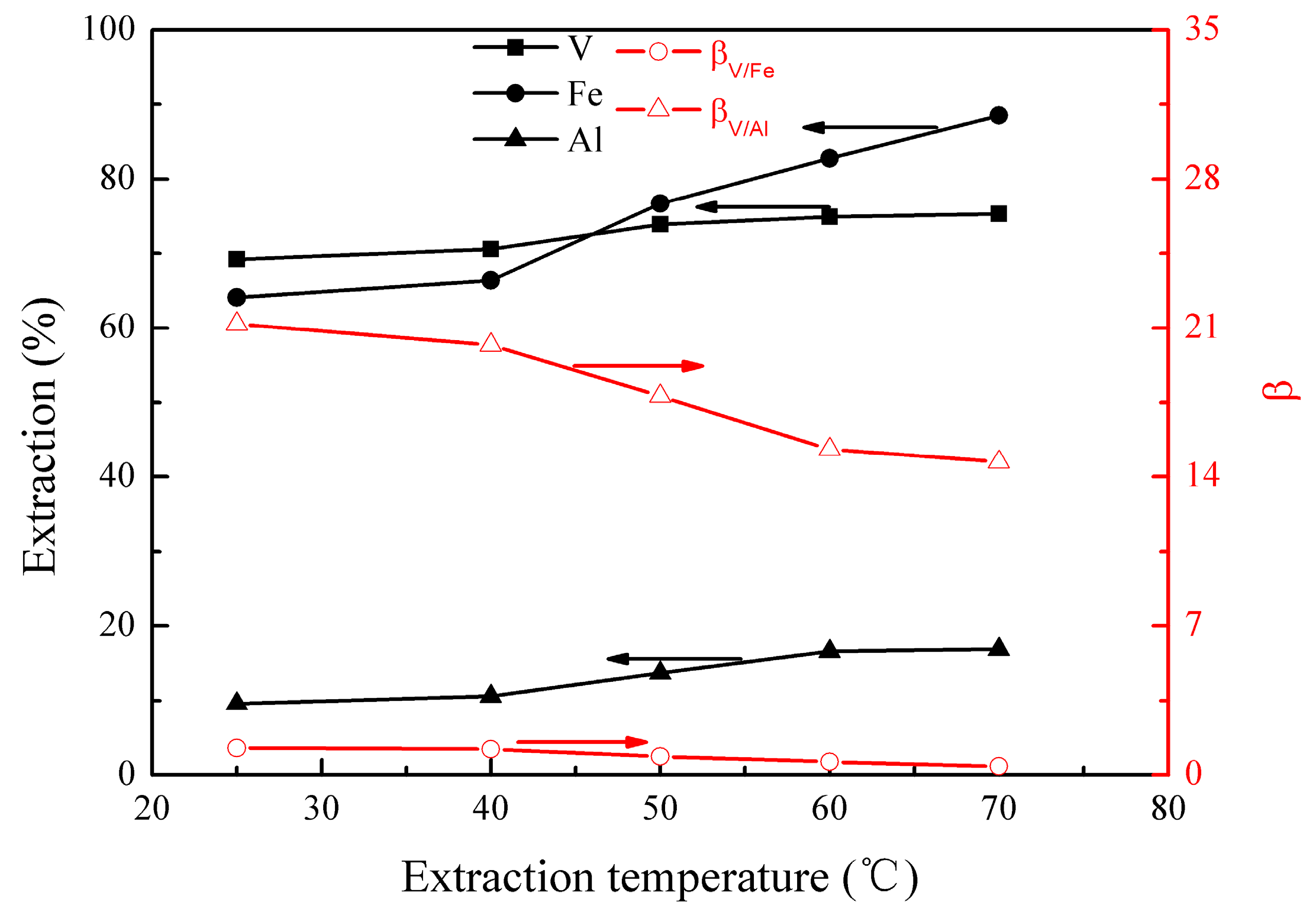
3.1.2. Effect of Extraction Temperature
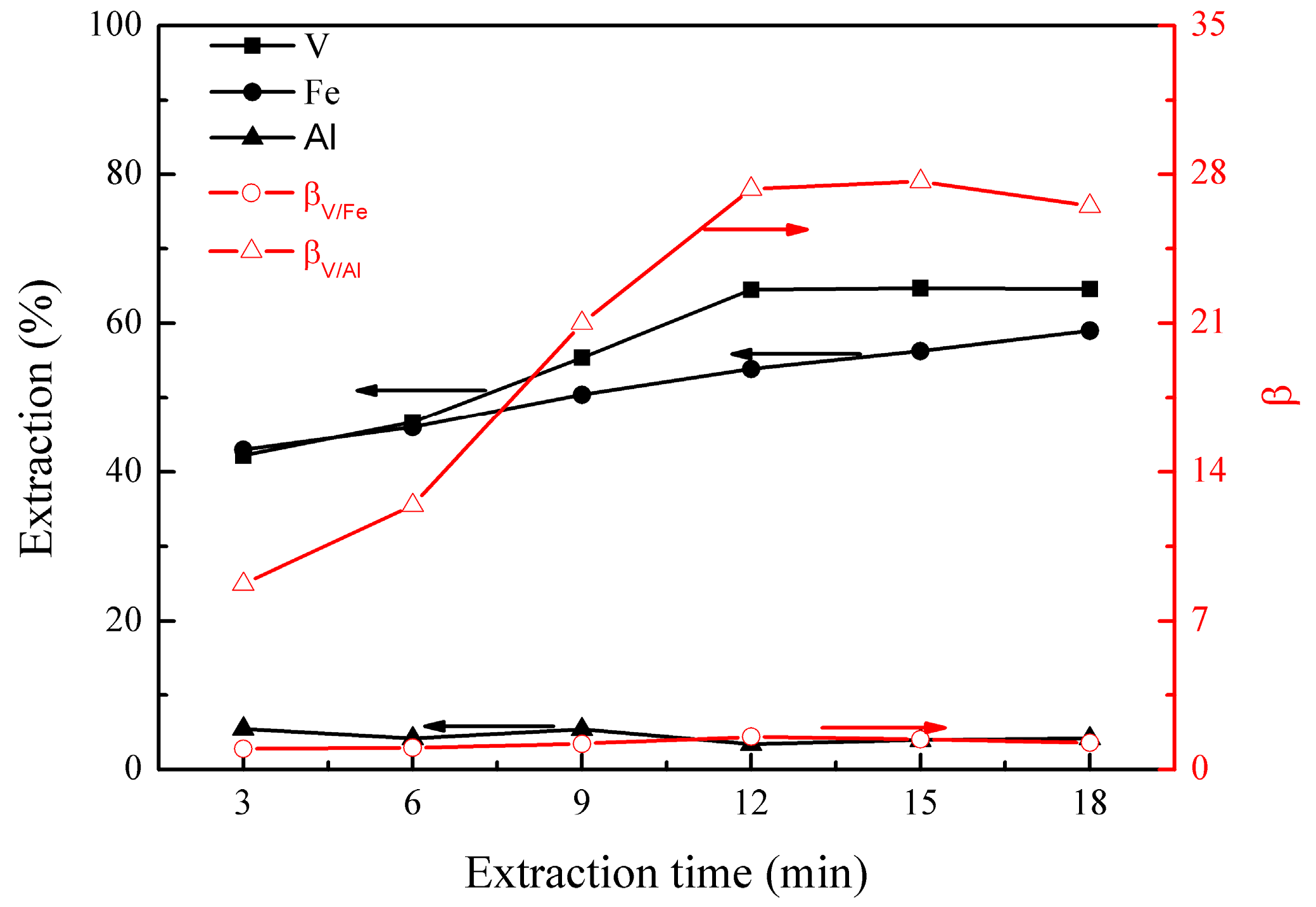
3.1.3. Effect of Extraction Time
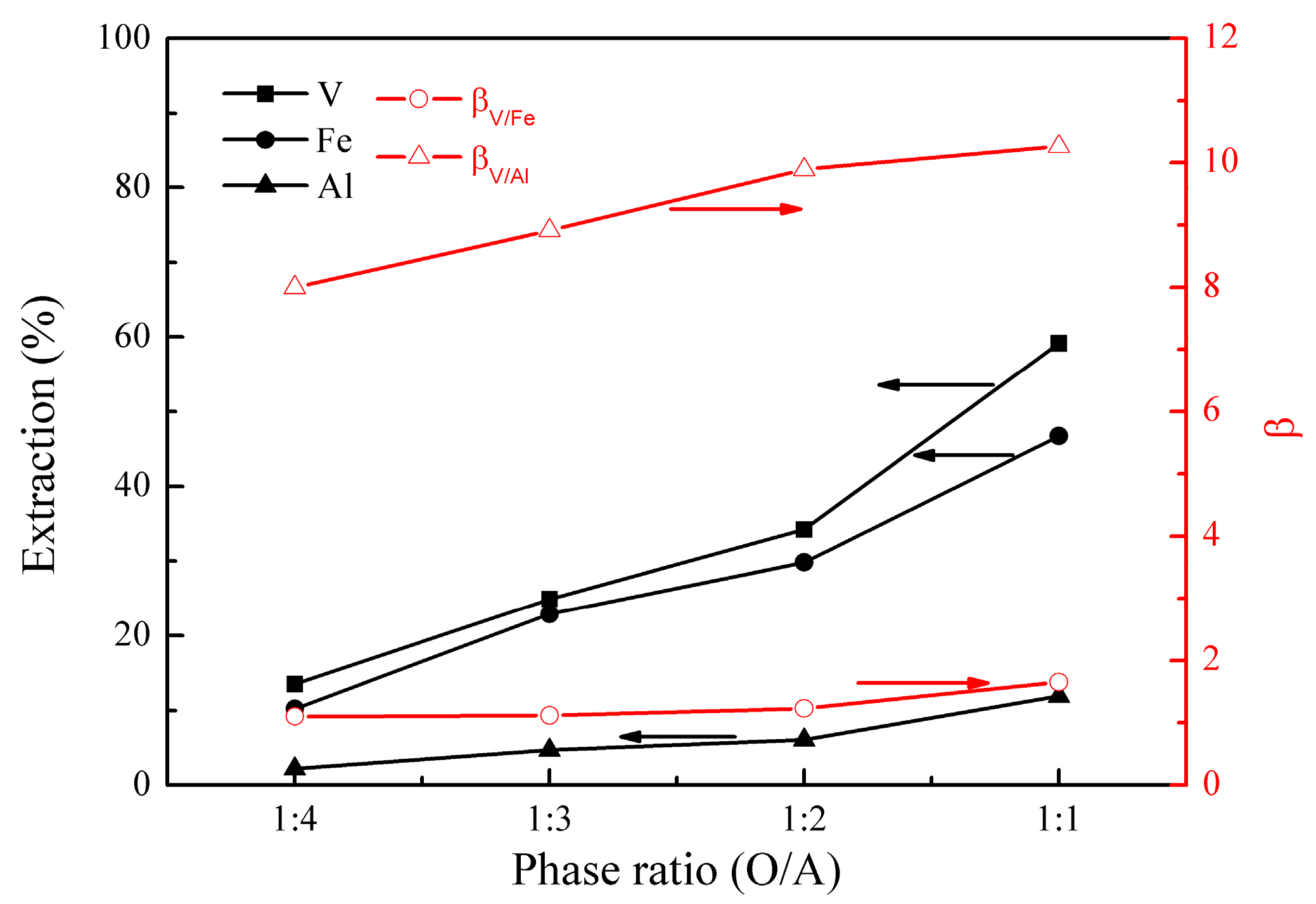
3.1.4. Effect of Phase Ratio (O/A)
3.1.5. Effect of EHEHPA Concentration
3.2. Stripping Process
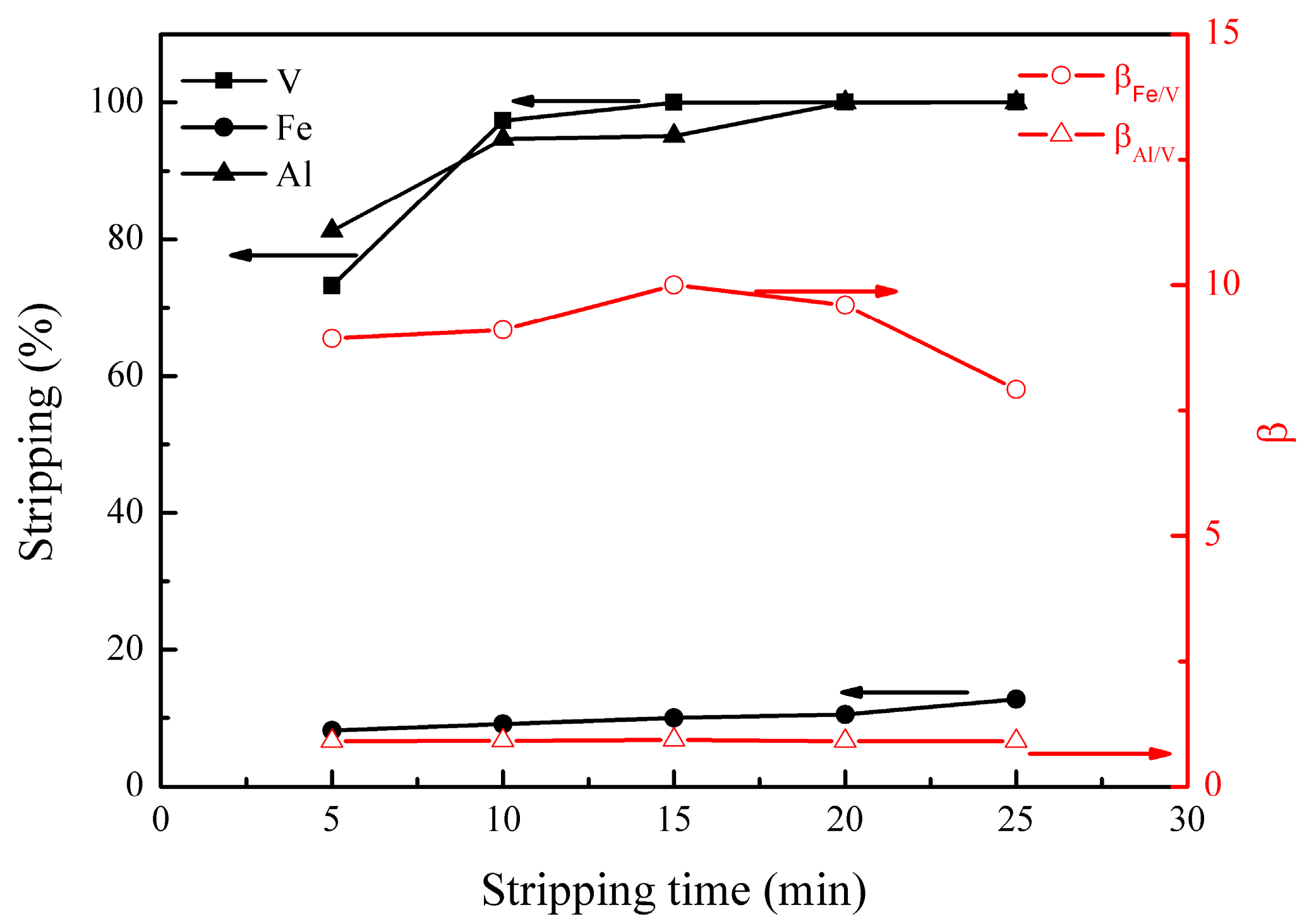
3.2.1. Effect of Stripping Time
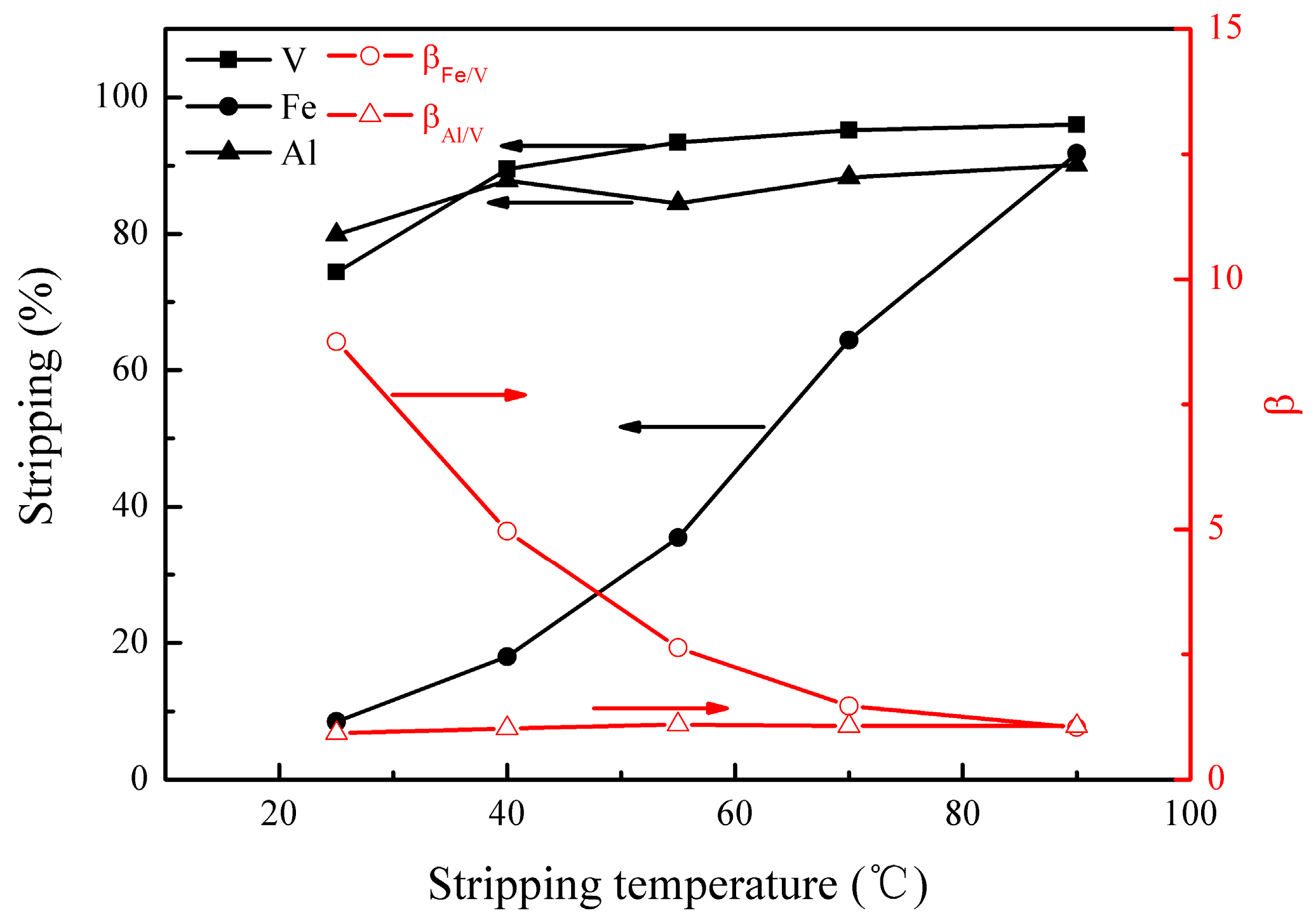
3.2.2. Effect of Stripping Temperature
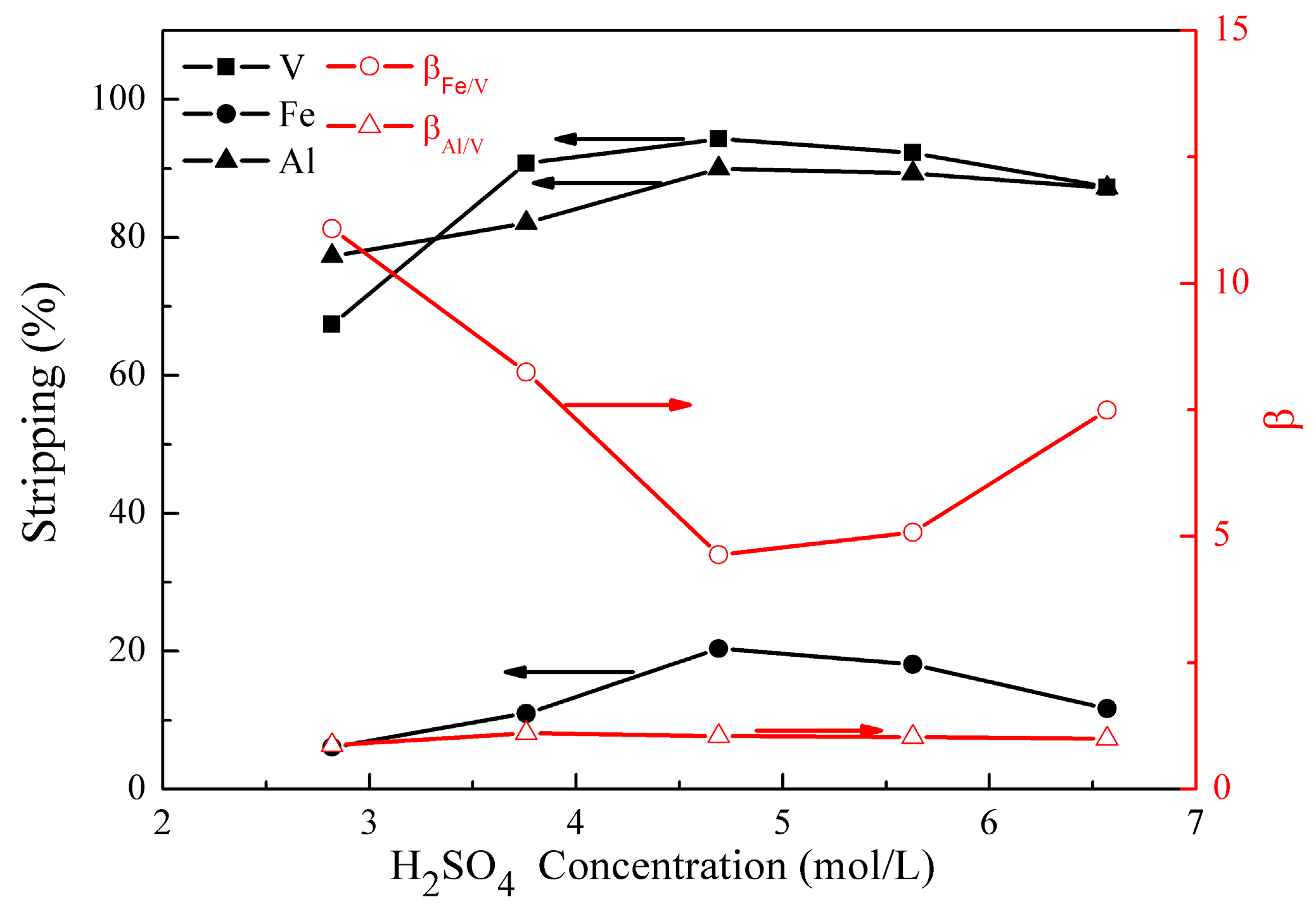
3.2.3. Effect of H2SO4 Concentration
3.2.4. Effect of Phase Ratio (O/A)
3.3. Contents of Fe and Al by Multiple Stages Extraction and Stripping
3.4. Organic Regeneration
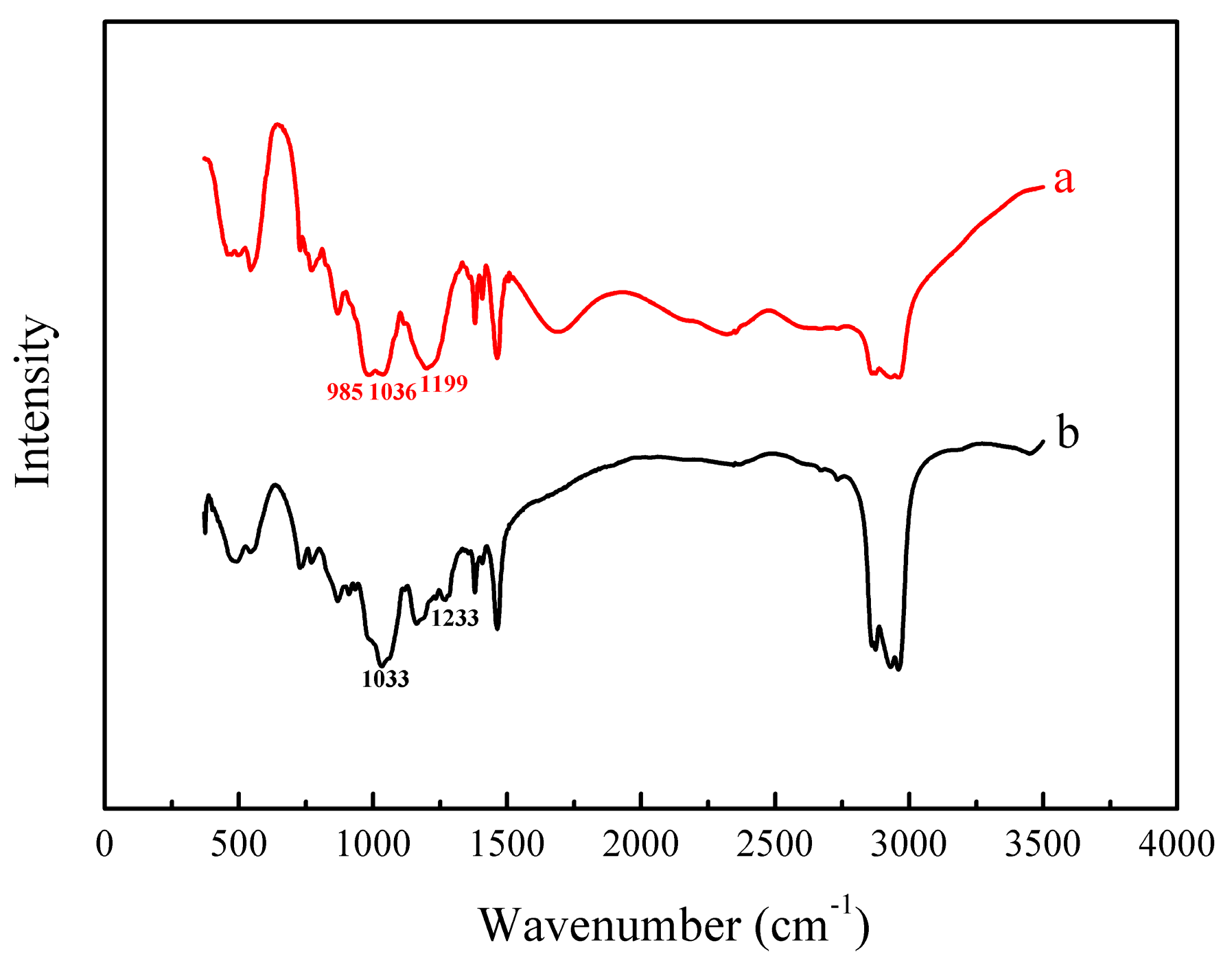
3.5. Metal Extraction Analysis by FT-IR Spectrum
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, G.; Chen, D.; Zhao, W.; Zhao, H.; Wang, L.; Li, D.; Qi, T. A novel synergistic extraction method for recovering vanadium (V) from high-acidity chloride leaching liquor. Sep. Purif. Technol. 2016, 165, 166–172. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, H.; Hu, G.; Qi, T.; Yu, H.; Zhang, G.; Wang, L.; Wang, W. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite. J. Hazard. Mater. 2015, 294, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Lin, X.; Wang, X.; Cao, H. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Nam, S.; Lee, D.; Kim, J.; Lee, D.G. Development of a fluoroelastomer/glass fiber composite flow frame for a vanadium redox flow battery (VRFB). Compos. Struct. 2016, 145, 113–118. [Google Scholar] [CrossRef]
- Huang, K.; Li, X.; Liu, S.; Tan, N.; Chen, L. Research progress of vanadium redox flow battery for energy storage in China. Renew. Energy 2008, 33, 186–192. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, S.; Zhang, Q.; Huang, K.; Li, Q.; Qin, D.; Wu, X.; Li, H.; Liu, W. A Method of Preparing Vanadyl Sulfate Applied to the Vanadium Redox Flow Battery. Chinese Patent No.201010140475.2, 26 March 2010. [Google Scholar]
- Mao, F.; Sun, Z.; Li, D.; Peng, H.; Yang, L.; Chen, W.; Cao, M. A Method of Preparing High-Purity Vanadyl Sulfate for the Vanadium Redox Flow Battery Electrolyte. Chinese Patent No.2013103722877.9, 23 August 2013. [Google Scholar]
- Xu, Y.; Wen, Y.; Cheng, J.; Yang, Y.; Cao, G. A Method of Two-Stage Extraction to Prepare High-Purity Vanadyl Sulfate Solution. Chinese Patent No.201210209648.0, 25 June 2010. [Google Scholar]
- Chagnes, A.; Rager, M.-N.; Courtaud, B.; Thiry, J.; Cote, G. Speciation of vanadium (V) extracted from acidic sulfate media by trioctylamine in n-dodecane modified with 1-tridecanol. Hydrometallurgy 2010, 104, 20–24. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Wu, J.; Li, C.; Li, M.; Deng, Z.; Xu, H. Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and Cyanex 272 in kerosene. Trans. Nonferr. Met. Soc. 2012, 22, 461–466. [Google Scholar] [CrossRef]
- Nekovar, P.; Schrotterova, D. Liquid-liquid extraction of Mo(VI) and V (V) by primene JMT. J. Radioanal. Nucl. Chem. 1998, 228, 95–98. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Bao, S.; Shen, C. Separation and recovery of vanadium from a sulfuric-acid leaching solution of stone coal by solvent extraction using trialkylamine. Sep. Purif. Technol. 2016, 164, 49–55. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Liu, W.; Liang, B.; Yue, H.; Li, C. Selective extraction of nitric and acetic acids from etching waste acid using N235 and MIBK mixtures. Sep. Purif. Technol. 2016, 169, 50–58. [Google Scholar] [CrossRef]
- Bal, Y.; Bal, K.E.; Cote, G. Kinetics of the alkaline stripping of vanadium(V) previously extracted by Aliquat® 336. Miner. Eng. 2002, 15, 377–379. [Google Scholar] [CrossRef]
- Cheraghi, A.; Ardakani, M.S.; Keshavarz Alamdari, E.; Haghshenas Fatmesari, D.; Darvishi, D.; Sadrnezhaad, S.K. Thermodynamics of vanadium (V) solvent extraction by mixture of D2EHPAand TBP. Int. J. Miner. Process. 2015, 138, 49–54. [Google Scholar] [CrossRef]
- Sole, K.C.; Feather, A.; O’Connell, J.P. Recovery of titanium from the leach liquors of titaniferous magnetites by solvent extraction: Part 3. Continuous mini-plant trials. Hydrometallurgy 1999, 51, 275–284. [Google Scholar] [CrossRef]
- Narayanan Remya, P.; Lakshmipathy Reddy, M. Solvent extraction separation of titanium(IV), vanadium(V) and iron(III) from simulated waste chloride liquors of titanium minerals processing industry by the trialkylphosphine oxide Cyanex 923. J. Chem. Technol. Biotechnol. 2004, 79, 734–741. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, C.Y. Recovery of molybdenum and vanadium from synthetic sulphuric acid leach solutions of spent hydrodesulphurisation catalysts using solvent extraction. Hydrometallurgy 2010, 101, 141–147. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Wu, J.; Li, M.; Deng, Z.; Li, C.; Xu, H. Co-extraction and selective stripping of vanadium (IV) and molybdenum (VI) from sulphuric acid solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Sep. Purif. Technol. 2012, 86, 64–69. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Solvent extraction of vanadium (V) from sulfate solutions using LIX63 and PC88A. J. Ind. Eng. Chem. 2015, 31, 118–123. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, H.; Zhou, Y. Selective separation and extraction of vanadium(IV) and manganese(II) from co-leaching solution of roasted stone coal and pyrolusite via solvent extraction. Ind. Eng. Chem. Res. 2013, 52, 13768–13776. [Google Scholar] [CrossRef]
- Tavakoli, M.R.; Dreisinger, D.B. Separation of vanadium from iron by solvent extraction using acidic and neutral organophosporus extractants. Hydrometallurgy 2014, 141, 17–23. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Zhu, B.; Yang, Y.; Wang, L. Adsorption and selective separation of neodymium with magnetic alginate microcapsules containing the extractant 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. J.Chem. Eng. Data 2011, 56, 2280–2289. [Google Scholar] [CrossRef]
- Jayadas, S.; Reddy, M.L. Solvent extraction separation of vanadium(V) from multivalent metal chloride solutions using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. J. Chem. Technol. Biotechnol. 2002, 77, 1149–1156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Lv, G.; Zhang, G.; Liu, Y.; Zhang, W. Synergistic extraction of vanadium(IV) in sulfuric acid media using amixture of D2EHPA and EHEHPA. Hydrometallurgy 2016, 166, 87–93. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]



| Contents of Impurities (mg/L) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Fe | 275 | 221 | 154 | 80 | 12 |
| Al | 196 | 165 | 117 | 60 | 10 |
| Functional Groups | P–O–H | P=O | P–O–C |
|---|---|---|---|
| before loading (cm−1) | 985 | 1199 | 1036 |
| after loading (cm−1) | Disappeared | 1233 | 1033 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Chen, D.; Zhang, G.; Zhao, H.; Qi, T.; Wang, W.; Wang, L.; Liu, Y. Preparation of Highly Pure Vanadyl Sulfate from Sulfate Solutions Containing Impurities of Iron and Aluminum by Solvent Extraction Using EHEHPA. Metals 2017, 7, 106. https://doi.org/10.3390/met7030106
Li D, Chen D, Zhang G, Zhao H, Qi T, Wang W, Wang L, Liu Y. Preparation of Highly Pure Vanadyl Sulfate from Sulfate Solutions Containing Impurities of Iron and Aluminum by Solvent Extraction Using EHEHPA. Metals. 2017; 7(3):106. https://doi.org/10.3390/met7030106
Chicago/Turabian StyleLi, Dan, Desheng Chen, Guozhi Zhang, Hongxin Zhao, Tao Qi, Weijing Wang, Lina Wang, and Yahui Liu. 2017. "Preparation of Highly Pure Vanadyl Sulfate from Sulfate Solutions Containing Impurities of Iron and Aluminum by Solvent Extraction Using EHEHPA" Metals 7, no. 3: 106. https://doi.org/10.3390/met7030106





